Durability of Lubricated Icephobic Coatings under Various Environmental Stresses
Abstract
:1. Introduction
2. Materials and Methods
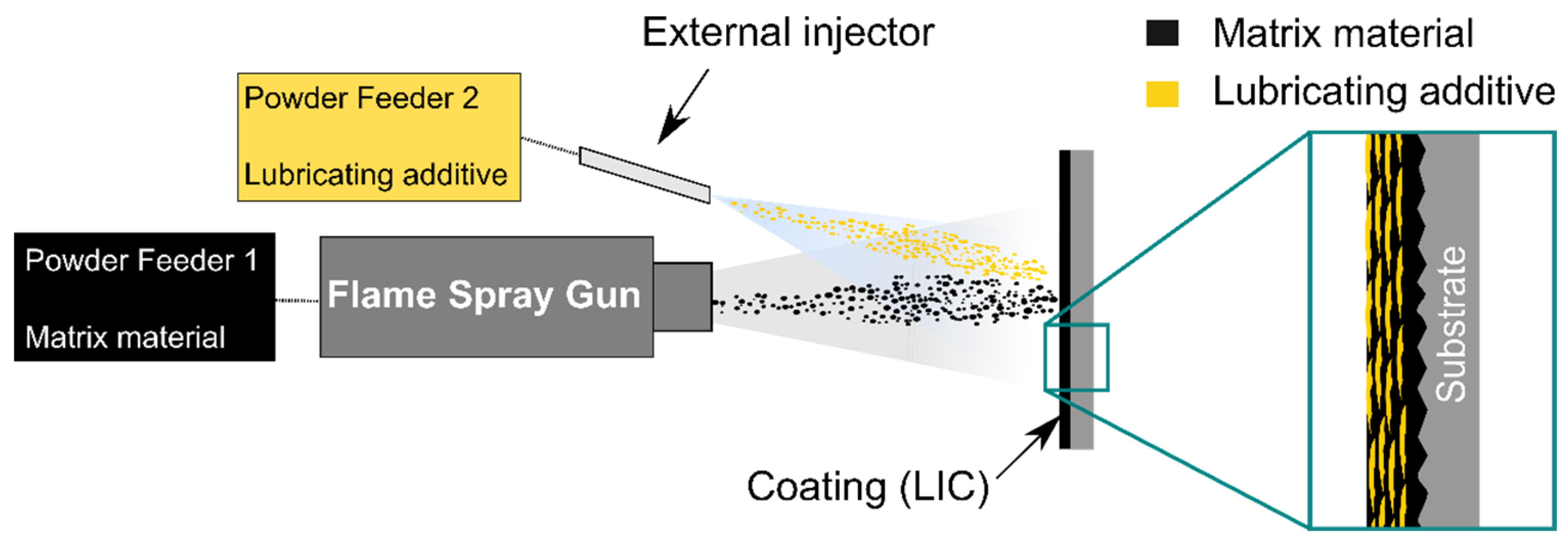
2.1. Materials and Coating Fabrication
2.2. Characterisation Methods
2.2.1. Feedstock Material Characterization
2.2.2. Coating Characterisation
2.3. Durability Tests
2.3.1. Durability under Repeated Icing/Deicing Cycles
2.3.2. Durability in Acidic, Saline and Basic Solutions
2.3.3. Durability under Exposure to Ultraviolet (UV) Irradiation
3. Results and Discussion
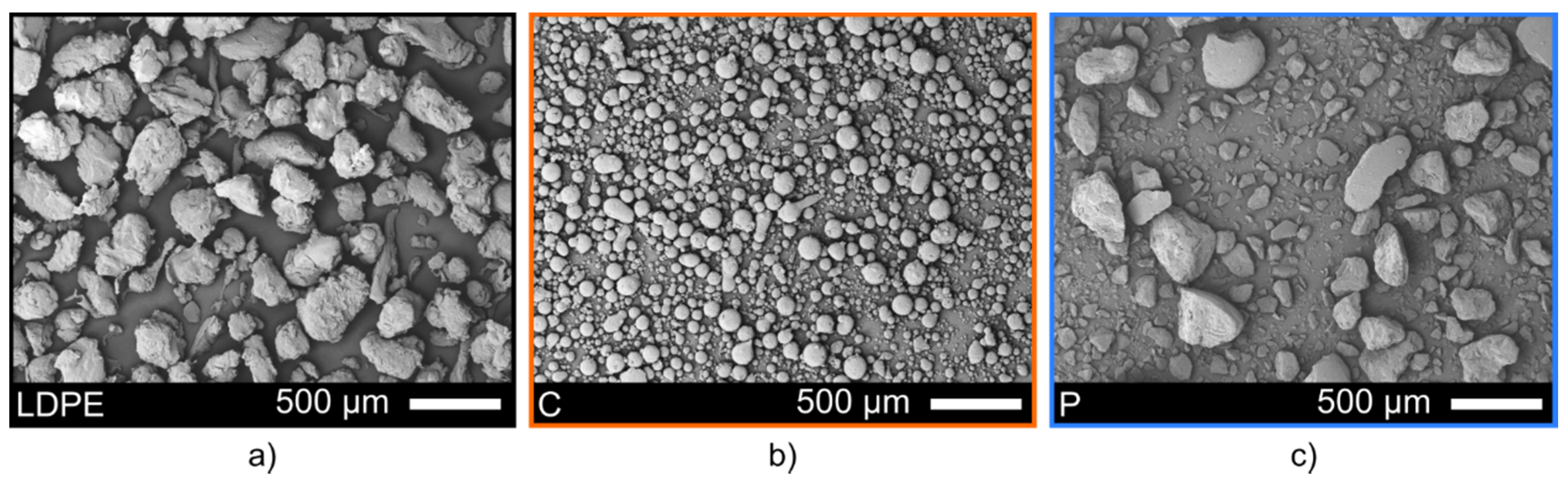
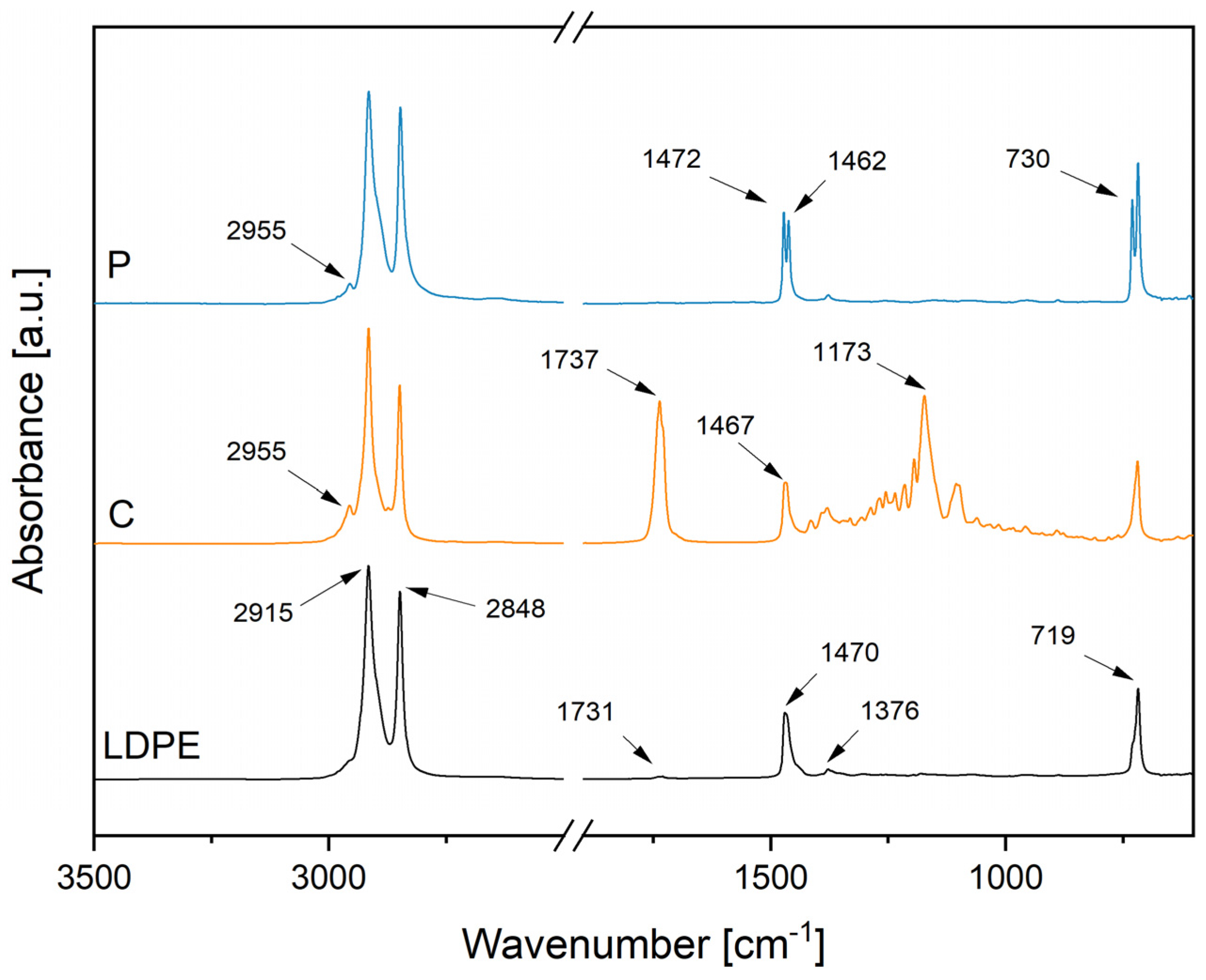
3.1. Properties of the Feedstock Powders
3.2. Composition and Surface Properties of the Coatings
3.3. Icephobic Behaviour of Coatings
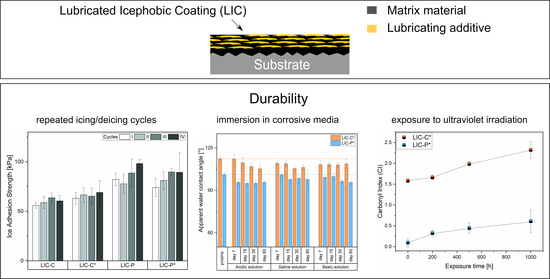
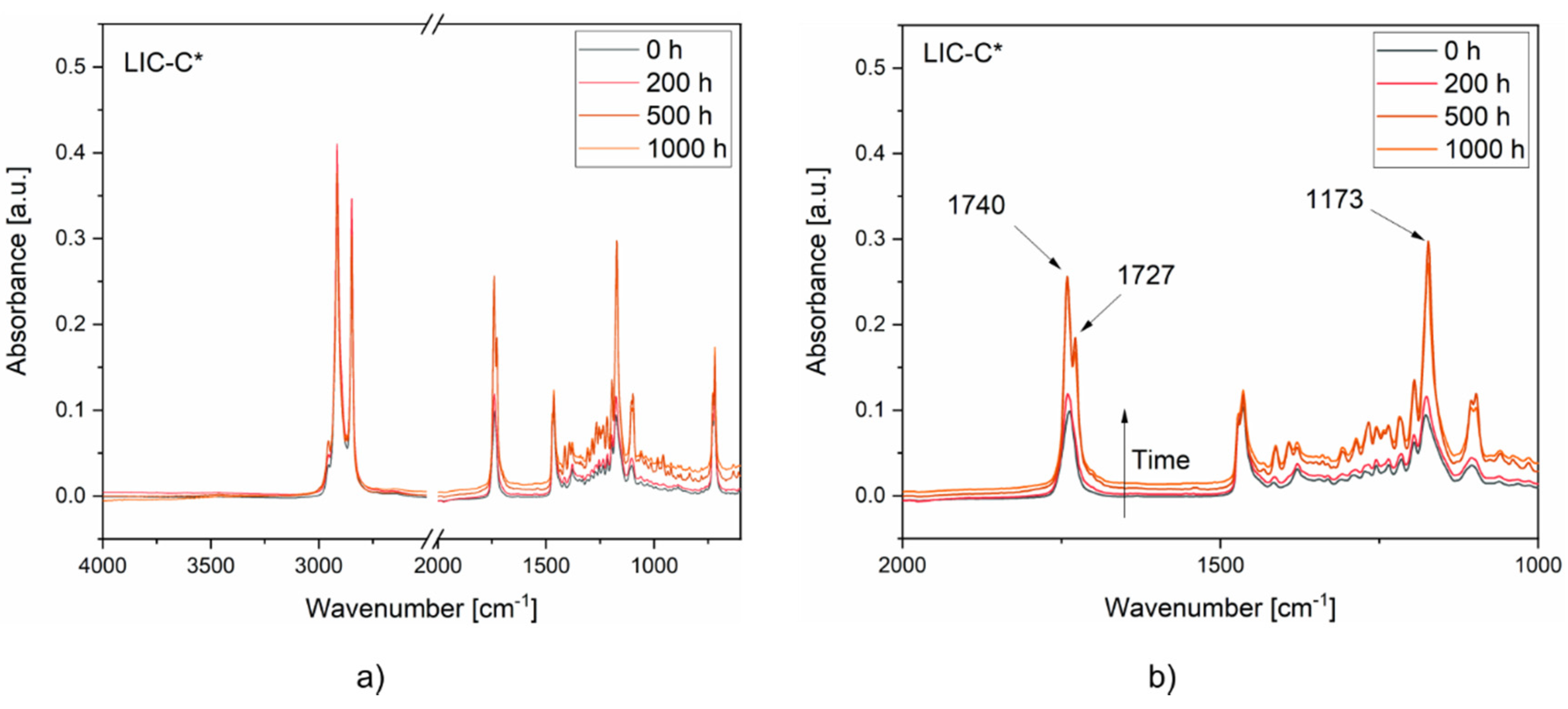
3.4. Coating Performance under Cyclic Icing/Deicing
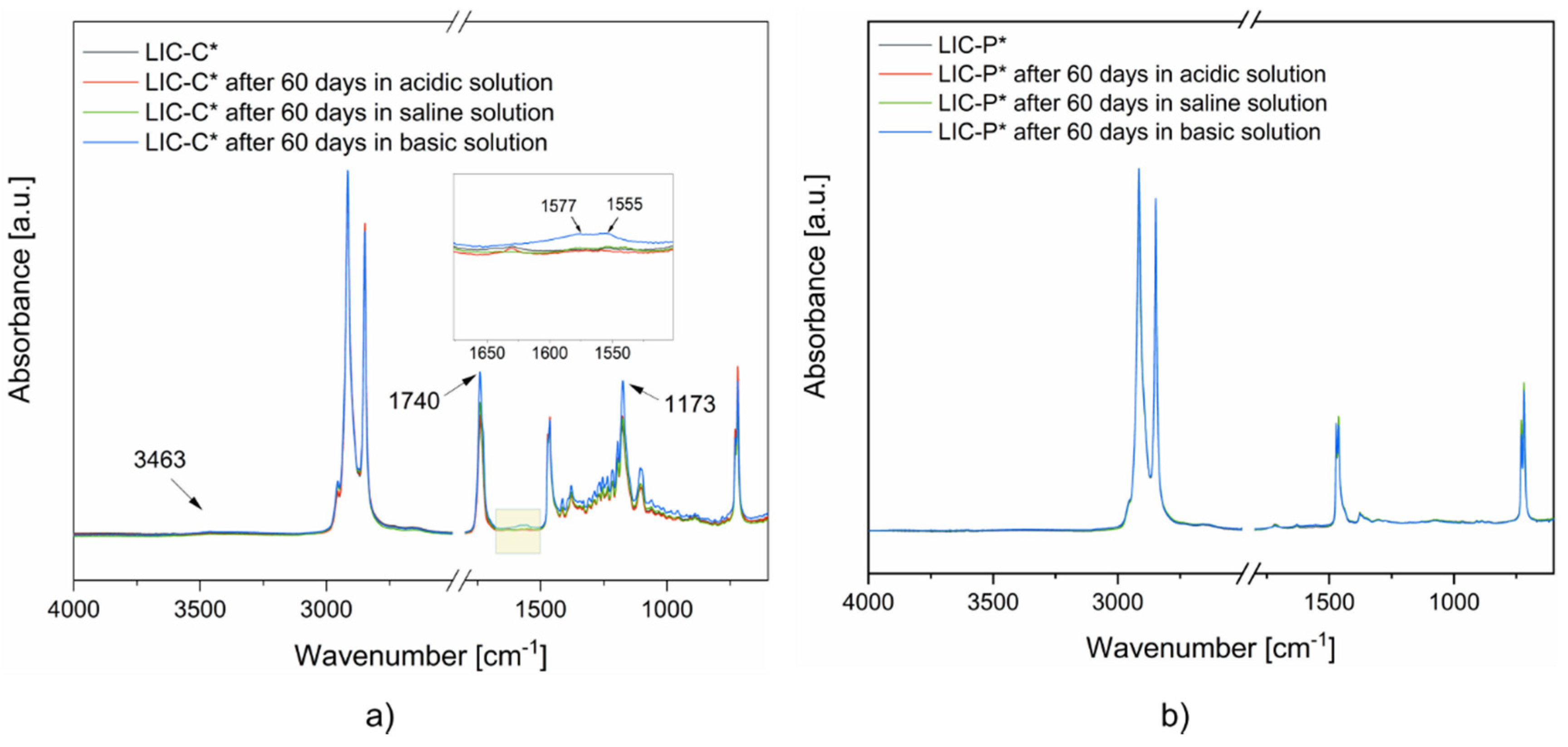
3.5. Coating Performance in Acidic, Saline and Basic Environments
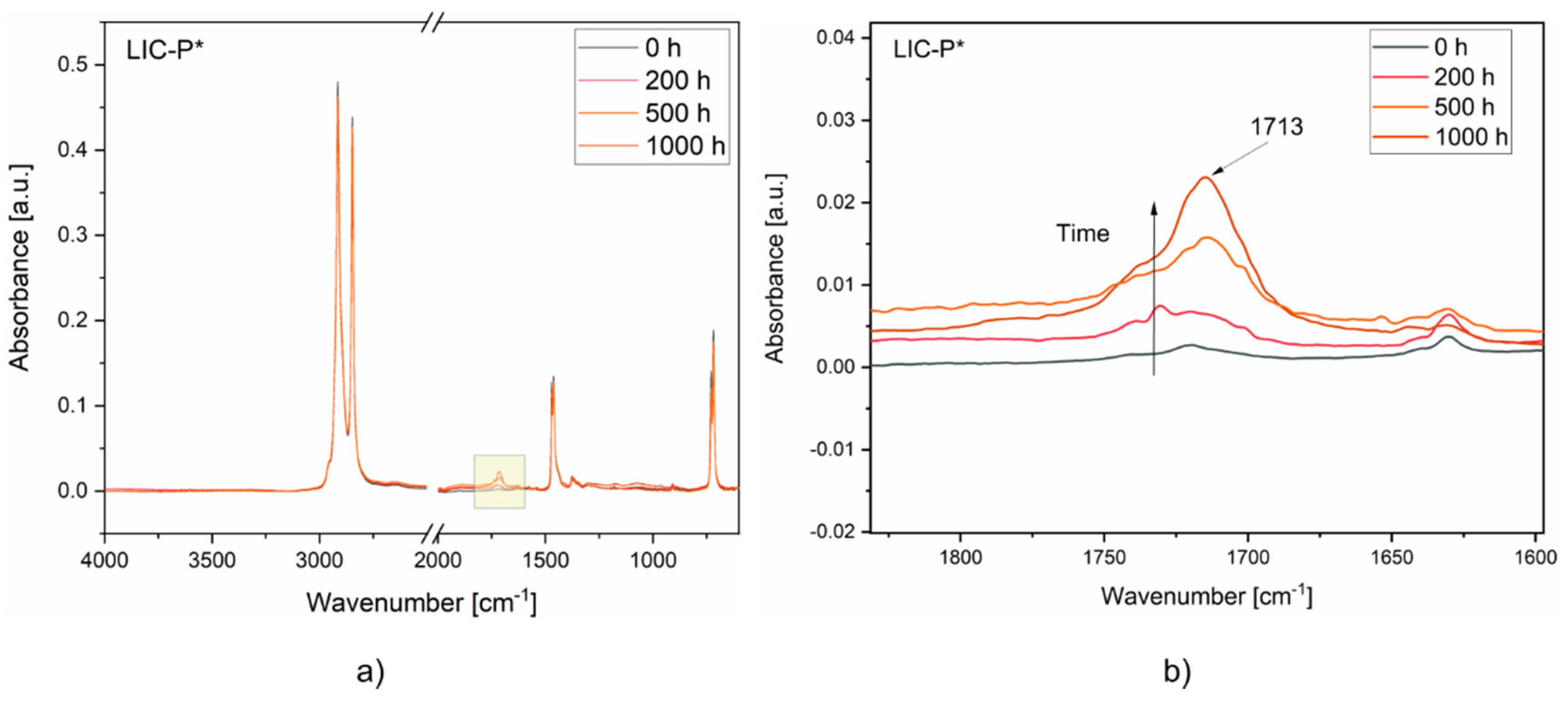
3.6. Coating Performance under Exposure to Ultraviolet (UV) Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic Materials: Fundamentals, Performance Evaluation, and Applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Shu, L.; Li, H.; Hu, Q.; Jiang, X.; Qiu, G.; McClure, G.; Yang, H. Study of Ice Accretion Feature and Power Characteristics of Wind Turbines at Natural Icing Environment. Cold Reg. Sci. Technol. 2018, 147, 45–54. [Google Scholar] [CrossRef]
- Fillion, R.M.; Riahi, A.R.; Edrisy, A. A Review of Icing Prevention in Photovoltaic Devices by Surface Engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Shi, K.; Duan, X. A Review of Ice Protection Techniques for Structures in the Arctic and Offshore Harsh Environments. J. Offshore Mech. Arct. Eng. 2021, 143, 1–27. [Google Scholar] [CrossRef]
- Luo, S.; Yang, X. Performance Evaluation of High-Elastic Asphalt Mixture Containing Deicing Agent Mafilon. Constr. Build. Mater. 2015, 94, 494–501. [Google Scholar] [CrossRef]
