Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Cell Culture Maintenance
2.3. Cytotoxicity Assays
2.4. Osmolality Measurement
2.5. Flow Cytometry Analysis
2.6. Autophagy Assay
2.7. G. mellonella Larvae Survivability Tests
2.8. Statistical Analysis
3. Results
3.1. Osmolality Results
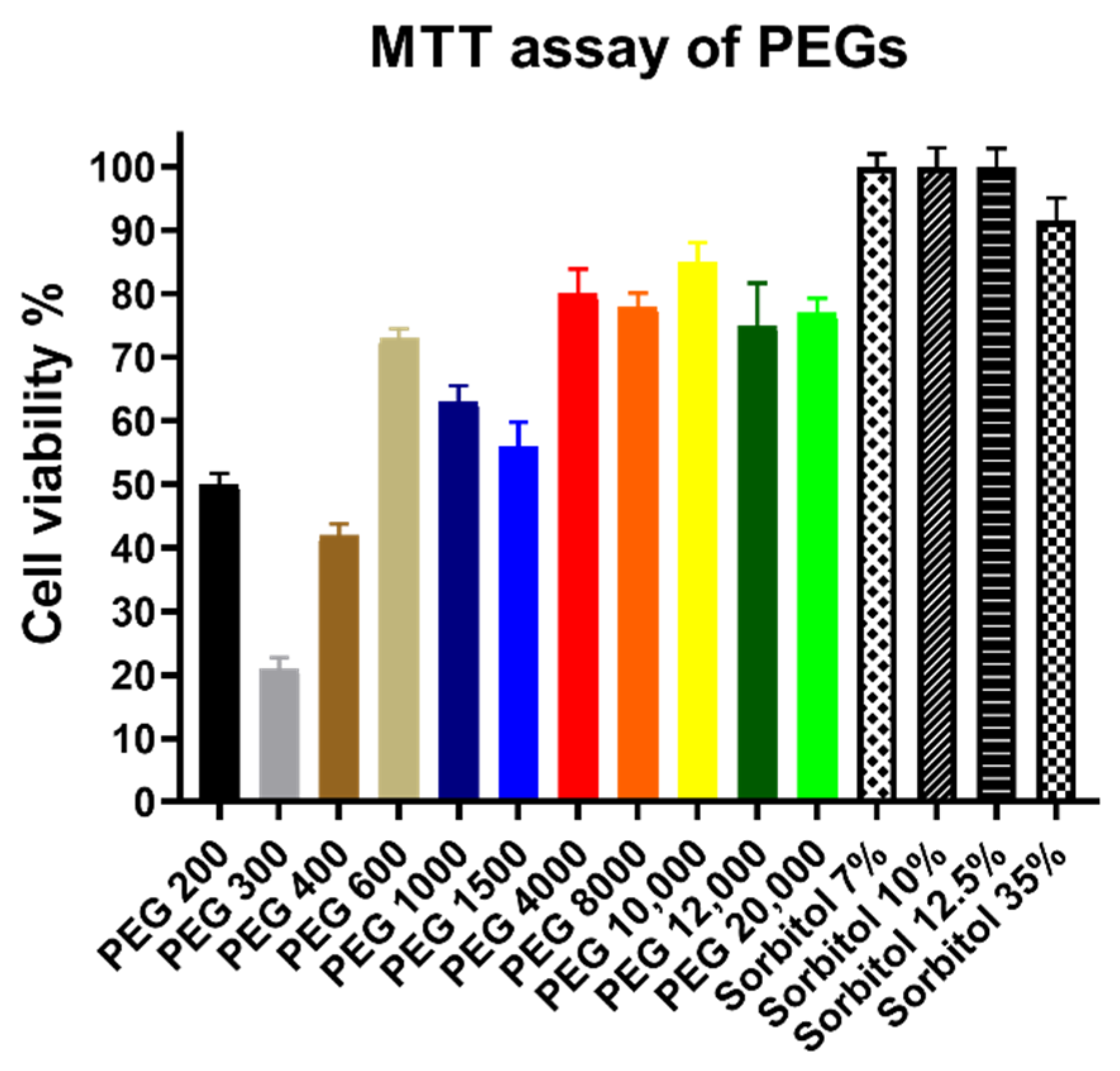
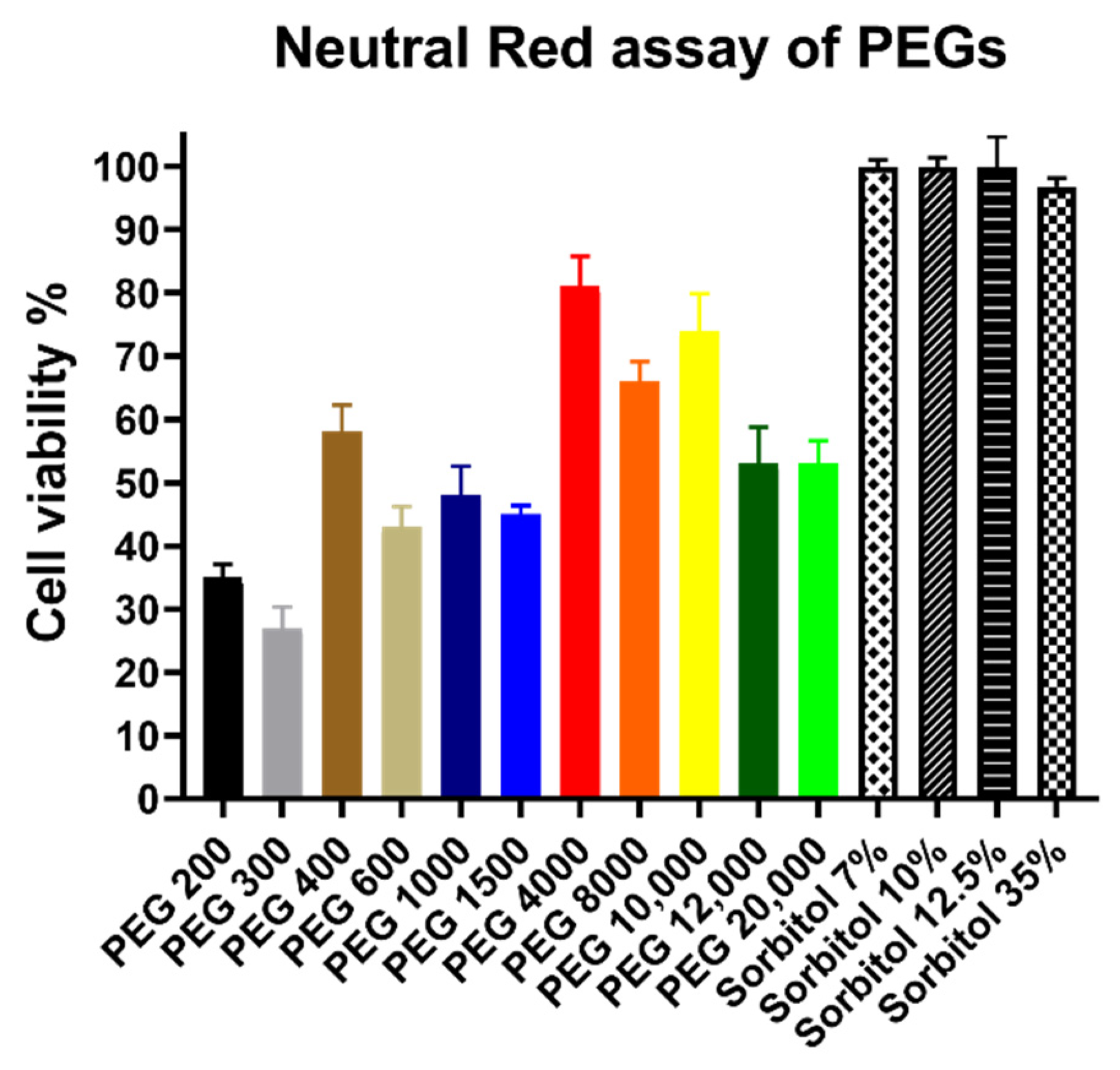
3.2. Cytotoxicity Assay Results
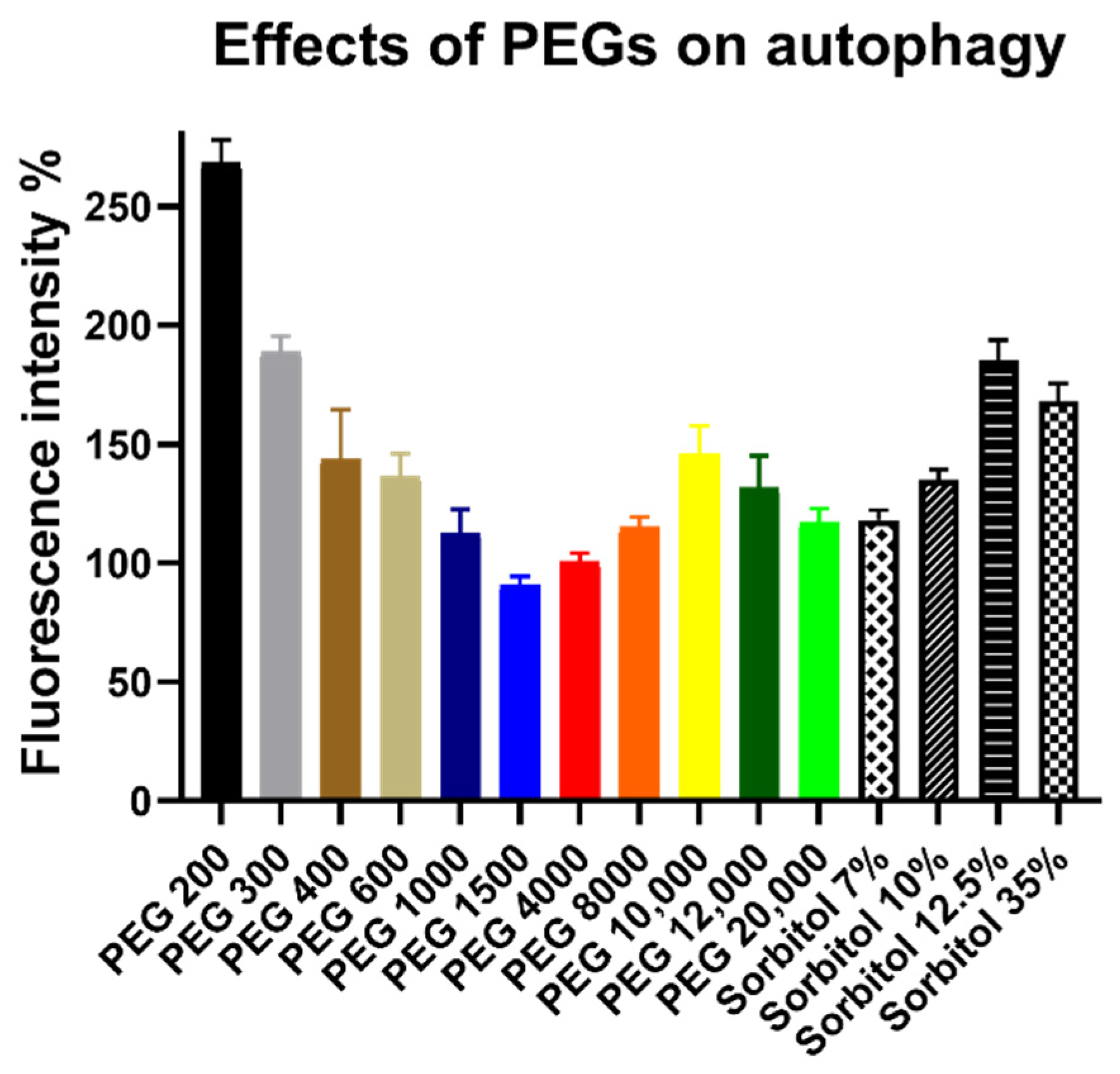
3.3. Autophagy Assay Results
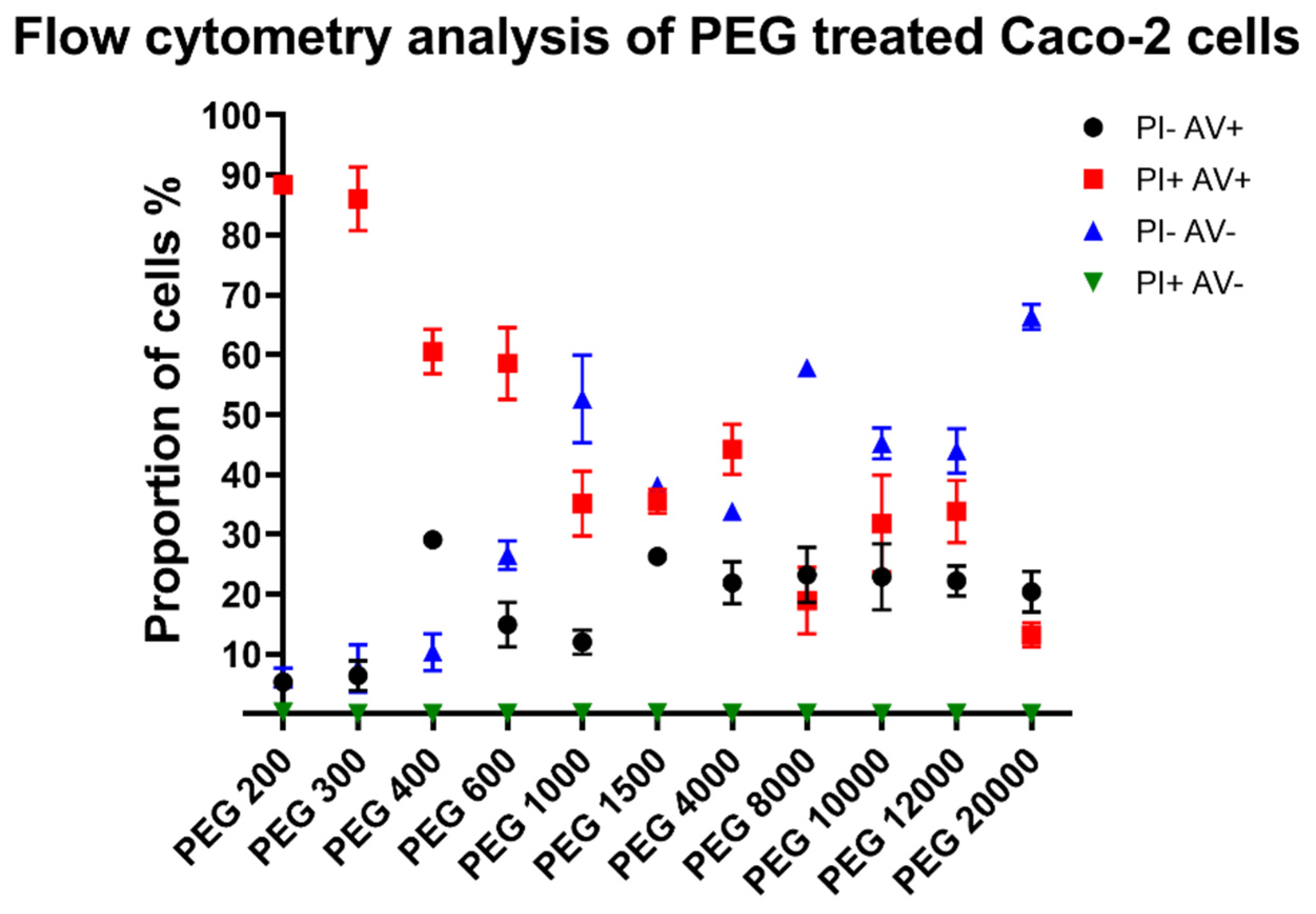
3.4. Flow Cytometry Results
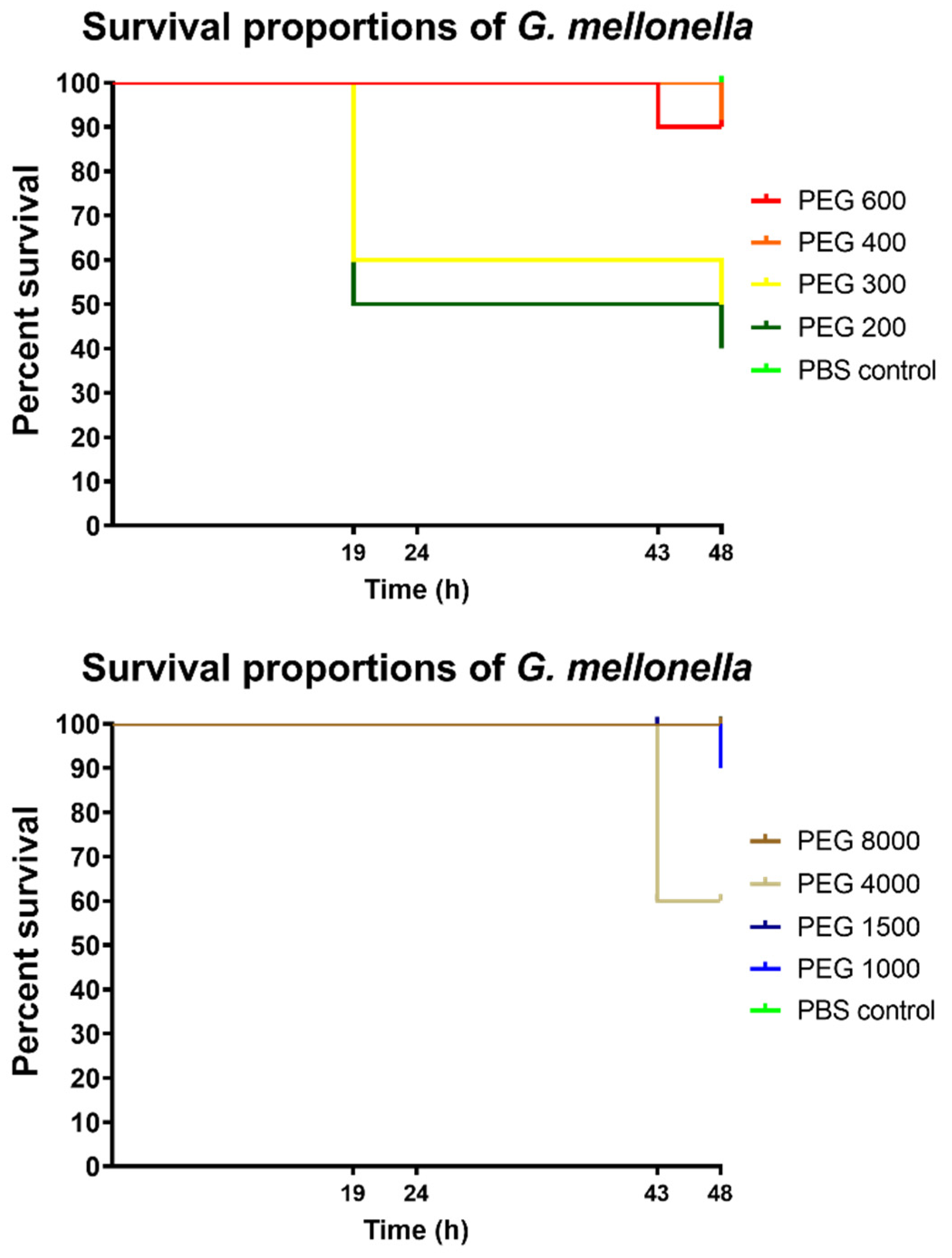
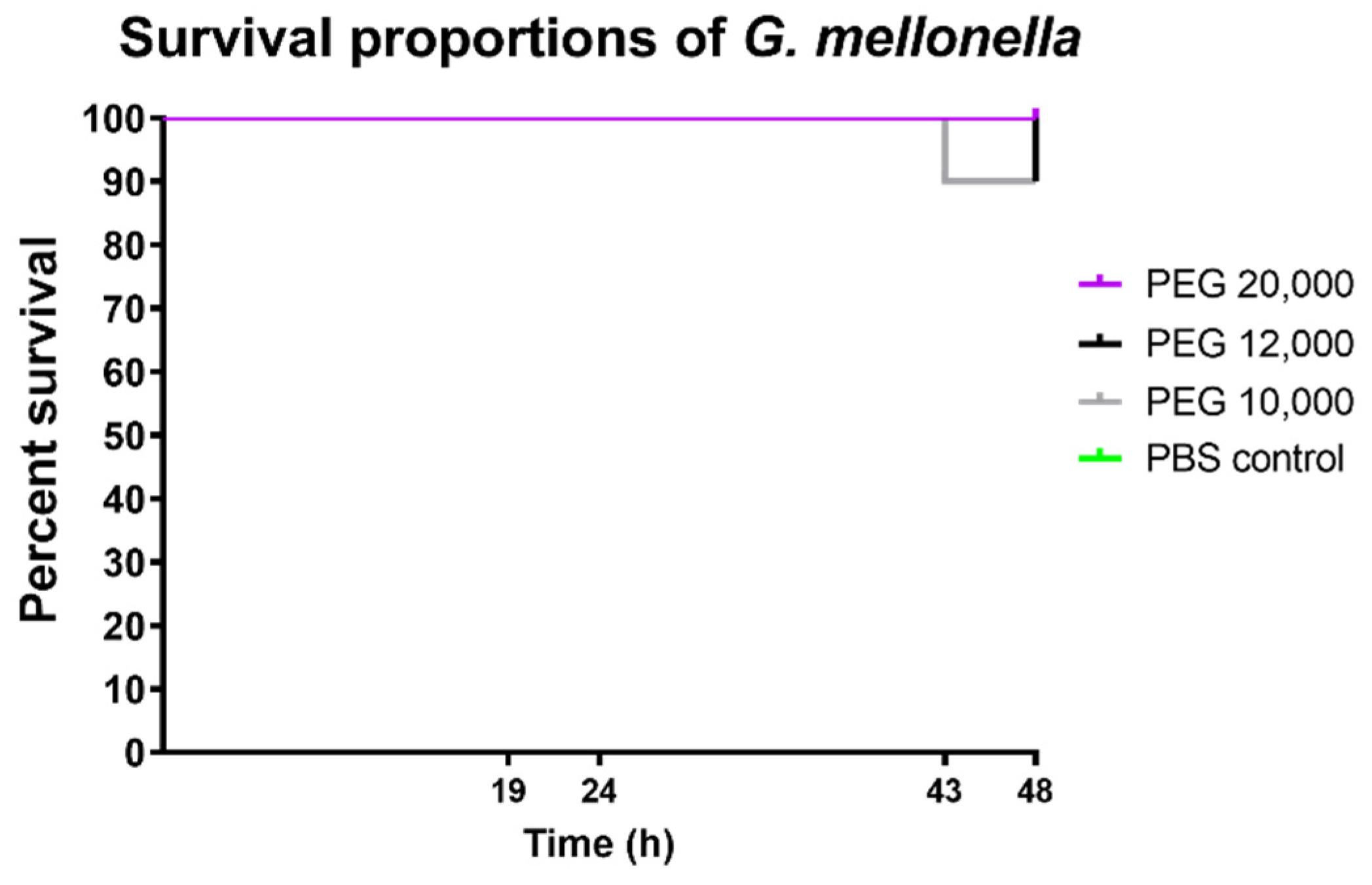
3.5. In Vivo Toxicity Test
3.6. Correlation of Results with Molecular Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herzberger, J.; Kerstin, N.; Pohlit, H.; Seiwert, J.; Worm, M.; Frederik, R.; Wurm, H.F. Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: Synthesis, novel polymer architectures, and bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullapalli, R.P.; Mazzitelli, C.L. Polyethylene Glycols in Oral and Parenteral Formulations—A Critical Review; Elsevier, B.V.: Amsterdam, The Netherlands, 2015; Volume 496, ISBN 9143164935. [Google Scholar]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Torsten, H. Polyethylene glycols (PEGs) and the pharmaceutical industry. Pharma Chem. 2002, 57–59. [Google Scholar]
