Preparation of Transparent Fast-Growing Poplar Veneers with a Superior Optical Performance, Excellent Mechanical Properties, and Thermal Insulation by Acetylation Modification Using a Green Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Materials
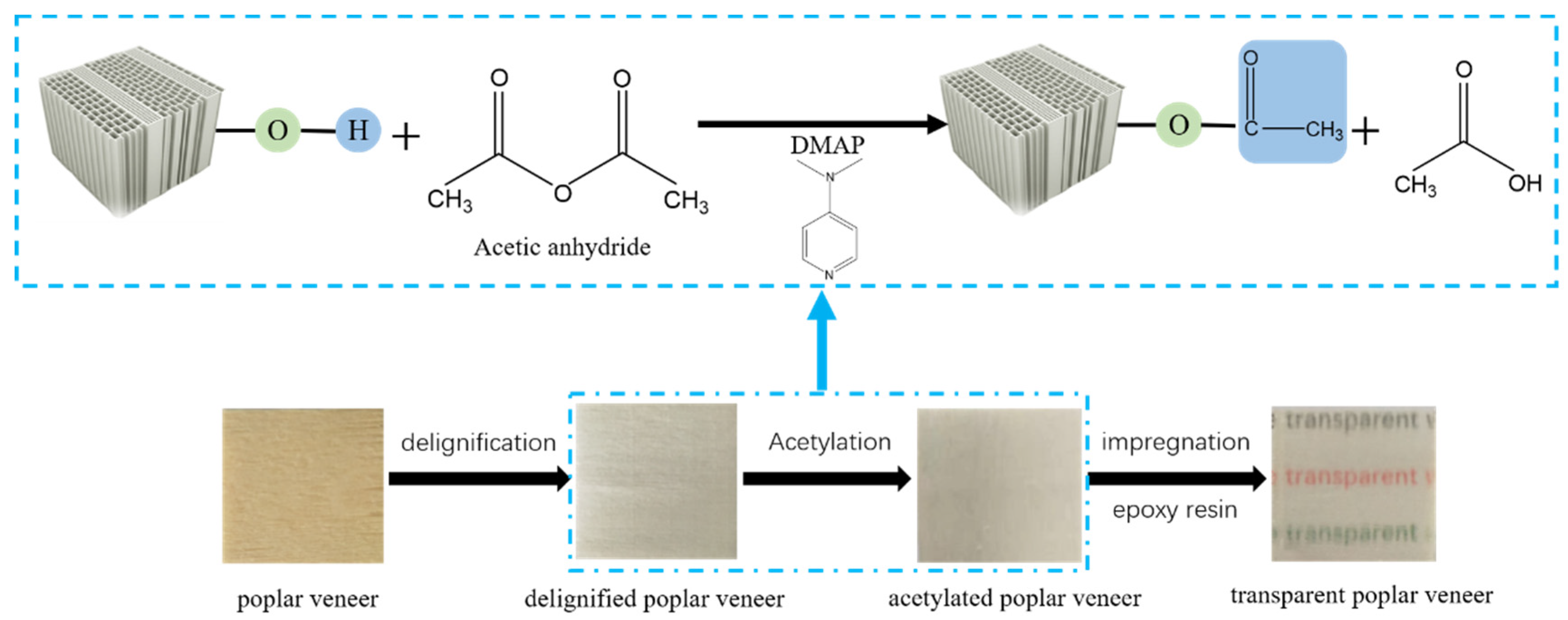
2.2. Preparation of Transparent Poplar Veneers
2.3. Characterization
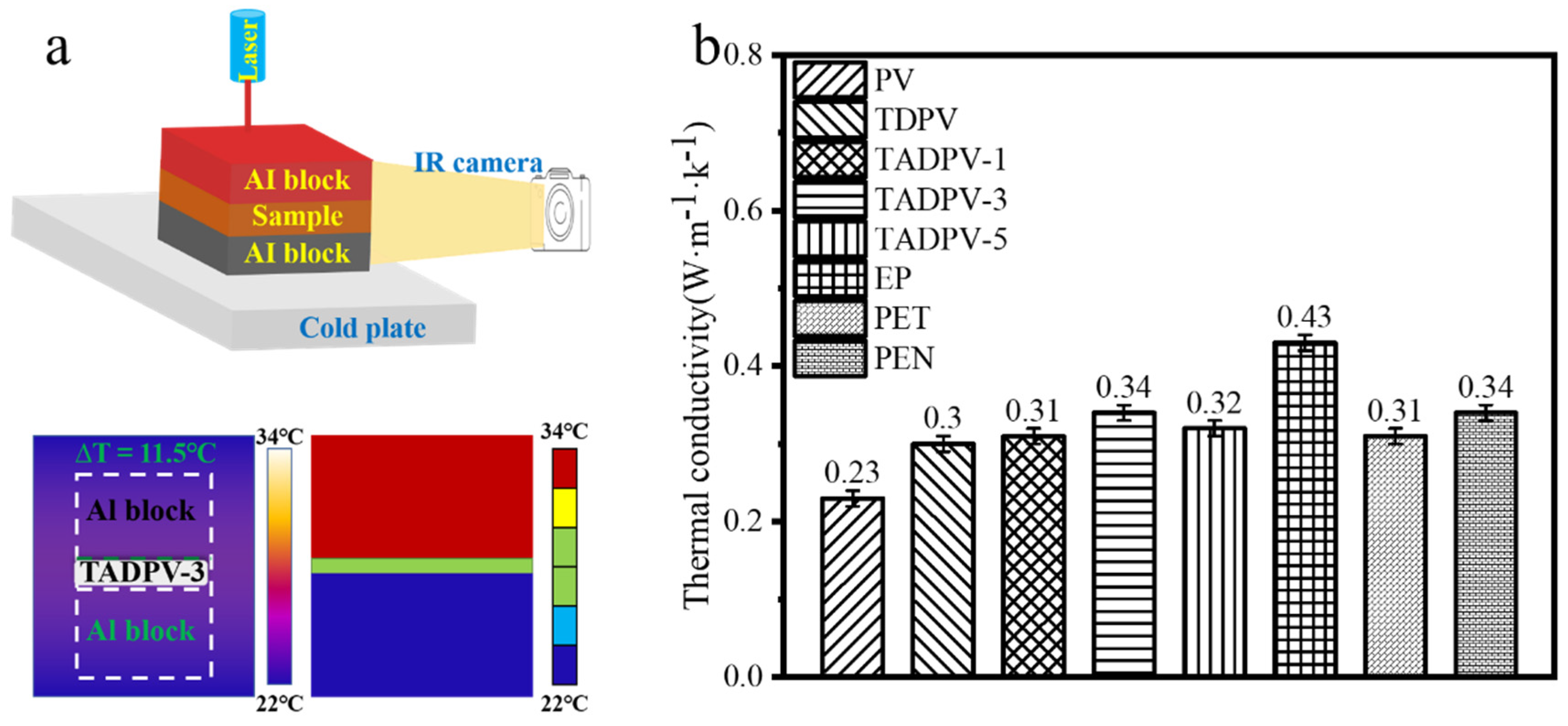
3. Results and Discussion
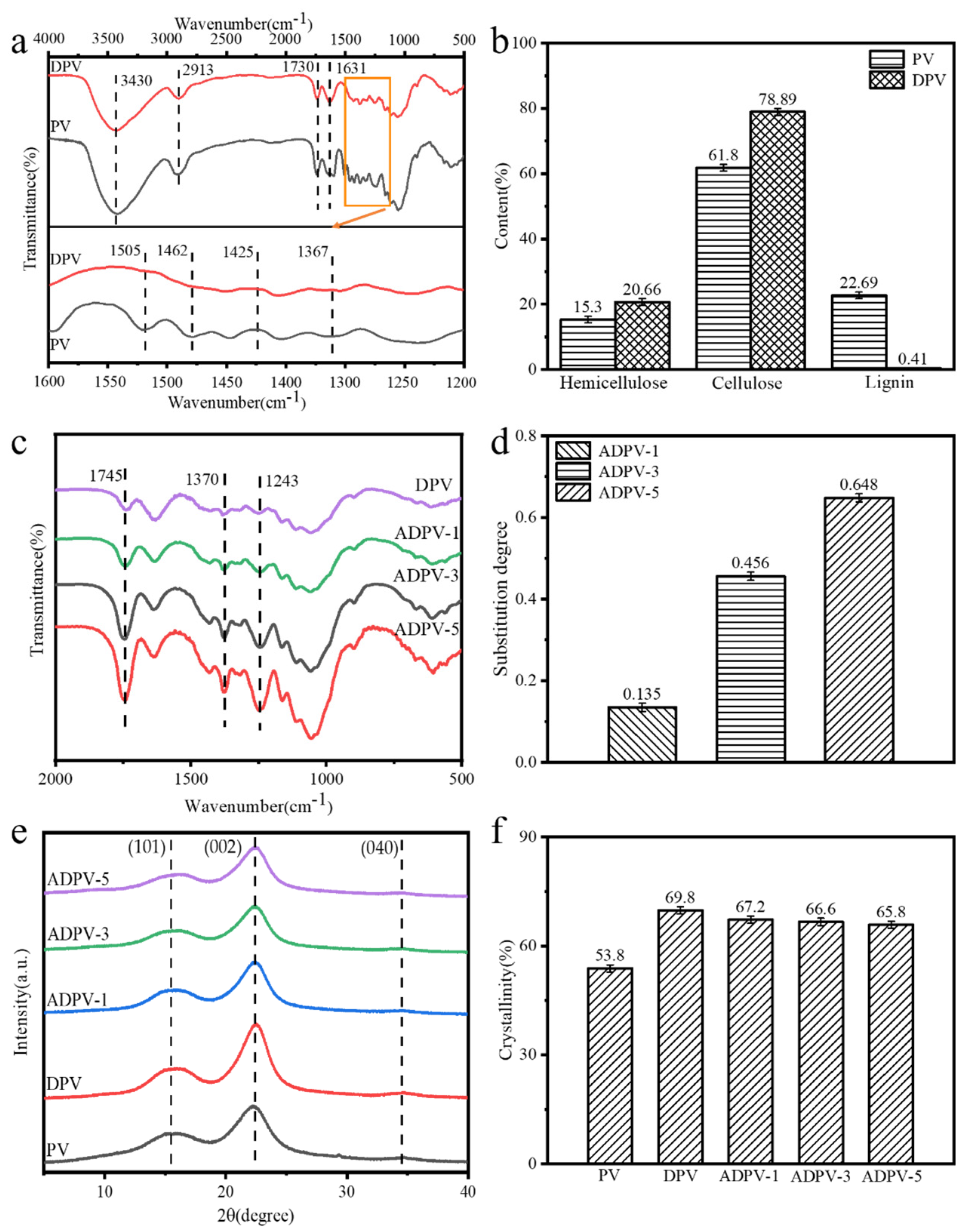
3.1. Morphological Analysis
3.2. Chemical Components and Crystal Structure Analysis
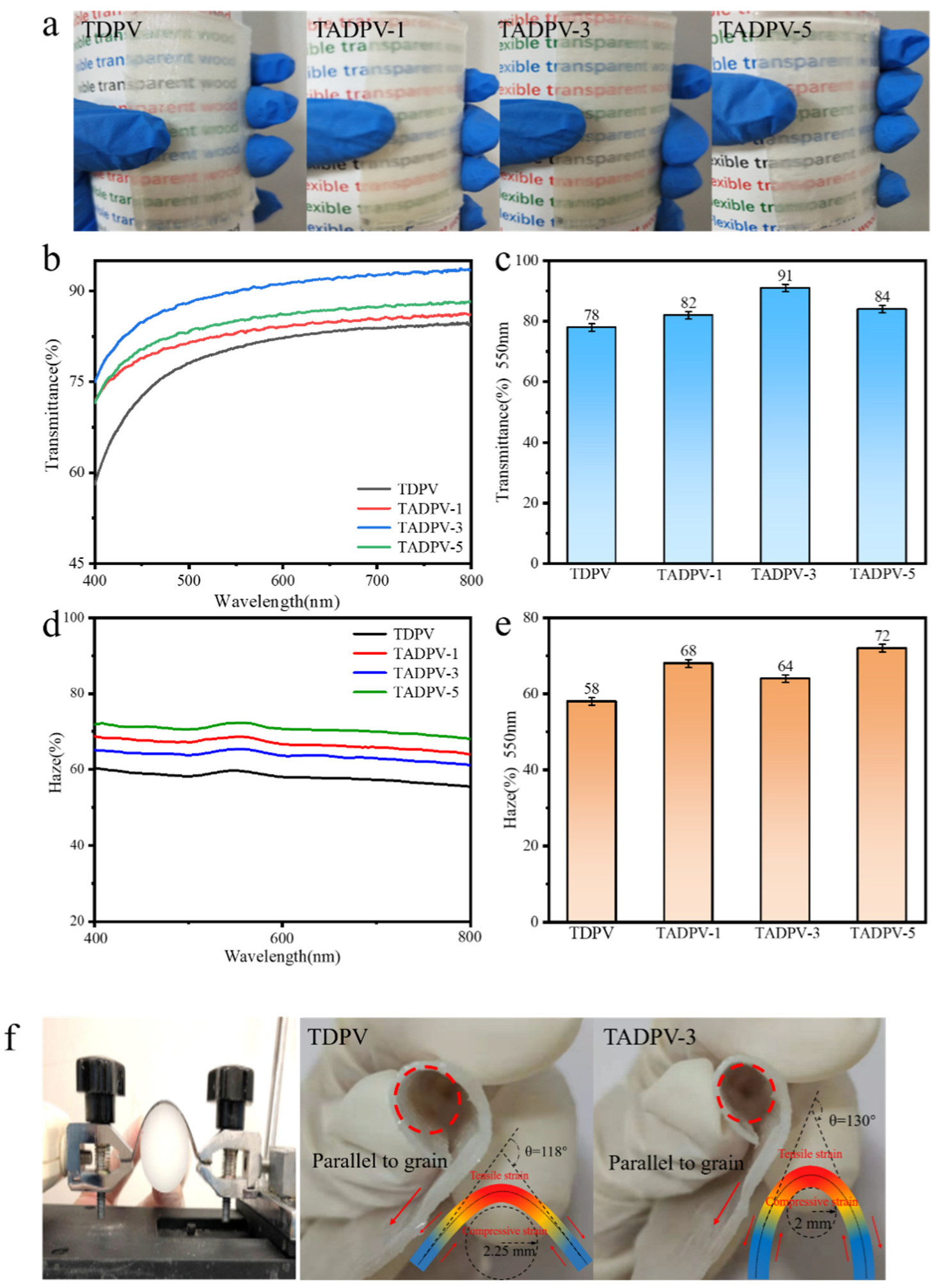
3.3. Optical Performance and Flexibility of TADPVs
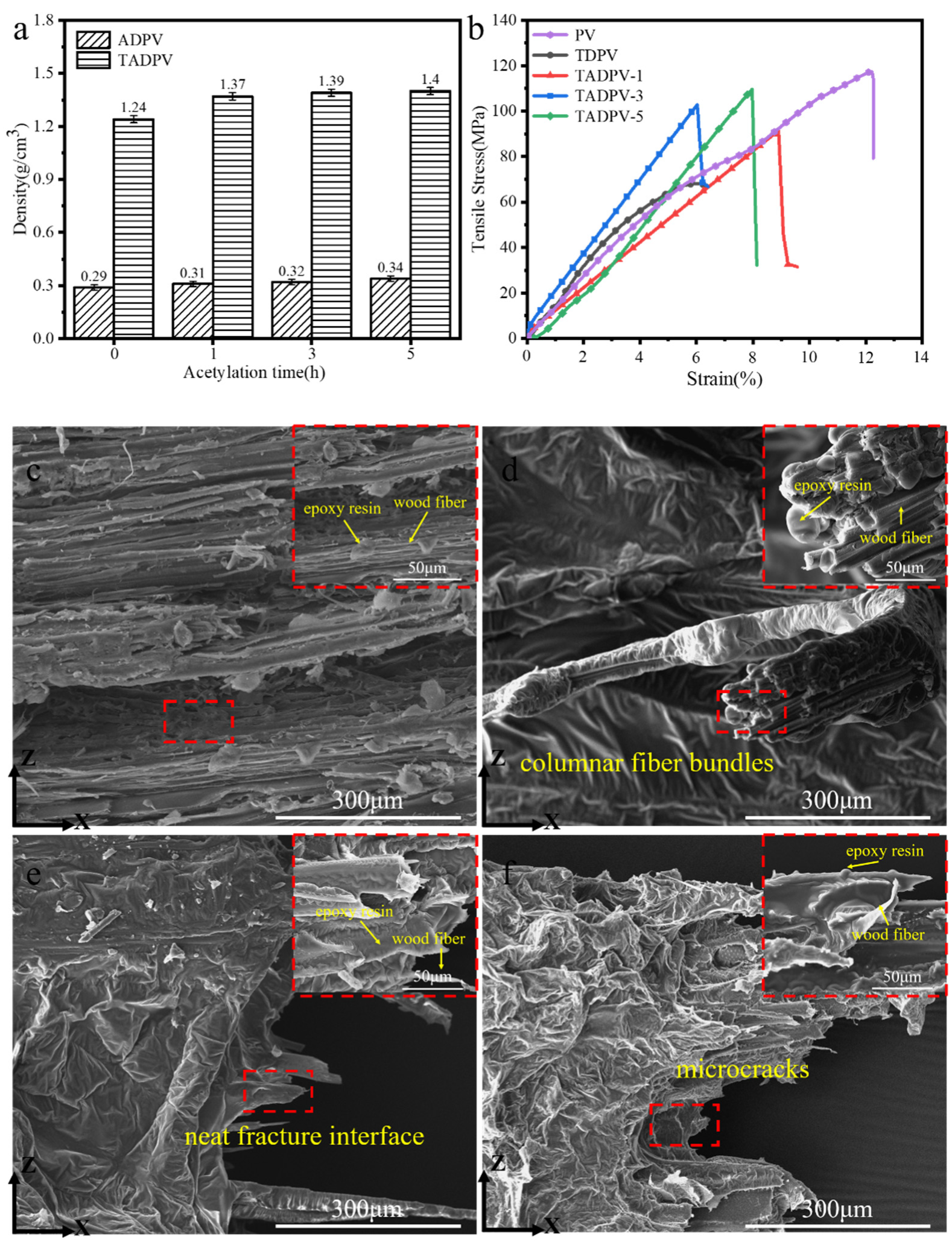
3.4. Mechanical Properties
3.5. Thermal Conductivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable Inorganic Thin-Film Battery for Fully Flexible Electronic Systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.N.; Bikiaris, D.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yang, P.; Li, Y.; Cao, Y.; Zhou, Y.; Chen, M.; Zhu, Z.; Chen, W.; Zhou, X. Flexible Transparent Sliced Veneer for Alternating Current Electroluminescent Devices. ACS Sustain. Chem. Eng. 2019, 7, 11464–11473. [Google Scholar] [CrossRef]