- Habibi, H.; Edwards, G.; Sannassy, C.; Kappatos, V.; Lage, Y.; Stein, J.; Selcuk, C.; Gan, T.H. Modelling and Empirical Development of an Anti/de-Icing Approach for Wind Turbine Blades through Superposition of Different Types of Vibration. Cold Reg. Sci. Technol. 2016, 128, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, V.F.; Sullivan, C.R.; Kozlyuk, V.; Petrenko, F.V.; Veerasamy, V. Pulse Electro-Thermal de-Icer (PETD). Cold Reg. Sci. Technol. 2011, 65, 70–78. [Google Scholar] [CrossRef]
- Dalili, N.; Edrisy, A.; Carriveau, R. A Review of Surface Engineering Issues Critical to Wind Turbine Performance. Renew. Sustain. Energy Rev. 2009, 13, 428–438. [Google Scholar] [CrossRef]
- Hochart, C.; Fortin, G.; Perron, J.; Ilinca, A. Wind Turbine Performance under Icing Conditions. Wind Energy 2008, 11, 319–333. [Google Scholar] [CrossRef]
- Rehfeld, N.; Speckmann, B.; Schreiner, C.; Stenzel, V. Assessment of Icephobic Coatings—How Can We Monitor Performance Durability? Coatings 2021, 11, 614. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Masson, D.; Du, X.; Emelyanenko, A.M. Testing the Durability of Anti-Icing Coatings. In Ice Adhesion: Mechanism, Measurement and Mitigation; Mittal, K.L., Choi, C.-H., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 495–520. [Google Scholar]
- Hakimian, A.; Nazifi, S.; Ghasemi, H. Durability Assessment of Icephobic Coatings. In Ice Adhesion: Mechanism, Measurement and Mitigation; Mittal, K.L., Choi, C.-H., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 521–545. [Google Scholar]
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing Durable Icephobic Surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of Slippery Liquid Infused Porous Surfaces under Multiple Freeze-Thaw and Ice Accretion-Detachment Cycles. Adv. Mater. Interfaces 2018, 5, 1800828. [Google Scholar] [CrossRef]