- Jang, H.J.; Shin, C.Y.; Kim, K.B. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magdalena, K.; Potemski, P.; Drabczyk, A.; Kudłacik-kramarczyk, S.; Gł, M.; Grabowska, B.; Mierzwi, D. The Synthesis Methodology of PEGylated Fe3O4@Ag Nanoparticles Supported by Their Physicochemical Evaluation. Molecules 2021, 26, 1744. [Google Scholar]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Herold, D.A.; Rodeheaver, G.T.; Bellamy, W.T.; Fitton, L.A.; Bruns, D.E.; Edlich, R.F. Toxicity of topical polyethylene glycol. Toxicol. Appl. Pharmacol. 1982, 65, 329–335. [Google Scholar] [CrossRef]
- Prentice, D.E.; Majeed, S.K. Oral toxicity of polyethylene glycol (PEG 200) in monkeys and rats. Toxicol. Lett. 1978, 2, 119–122. [Google Scholar] [CrossRef]
- Bruns, D.E.; Herold, D.A.; Rodeheaver, G.T.; Edlich, R.F. Polyethylene glycol intoxication in burn patients. Burns 1982, 9, 49–52. [Google Scholar] [CrossRef]
- Hodaei, D.; Baradaran, B.; Valizadeh, H.; Zakeri-Milani, P. Effects of polyethylene glycols on intestinal efflux pump expression and activity in Caco-2 cells. Braz. J. Pharm. Sci. 2015, 51, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Parnaud, G.; Corpet, D.E.; Gamet-Payrastre, L. Cytostatic effect of polyethylene glycol on human colonic adenocarcinoma cells. Int. J. Cancer 2001, 92, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Nemes, D.; Kovács, R.; Nagy, F.; Tóth, Z.; Herczegh, P.; Borbás, A.; Kelemen, V.; Pfliegler, W.P.; Rebenku, I.; Hajdu, P.B.; et al. Comparative biocompatibility and antimicrobial studies of sorbic acid derivates. Eur. J. Pharm. Sci. 2020, 143, 105162. [Google Scholar] [CrossRef]