- Hsu, Y.-I.; Huang, L.; Asoh, T.-A.; Uyama, H. Anhydride-cured epoxy resin reinforcing with citric acid-modified cellulose. Polym. Degrad. Stab. 2020, 178, 109213. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Zhai, L.; Li, Y.; Kim, J. Preparation and characterization of hydrogels from polyvinyl alcohol and cellulose and their electroactive behavior. Soft Mater. 2016, 15, 64–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Ding, L.; De La Cruz, J.A.; Wang, B.; Feng, X.; Chen, Z.; Mao, Z.; Sui, X. Regenerated cellulose-dispersed polystyrene composites enabled via Pickering emulsion polymerization. Carbohydr. Polym. 2019, 223, 115079. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing bulk natural wood into a high-performance structural material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Subba Rao, A.N.; Nagarajappa, G.B.; Nair, S.; Chathoth, A.M.; Pandey, K.K. Flexible transparent wood prepared from poplar veneer and polyvinyl alcohol. Compos. Sci. Technol. 2019, 182, 107719. [Google Scholar] [CrossRef]
- Foster, K.E.O.; Hess, K.M.; Miyake, G.M.; Srubar, W.V., 3rd. Optical Properties and Mechanical Modeling of Acetylated Transparent Wood Composite Laminates. Materials 2019, 12, 2256. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Baitenov, A.; Li, Y.; Vasileva, E.; Popov, S.; Sychugov, I.; Yan, M.; Berglund, L. Thickness Dependence of Optical Transmittance of Transparent Wood: Chemical Modification Effects. ACS Appl. Mater. Interfaces 2019, 11, 35451–35457. [Google Scholar] [CrossRef]