- Asadollahi, S.; Farzaneh, M.; Stafford, L. On the Icephobic Behavior of Organosilicon-Based Surface Structures Developed Through Atmospheric Pressure Plasma Deposition in Nitrogen Plasma. Coatings 2019, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Vercillo, V.; Cardoso, J.T.; Huerta-Murillo, D.; Tonnicchia, S.; Laroche, A.; Mayén Guillén, J.A.; Ocaña, J.L.; Lasagni, A.F.; Bonaccurso, E. Durability of Superhydrophobic Laser-Treated Metal Surfaces under Icing Conditions. Mater. Lett. X 2019, 3, 100021. [Google Scholar] [CrossRef]
- Sharifi, N.; Dolatabadi, A.; Pugh, M.; Moreau, C. Anti-Icing Performance and Durability of Suspension Plasma Sprayed TiO2 Coatings. Cold Reg. Sci. Technol. 2019, 159, 1–12. [Google Scholar] [CrossRef]
- Memon, H.; De Focatiis, D.S.A.; Choi, K.S.; Hou, X. Durability Enhancement of Low Ice Adhesion Polymeric Coatings. Prog. Org. Coat. 2021, 151, 106033. [Google Scholar] [CrossRef]
- Veronesi, F.; Boveri, G.; Raimondo, M. Amphiphobic Nanostructured Coatings for Industrial Applications. Materials 2019, 12, 787. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Ma, L.; Zhang, F.; Qian, H.; Minhas, B.; Yang, Y.; Han, X.; Zhang, D. Durable Deicing Lubricant-Infused Surface with Photothermally Switchable Hydrophobic/Slippery Property. Mater. Des. 2020, 185, 108236. [Google Scholar] [CrossRef]
- Balordi, M.; Santucci de Magistris, G.; Chemelli, C. A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface. Coatings 2020, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Guarini, G.; Corozzi, A.; Raimondo, M. Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants. Coatings 2021, 11, 1170. [Google Scholar] [CrossRef]
- Brown, S.; Lengaigne, J.; Sharifi, N.; Pugh, M.; Moreau, C.; Dolatabadi, A.; Martinu, L.; Klemberg-Sapieha, J.E. Durability of Superhydrophobic Duplex Coating Systems for Aerospace Applications. Surf. Coat. Technol. 2020, 401, 126249. [Google Scholar] [CrossRef]
- Orlandini, M. Slippery Lubricant Infused Porous Surface Generated by Self-Assembly Mechanism of a Composite Organogel for Low Ice Adhesion. Ph.D. Thesis, Tampere University, Tampere, Finland, 2021. [Google Scholar]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Villedieu, P.; Bennani, L. A Survey of Icephobic Coatings and Their