- Rusznyák, Á.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Bácskay, I.; Vecsernyés, M.; Vasvári, G.; Haimhoffer, Á.; Fehér, P.; et al. Investigation of the cellular effects of beta-cyclodextrin derivatives on Caco-2 intestinal epithelial cells. Pharmaceutics 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Pampati, V.; Fogel, R. Treatment options for primary constipation. Curr. Treat. Options Gastroenterol. 2004, 7, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.C.; Di Palma, J.A. Lack of lasting effectiveness of PEG 3350 laxative treatment of constipation. J. Clin. Gastroenterol. 2005, 39, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth coating of Nanoparticles in drug-delivery systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Li, S.D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Schiller, L.R.; Emmett, M.; Santa Ana, C.A.; Fordtran, J.S. Osmotic effects of polyethylene glycol. Gastroenterology 1988, 94, 933–941. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Gibson, D.J.; Yang, Q.; Kerekes, D.T.; Schultz, G.S. Medical honey and silver dressings do not interfere with each other’s key functional attributes. Wounds 2014, 26, 309–316. [Google Scholar]
- Misichronis, K.; Rangou, S.; Avgeropoulos, A. Synthesis and molecular and morphological characterization of poly(p-trimethylsilyl styrene) and diblock copolymers with poly(1,3- cyclohexadiene). Int. J. Polym. Anal. Charact. 2008, 13, 136–148. [Google Scholar] [CrossRef]
- Marsano, E.; Bianchi, E.; Chimico-fisici, S.; Sintetiche, M.; Europa, C.; Chimica, D. Synthesis and characterization of block copolymers based on poly(p-benzamide) and poly(terephthalamide of p-aminobenzhydrazide). Polym. Commun. 1991, 32, 45. [Google Scholar]