- Montanari, C.; Olsén, P.; Berglund, L.A. Interface tailoring by a versatile functionalization platform for nanostructured wood biocomposites. Green Chem. 2020, 22, 8012–8023. [Google Scholar] [CrossRef]
- Avila Ramirez, J.A.; Gomez Hoyos, C.; Arroyo, S.; Cerrutti, P.; Foresti, M.L. Acetylation of bacterial cellulose catalyzed by citric acid: Use of reaction conditions for tailoring the esterification extent. Carbohydr. Polym. 2016, 153, 686–695. [Google Scholar] [CrossRef]
- Lutz, V.; Glatthaar, J.; Wurtele, C.; Serafin, M.; Hausmann, H.; Schreiner, P.R. Structural analyses of N-acetylated 4-(dimethylamino)pyridine (DMAP) salts. Chemistry 2009, 15, 8548–8557. [Google Scholar] [CrossRef]
- Ávila Ramírez, J.A.; Cerrutti, P.; Bernal, C.; Errea, M.I.; Foresti, M.L. Nanocomposites Based on Poly(lactic acid) and Bacterial Cellulose Acetylated by an α-Hydroxyacid Catalyzed Route. J. Polym. Environ. 2019, 27, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Alfassi, G.; Rein, D.M.; Cohen, Y. Partial cellulose acetylation in 1-ethyl-3-methylimidazolium acetate induced by dichloromethane. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2458–2462. [Google Scholar] [CrossRef]
- Chen, C.; Luo, J.; Qin, W.; Tong, Z. Elemental analysis, chemical composition, cellulose crystallinity, and FT-IR spectra of Toona sinensis wood. Mon. Für Chem. Chem. Mon. 2013, 145, 175–185. [Google Scholar] [CrossRef]