Potential Use in a Hybrid Coating/Active Ice Protection System for Aerospace Applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef] [Green Version]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F.; Wetterwald, M.; Lasagni, A.F.; Bonaccurso, E.; Kunze, T. Self-Limited Ice Formation and Efficient De-Icing on Superhydrophobic Micro-Structured Airfoils through Direct Laser Interference Patterning. Adv. Mater. Interfaces 2020, 7, 2001231. [Google Scholar] [CrossRef]
- Bottone, D.; Donadei, V.; Niemelä, H.; Koivuluoto, H.; Seeger, S. Coral-like Silicone Nanofilament Coatings with Extremely Low Ice Adhesion. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Wong, W.S.Y.; Hegner, K.I.; Donadei, V.; Hauer, L.; Naga, A.; Vollmer, D. Capillary Balancing: Designing Frost-Resistant Lubricant-Infused Surfaces. Nano Lett. 2020, 20, 8508–8515. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Emelyanenko, A.M.; Pashinin, A.S. Analysis of Long-Term Durability of Superhydrophobic Properties under Continuous Contact with Water. ACS Appl. Mater. Interfaces 2010, 2, 1754–1758. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable Icephobic Coating for Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Laroche, A.; Bottone, D.; Seeger, S.; Bonaccurso, E. Silicone Nanofilaments Grown on Aircraft Alloys for Low Ice Adhesion. Surf. Coatings Technol. 2021, 410, 126971. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-Icing Performance of Superhydrophobic Surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Stenroos, C.; Kylmälahti, M.; Apostol, M.; Kiilakoski, J.; Vuoristo, P. Anti-Icing Behavior of Thermally Sprayed Polymer Coatings. J. Therm. Spray Technol. 2017, 26, 150–160. [Google Scholar] [CrossRef]
- Janjua, Z.A.; Turnbull, B.; Choy, K.L.; Pandis, C.; Liu, J.; Hou, X.; Choi, K.S. Performance and Durability Tests of Smart Icephobic Coatings to Reduce Ice Adhesion. Appl. Surf. Sci. 2017, 407, 555–564. [Google Scholar] [CrossRef]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Lubricated Icephobic Coatings Prepared by Flame Spraying with Hybrid Feedstock Injection. Surf. Coatings Technol. 