- Wang, T.; Guo, Y.; He, Y.; Ren, T.; Yin, L.; Fawcett, J.P.; Gu, J.; Sun, H. Impact of molecular weight on the mechanism of cellular uptake of polyethylene glycols (PEGs) with particular reference to P-glycoprotein. Acta Pharm. Sin. B 2020, 10, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Rojas, M.; Cascione, M.; Mazzotta, S.; Di Sansebastiano, G.P.; Rinaldi, R. Physico-chemical properties of inorganic NPs influence the absorption rate of aquatic mosses reducing cytotoxicity on intestinal epithelial barrier model. Molecules 2021, 26, 2885. [Google Scholar] [CrossRef]
- Florkiewicz, W.; Malina, D.; Pluta, K.; Rudnicka, K.; Gajewski, A.; Olejnik, E.; Tyliszczak, B.; Sobczak-Kupiec, A. Assessment of cytotoxicity and immune compatibility of phytochemicals-mediated biosynthesised silver nanoparticles using Cynara scolymus. IET Nanobiotechnol. 2019, 13, 726–735. [Google Scholar] [CrossRef]
- George, B.P.A.; Tynga, I.M.; Abrahamse, H. In vitro antiproliferative effect of the acetone extract of rubus fairholmianus gard. Root on human colorectal cancer cells. Biomed. Res. Int. 2015, 2015, 165037. [Google Scholar]
- Alam, F.; us Saqib, Q.N.; Waheed, A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Dino, P.; D’Anna, C.; Sangiorgi, C.; Di Sano, C.; Di Vincenzo, S.; Ferraro, M.; Pace, E. Cigarette smoke extract modulates E-Cadherin, Claudin-1 and miR-21 and promotes cancer invasiveness in human colorectal adenocarcinoma cells. Toxicol. Lett. 2019, 317, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nozari, S.; Faridvand, Y.; Etesami, A.; Ahmad Khan Beiki, M.; Miresmaeili Mazrakhondi, S.A.; Abdolalizadeh, J. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett. Appl. Microbiol. 2019, 69, 148–154. [Google Scholar] [CrossRef]
- Lauzier, A.; Normandeau-Guimond, J.; Vaillancourt-Lavigueur, V.; Boivin, V.; Charbonneau, M.; Rivard, N.; Scott, M.S.; Dubois, C.M.; Jean, S. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci. Rep. 2019, 9, 11316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Qiu, S.Z.; He, J.; Peng, Y.; Xu, H.; Feng, Z.; Huang, H.; Du, Y.; Zhou, Y.; Nie, Y. Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death. Cancer Lett. 2020, 481, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Atique Ullah, A.K.M.; Banik, S.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Green Synthesized Silver Nanoparticles-Mediated Cytotoxic Effect in Colorectal Cancer Cells: NF-κB Signal Induced Apoptosis Through Autophagy. Biol. Trace Elem. Res. 2021, 199, 3272–3286. [Google Scholar] [CrossRef]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 428. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, G.K.; Maia, F.C.; de Oliveira, T.R.; de Feiria, S.N.B.; Joia, F.; Barbosa, J.P.; Boni, G.C.; de Cássia Orlandi Sardi, J.; Rosalen, P.L.; Höfling, J.F. Effect of cinnamomum verum leaf essential oil on virulence factors of candida species and determination of the in-vivo toxicity with galleria mellonella model. Mem. Inst. Oswaldo Cruz 2020, 115, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moya-Andérico, L.; Vukomanovic, M.; del