- Nilsson, T.; Rowell, R. Historical wood—Structure and properties. J. Cult. Herit. 2012, 13, S5–S9. [Google Scholar] [CrossRef]
- Dong, A.; Fan, X.; Wang, Q.; Yu, Y.; Wang, P.; Yuan, J.; Cavaco-Paulo, A. Changes on Content, Structure and Surface Distribution of Lignin in Jute Fibers After Laccase Treatment. J. Nat. Fibers 2017, 15, 384–395. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.-M.; Runge, T.; Feng, J.; Feng, J.; Hu, S.; Shi, Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 16971–16978. [Google Scholar] [CrossRef]
- Beaumont, M.; Winklehner, S.; Veigel, S.; Mundigler, N.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. Wet esterification of never-dried cellulose: A simple process to surface-acetylated cellulose nanofibers. Green Chem. 2020, 22, 5605–5609. [Google Scholar] [CrossRef]
- Ioelovich, M. Adjustment of Hydrophobic Properties of Cellulose Materials. Polymers 2021, 13, 1241. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.; Charlet, K.; Petit, E.; Béakou, A.; Dupont, C. Fluorination/Torrefaction Combination to Further Improve the Hydrophobicity of Wood. Macromol. Chem. Physics 2019, 220, 1900041. [Google Scholar] [CrossRef]
- Xie, Y.; Kurita, H.; Ishigami, R.; Narita, F. Assessing the Flexural Properties of Epoxy Composites with Extremely Low Addition of Cellulose Nanofiber Content. Appl. Sci. 2020, 10, 1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, X.; Xie, X.; Cai, S.; Yuan, Z.; Li, Y. Multi-Scale Evaluation of the Effect of Phenol Formaldehyde Resin Impregnation on the Dimensional Stability and Mechanical Properties of Pinus Massoniana Lamb. Forests 2019, 10, 646. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Chen, M.J.; Zhang, X.Q.; Liu, C.F.; Sun, R.C. Per-O-acetylation of cellulose in dimethyl sulfoxide with catalyzed transesterification. J. Agric. Food Chem. 2014, 62, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Sohma, M. Effects of Long-Chain Acyl Substituents on the Thermoplasticity and Mechanical Properties of Paramylon Mixed Esters. J. Polym. Environ. 2020, 28, 2263–2276. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; He, Y.; Zheng, R. A green steam-modified delignification method to prepare low-lignin delignified wood for thick, large highly transparent wood composites. J. Mater. Res. 2019, 34, 932–940. [Google Scholar] [CrossRef]
- Liang, R.; Zhu, Y.-H.; Wen, L.; Zhao, W.-W.; Kuai, B.-B.; Zhang, Y.-L.; Cai, L.-P. Exploration of effect of delignification on the mesopore structure in poplar cell wall by nitrogen absorption method. Cellulose 2019, 27, 1921–1932. [Google Scholar] [CrossRef]
- Cabane, E.; Keplinger, T.; Merk, V.; Hass, P.; Burgert, I. Renewable and functional wood materials by grafting polymerization within cell walls. ChemSusChem 2014, 7, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, Z.; Liu, Z.; Zhi, C. Evaluating Flexibility and Wearability of Flexible Energy Storage Devices. Joule 2019, 3, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Kim, E.