2020, 403, 126396. [Google Scholar] [CrossRef]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Durability of Lubricated Icephobic Coatings under Multiple Icing/Deicing Cycles. In Proceedings of the International Thermal Spray Conference (ITSC), Online, 24–28 May 2021; Azarmi, F., Chen, X., Cizek, J., Cojocaru, C., Jodoin, B., Koivuluoto, H., Lau, Y., Fernandez, R., Ozdemir, O., Salami Jazi, H., Eds.; ASM International: Anaheim, CA, USA; Quebec City, QC, Canada, 2021; pp. 473–481. [Google Scholar]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Niemelä-Anttonen, H.; Varis, T.; Vuoristo, P. The Effect of Mechanical and Thermal Stresses on the Performance of Lubricated Icephobic Coatings during Cyclic Icing/Deicing Tests. Prog. Org. Coat. 2021, 106614. [Google Scholar] [CrossRef]
- Tracton, A. Coatings Technology Handbook, 3rd ed.; Tracton, A.A., Ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429119644. [Google Scholar]
- Mittal, K.; Lee, K. Polymer Surfaces and Interfaces: Characterization, Modification and Application; Mittal, K., Lee, K., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Novák, I.; Krupa, I.; Luyt, A.S. Modification of a Fischer-Tropsch Wax by Grafting with Maleic Anhydride. J. Appl. Polym. Sci. 2004, 93, 662–668. [Google Scholar] [CrossRef]
- Ozbay, S.; Erbil, H.Y. On the Relationship between Surface Free Energy and Ice Adhesion of Flat Anti-Icing Surfaces. In Ice Adhesion: Mechanism, Measurement and Mitigation; Mittal, K.L., Choi, C.-H., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 187–215. [Google Scholar]
- ISO 4288:1996 Geometrical Product Specifications (GPS). Surface Texture: Profile Method for the Assessment of Surface Texture. Available online: https://www.iso.org/standard/2096.html (accessed on 20 October 2021).
- ISO 25178-3:2012-Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 3: Specification Operators . Available online: https://www.iso.org/standard/42895.html (accessed on 2 June 2020).