Mar Cendra, M.; Segura-Feliu, M.; Gil, V.; del Río, J.A.; Torrents, E. Utility of Galleria mellonella larvae for evaluating nanoparticle toxicology. Chemosphere 2021, 266, 129235. [Google Scholar] [CrossRef]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef] [Green Version]
- Ivana Postic, H.S. Poly(ethylene glycol) induces cell toxicity in melanoma cells by producing a hyperosmotic extracellular medium. J. Biomater. Appl. 2018, 33, 693–706. [Google Scholar] [CrossRef]
- Ahmadyan, S.; Kabiri, M.; Hanaee-Ahvaz, H.; Farazmand, A. Osmolyte Type and the Osmolarity Level Affect Chondrogenesis of Mesenchymal Stem Cells. Appl. Biochem. Biotechnol. 2018, 185, 507–523. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Gao, N.; Li, J.; Pflugfelder, S.C.; Li, D.Q. Autophagy activation protects ocular surface from inflammation in a dry eye model in vitro. Int. J. Mol. Sci. 2020, 21, 8966. [Google Scholar] [CrossRef]
- Nunes, P.; Ernandez, T.; Roth, I.; Qiao, X.; Strebel, D.; Bouley, R.; Charollais, A.; Ramadori, P.; Foti, M.; Meda, P.; et al. Hypertonic stress promotes autophagy and microtubule-dependent autophagosomal clusters. Autophagy 2013, 9, 550–567. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Closa, D.; Folch-puy, E. Polyethylene Glycol 35 (PEG35) Modulates Exosomal Uptake and Function. Polymers 2020, 12, 3044. [Google Scholar]