-S.; Choi, K.; Cho, J.K.; Sun, H.; Yoo, J.W.; Park, I.-K.; Lee, Y.; Choi, H.R.; Kim, T.; et al. Rheological and mechanical properties of polypropylene composites containing microfibrillated cellulose (MFC) with improved compatibility through surface silylation. Cellulose 2018, 26, 1085–1097. [Google Scholar] [CrossRef]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bartolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, I.; Takahashi, Y.; Shimizu, H. Mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol. Scand. 2011, 69, 75–79. [Google Scholar] [CrossRef]
- Jia, C.; Li, Y.; Yang, Z.; Chen, G.; Yao, Y.; Jiang, F.; Kuang, Y.; Pastel, G.; Xie, H.; Yang, B.; et al. Rich Mesostructures Derived from Natural Woods for Solar Steam Generation. Joule 2017, 1, 588–599. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Chen, C.; Mi, R.; Li, T.; Dai, J.; Yang, Z.; Pei, Y.; He, S.; Bian, H.; Jang, S.H.; et al. Clear Wood toward High-Performance Building Materials. ACS Nano 2019, 13, 9993–10001. [Google Scholar] [CrossRef]
- Poonyakan, A.; Rachakornkij, M.; Wecharatana, M.; Smittakorn, W. Potential Use of Plastic Wastes for Low Thermal Conductivity Concrete. Materials 2018, 11, 1938. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Wang, R.; Guo, F.; Cao, J.; Guo, Z.; Qiang, H.; Liang, S.; Pang, Q.; Wei, B. Preparation of Transparent Fast-Growing Poplar Veneers with a Superior Optical Performance, Excellent Mechanical Properties, and Thermal Insulation by Acetylation Modification Using a Green Catalyst. Polymers 2022, 14, 257. https://doi.org/10.3390/polym14020257
He W, Wang R, Guo F, Cao J, Guo Z, Qiang H, Liang S, Pang Q, Wei B. Preparation of Transparent Fast-Growing Poplar Veneers with a Superior Optical Performance, Excellent Mechanical Properties, and Thermal Insulation by Acetylation Modification Using a Green Catalyst. Polymers. 2022; 14(2):257. https://doi.org/10.3390/polym14020257
Chicago/Turabian StyleHe, Wen, Rui Wang, Feiyu Guo, Jizhou Cao, Zhihao Guo, Han Qiang, Shuang Liang, Qunyan Pang, and Bairen Wei. 2022. "Preparation of Transparent Fast-Growing Poplar Veneers with a Superior Optical Performance, Excellent Mechanical Properties, and Thermal Insulation by Acetylation Modification Using a Green Catalyst" Polymers 14, no. 2: 257. https://doi.org/10.3390/polym14020257
APA StyleHe, W., Wang, R., Guo, F., Cao, J., Guo, Z., Qiang, H., Liang, S., Pang, Q., & Wei, B. (2022). Preparation of Transparent Fast-Growing Poplar Veneers with a Superior Optical Performance, Excellent Mechanical Properties, and Thermal Insulation by Acetylation Modification Using a Green Catalyst. Polymers, 14(2), 257. https://doi.org/10.3390/polym14020257