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte Der Benetzungstheorie Und Ihre Anwendung Auf Die Untersuchung Und Veränderung Der Oberflächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Fowkes, F.M. Attractive Forces at Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Stenroos, C.; Ruohomaa, R.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Research on Icing Behavior and Ice Adhesion Testing of Icephobic Surfaces. In Proceedings of the International Workshop on Atmospheric Icing of Structures (IWAIS), Uppsala, Sweden, 28 June–3 July 2015; pp. 183–188. [Google Scholar]
- Niemelä-Anttonen, H.; Kiilakoski, J.; Vuoristo, P.; Koivuluoto, H. Icephobic Performance of Different Surface Designs and Materials. In Proceedings of the International Workshop on Atmospheric Icing of Structures (IWAIS), Reykjavík, Iceland, 23–27 June 2019; pp. 1–5. [Google Scholar]
- Likens, G.E.; Bormann, F.H. Acid Rain: A Serious Regional Environmental Problem. Science 1974, 184, 1176–1179. [Google Scholar] [CrossRef]
- Fedor, G.R.; Brennan, P.J. Comparison between Natural Weathering and Fluorescent UV Exposures: UVA-340 Lamp Test Results. ASTM Spec. Tech. Publ. 1996, 1294, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Mylläri, V.; Ruoko, T.P.; Järvelä, P. The Effects of UV Irradiation to Polyetheretherketone Fibres - Characterization by Different Techniques. Polym. Degrad. Stab. 2014, 109, 278–284. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. E-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Identifying Color Differences Using L*a*b* or L*C*H* Coordinates. Available online: https://sensing.konicaminolta.us/us/blog/identifying-color-differences-using-l-a-b-or-l-c-h-coordinates/ (accessed on 21 June 2021).
- ISO/CIE 11664-4:2019-Colorimetry-Part 4: CIE 1976 L*a*b* Colour Space. Available online: https://www.iso.org/standard/74166.html (accessed on 25 October 2021).
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; iSmithers Rapra Publishing: Shawbury, UK, 2005; ISBN 9783527680856. [Google Scholar]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials Fromworks of Art: SAXS Characterization and Application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef] [Green Version]
- Kühn, H. Detection and Identification of Waxes, Including Punic Wax, by Infra-Red Spectrography. Stud. Conserv. 1960, 5, 71–81. [Google Scholar] [CrossRef]
- Masae, M.; Pitsuwan, P.; Sikong, L.; Kongsong, P.; Kooptarnond, K.; Phoempoon, P. Thermo- Physical Characterization of Paraffin and Beeswax on Cotton Fabric. Thammasat Int. J. Sci. Technol. 2014, 19, 69–77. [Google Scholar]
- Varshney, D.; Ahmadi, M.; Guinel, M.J.-F.; Weiner, B.R.; Morell, G. Single-Step Route to Diamond-Nanotube Composite. Nanoscale Res. Lett. 2012, 7, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsson, A.C.; Barenstedt, C.; Karlsson, S. Susceptibility of Enhanced Environmentally Degradable Polyethylene to Thermal and Photo-Oxidation. Polym. Degrad. Stab. 1992, 37, 163–171. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Gajger, I.T. An Approach for Routine Analytical Detection of Beeswax Adulteration Using Ftir-Atr Spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.H.M.; Rakib, F.; Nischwitz, V.; Ullah, E.; Mall, R.; Shraim, A.M.; Ahmad, M.I.; Ghouri, Z.K.; McNaughton, D.; Küppers, S.; et al. Application of FTIR and LA-ICPMS Spectroscopies as a Possible Approach for Biochemical Analyses of Different Rat Brain Regions. Appl. Sci. 2018, 8, 2436. [Google Scholar] [CrossRef] [Green Version]
- Uznanski, P.; Zakrzewska, J.; Favier, F.; Kazmierski, S.; Bryszewska, E. Synthesis and Characterization of Silver Nanoparticles from (Bis)Alkylamine Silver Carboxylate Precursors. J. Nanoparticle Res. 2017, 19, 121. [Google Scholar] [CrossRef] [Green Version]