- de Sousa, A.K.A.; Ribeiro, F.O.S.; de Oliveira, T.M.; de Araújo, A.R.; Dias, J.d.N.; Albuquerque, P.; Silva-Pereira, I.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Leite, J.R.S.A.; et al. Quaternization of angico gum and evaluation of anti-staphylococcal effect and toxicity of their derivatives. Int. J. Biol. Macromol. 2020, 150, 1175–1183. [Google Scholar] [CrossRef]
- Vyas, K.; Brassard, D.L.; DeLorenzo, M.M.; Sun, Y.; Grace, M.J.; Borden, E.C.; Leaman, D.W. Biologic activity of polyethylene glycol12000-Interferon-α2b Compared with Interferon-α2b: Gene Modulatory and Antigrowth Effects in Tumor Cells. J. Immunother. 2003, 26, 2489. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Ferreira de Oliveira, J.M.P.; Brown, D.; Jonhston, H.; Malheiro, E.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. The influence of Citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line. Toxicol. Lett. 2016, 249, 29–41. [Google Scholar] [CrossRef] [PubMed]

| Spearman Correlation Coefficient | Level of Significance | |
|---|---|---|
| Osmolality | −0.8091 | ** |
| Cell viability—MTT | 0.7909 | ** |
| Cell viability—NR | 0.6241 | * |
| Autophagy | ns | |
| Proportion of PI−AV+ cells | ns | |
| Proportion of PI−AV+ cells | −0.9273 | *** |
| Proportion of PI−AV− cells | 0.8455 | ** |
| Total larvae mortality | −0.6357 | * |
| Osmolality | Cell Viability—MTT | Cell Viability—NR | Autophagy | Proportion of PI− AV+ Cells | Proportion of PI+ AV+ Cells | Proportion of PI− AV− Cells | Total Larvae Mortality | |
|---|---|---|---|---|---|---|---|---|
| Osmolality | - | 0.909/**** | −0.843/** | ns | ns | 0.755/* | −0.709/* | ns |
| Cell viability—MTT | −0.909/**** | - | 0.770/** | ns | ns | −0.736/* | 0.664/* | ns |