- Aykas, D.P.; Rodriguez-Saona, L.E. Assessing Potato Chip Oil Quality Using a Portable Infrared Spectrometer Combined with Pattern Recognition Analysis. Anal. Methods 2016, 8, 731–741. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M. Triacylglycerol Composition, Physico-Chemical Characteristics and Oxidative Stability of Interesterified Canola Oil and Fully Hydrogenated Cottonseed Oil Blends. Lipids Health Dis. 2015, 14, 138. [Google Scholar] [CrossRef] [Green Version]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Icephobic Behaviour and Thermal Stability of Flame-Sprayed Polyethylene Coating: The Effect of Process Parameters. J. Therm. Spray Technol. 2020, 29, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Jiang, Y.; Mielonen, K.; Pakkanen, T.A. The Verification of Icephobic Performance on Biomimetic Superhydrophobic Surfaces and the Effect of Wettability and Surface Energy. Appl. Surf. Sci. 2019, 466, 503–514. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; Mckinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Schawaller, D. Kinetic Study on the Thermal Degradation of Polypropylene and Polyethylene. J. Anal. Appl. Pyrolysis 1999, 48, 93–109. [Google Scholar] [CrossRef]
- Rønneberg, S.; Xiao, S.; He, J.; Zhang, Z. Nanoscale Correlations of Ice Adhesion Strength and Water Contact Angle. Coatings 2020, 10, 379. [Google Scholar] [CrossRef]
- He, Z.; Vågenes, E.T.; Delabahan, C.; He, J.; Zhang, Z. Room Temperature Characteristics of Polymer-Based Low Ice Adhesion Surfaces. Sci. Rep. 2017, 7, 42181. [Google Scholar] [CrossRef] [Green Version]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between Water Wettability and Ice Adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef]
- Dotan, A.; Dodiuk, H.; Laforte, C.; Kenig, S. The Relationship between Water Wetting and Ice Adhesion. J. Adhes. Sci. Technol. 2009, 23, 1907–1915. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of Anti-Icing Surfaces: Smooth, Textured or Slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Hartikainen, E.; Niemelä-Anttonen, H. Thermally Sprayed Coatings: Novel Surface Engineering Strategy towards Icephobic Solutions. Materials 2020, 13, 1434. [Google Scholar] [CrossRef] [Green Version]
- Memon, H.; Liu, J.; De Focatiis, D.S.A.; Choi, K.S.; Hou, X. Intrinsic Dependence of Ice Adhesion Strength on Surface Roughness. Surf. Coat. Technol. 2020, 385, 125382. [Google Scholar] [CrossRef]
- He, Z.; Xiao, S.; Gao, H.; He, J.; Zhang, Z. Multiscale Crack Initiator Promoted Super-Low Ice Adhesion Surfaces. Soft Matter 2017, 13, 6562–6568. [Google Scholar] [CrossRef] [PubMed]
- Irajizad, P.; Al-Bayati, A.; Eslami, B.; Shafquat, T.; Nazari, M.; Jafari, P.; Kashyap, V.; Masoudi, A.; Araya, D.; Ghasemi, H. Stress-Localized Durable Icephobic Surfaces. Mater. Horizons 2019, 6, 758–766. [Google Scholar] [CrossRef]
- Memon, H.; Mirshahidi, K.; Zarasvand, K.A.; Golovin, K.; De Focatiis, D.S.A.; Choi, K.S.; Hou, X. Comparative Study on the Influence of Surface Characteristics on De-Icing Evaluation. J. Mater. Sci. 2021, 56, 17337–17352. [Google Scholar] [CrossRef]
- Makkonen, L. Ice Adhesion - Theory, Measurements and Countermeasures. J. Adhes. Sci. Technol. 2012, 26, 413–445. [Google Scholar] [CrossRef]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M.A. Effects of Surface Roughness and Energy on Ice Adhesion Strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]