| Cell viability—NR | −0.843/** | 0.770/** | - | ns | 0.620/* | ns | ns | ns |
| Autophagy | ns | ns | ns | - | ns | ns | ns | ns |
| Proportion of PI−AV+ cells | ns | ns | 0.620/* | ns | - | ns | ns | −0.626/* |
| Proportion of PI+ AV+ cells | 0.755/* | −0.736/* | ns | ns | ns | - | −0.973/**** | 0.771/** |
| Proportion of PI−AV−cells | −0.709/* | 0.664/* | ns | ns | ns | −0.973/**** | - | −0.771/** |
| Larvae mortality | ns | ns | ns | ns | −0.626/* | 0.771/** | −0.771/** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham Le Khanh, H.; Nemes, D.; Rusznyák, Á.; Ujhelyi, Z.; Fehér, P.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Bácskay, I. Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives. Polymers 2022, 14, 279. https://doi.org/10.3390/polym14020279
Pham Le Khanh H, Nemes D, Rusznyák Á, Ujhelyi Z, Fehér P, Fenyvesi F, Váradi J, Vecsernyés M, Bácskay I. Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives. Polymers. 2022; 14(2):279. https://doi.org/10.3390/polym14020279
Chicago/Turabian StylePham Le Khanh, Ha, Dániel Nemes, Ágnes Rusznyák, Zoltán Ujhelyi, Pálma Fehér, Ferenc Fenyvesi, Judit Váradi, Miklós Vecsernyés, and Ildikó Bácskay. 2022. "Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives" Polymers 14, no. 2: 279. https://doi.org/10.3390/polym14020279
APA StylePham Le Khanh, H., Nemes, D., Rusznyák, Á., Ujhelyi, Z., Fehér, P., Fenyvesi, F., Váradi, J., Vecsernyés, M., & Bácskay, I. (2022). Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives. Polymers, 14(2), 279. https://doi.org/10.3390/polym14020279