- Fillion, R.M.; Riahi, A.R.; Edrisy, A. Design Factors for Reducing Ice Adhesion. J. Adhes. Sci. Technol. 2017, 31, 2271–2284. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost Formation and Ice Adhesion on Superhydrophobic Surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Drelich, J.W. Contact Angles: From Past Mistakes to New Developments through Liquid-Solid Adhesion Measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.; Loth, E. Reducing Static and Impact Ice Adhesion with a Self-Lubricating Icephobic Coating (SLIC). Coatings 2020, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Yang, S.; Xia, Q.; Zhu, L.; Xue, J.; Wang, Q.; Chen, Q.M. Research on the Icephobic Properties of Fluoropolymer-Based Materials. Appl. Surf. Sci. 2011, 257, 4956–4962. [Google Scholar] [CrossRef]
- Laforte, C.; Laforte, J.-L.J. Tensile, Torsional and Bending Strain at the Adhesive Rupture of an Iced Substrate. In Proceedings of the International Conference on Offshore Mechanics and Arctic Engineering-OMAE, ASME, Honolulu, HI, USA, 31 May–5 June 2009; Volume 5, pp. 79–86. [Google Scholar]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of Surface Structure on the Hydrophobicity and Sliding Behavior of Water Droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Ha, J.W.; Park, I.J.; Lee, S.B. Hydrophobicity and Sliding Behavior of Liquid Droplets on the Fluorinated Latex Films. Macromolecules 2005, 38, 736–744. [Google Scholar] [CrossRef]
- Della Volpe, C.; Siboni, S.; Morra, M. Comments on Some Recent Papers on Interfacial Tension and Contact Angles. Langmuir 2002, 18, 1441–1444. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Pongratz, S. Resistance and Stability of Polymers. In Resistance and Stability of Polymers; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2013; ISBN 9781569904565. [Google Scholar]
- Brown, W.H.; Poon, T. Introduction to Organic Chemistry, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 9781118083383. [Google Scholar]
- Kulinich, S.A.; Farzaneh, M. On Ice-Releasing Properties of Rough Hydrophobic Coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Psarski, M.; Pawlak, D.; Grobelny, J.; Celichowski, G. Relationships between Surface Chemistry, Nanotopography, Wettability and Ice Adhesion in Epoxy and SU-8 Modified with Fluoroalkylsilanes from the Vapor Phase. Appl. Surf. Sci. 2019, 479, 489–498. [Google Scholar] [CrossRef]
- White, J.R.; Turnbull, A. Weathering of Polymers: Mechanisms of Degradation and Stabilization, Testing Strategies and Modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Iring, M.; Tüdős, F. Thermal Oxidation of Polyethylene and Polypropylene: Effects of Chemical Structure and Reaction Conditions on the Oxidation Process. Prog. Polym. Sci. 1990, 15, 217–262. [Google Scholar] [CrossRef]
- Sugimoto, M.; Shimada, A.; Kudoh, H.; Tamura, K.; Seguchi, T. Product Analysis for Polyethylene Degradation by Radiation and Thermal Ageing. Radiat. Phys. Chem. 2013, 82, 69–73. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Ołdak, D.; Podgórski, A. Photochemical Properties of Polyethylene Modified by Low-Molecular Organic Compounds. Polym. J. 2003, 35, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Eom, T.J. Chemical Characteristics of Degraded Beeswax in the Waxed Volume of the Annals of King Sejong in the Joseon Dynasty. J. Cult. Herit. 2015, 16, 918–921. [Google Scholar] [CrossRef]
- Lesar, B.; Pavlič, M.; Petrič, M.; Škapin, A.S.; Humar, M. Wax Treatment of Wood Slows Photodegradation. Polym. Degrad. Stab. 2011, 96, 1271–1278. [Google Scholar] [CrossRef]
- Achhammer, G.B. Chemical Structure and Stability Relationships in Polymers. Mod. Plast. 1959, 37, 131–227. [Google Scholar]
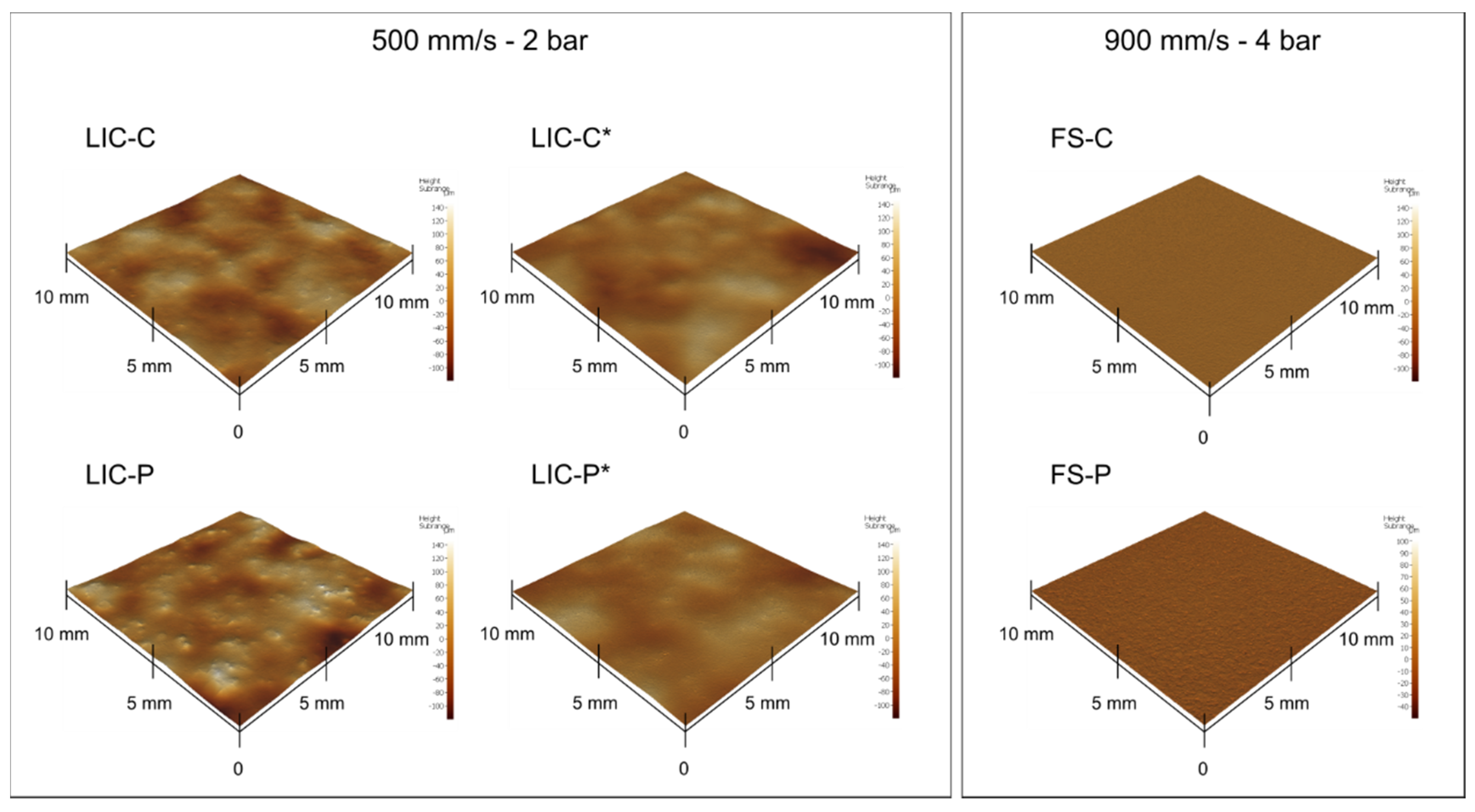

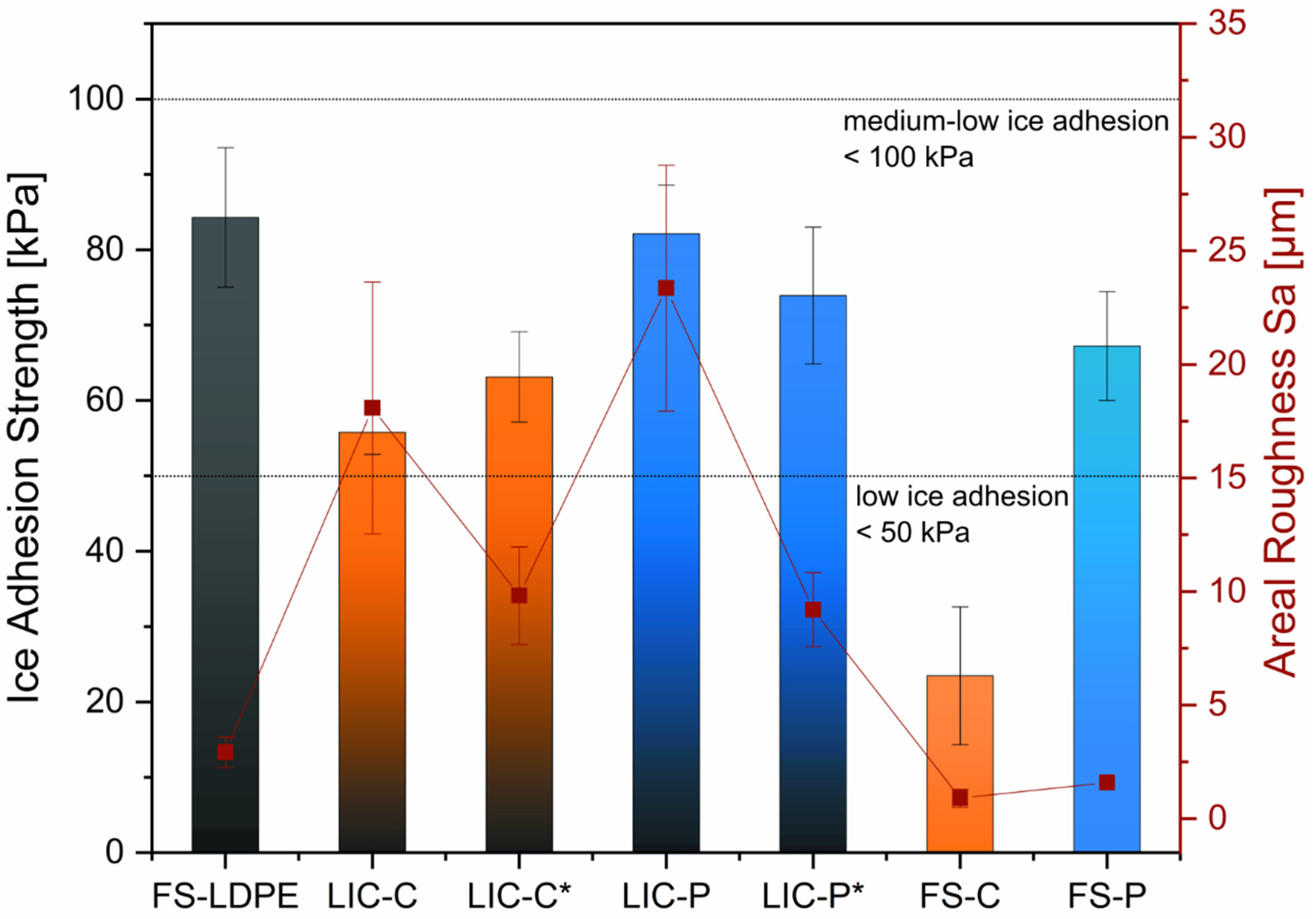
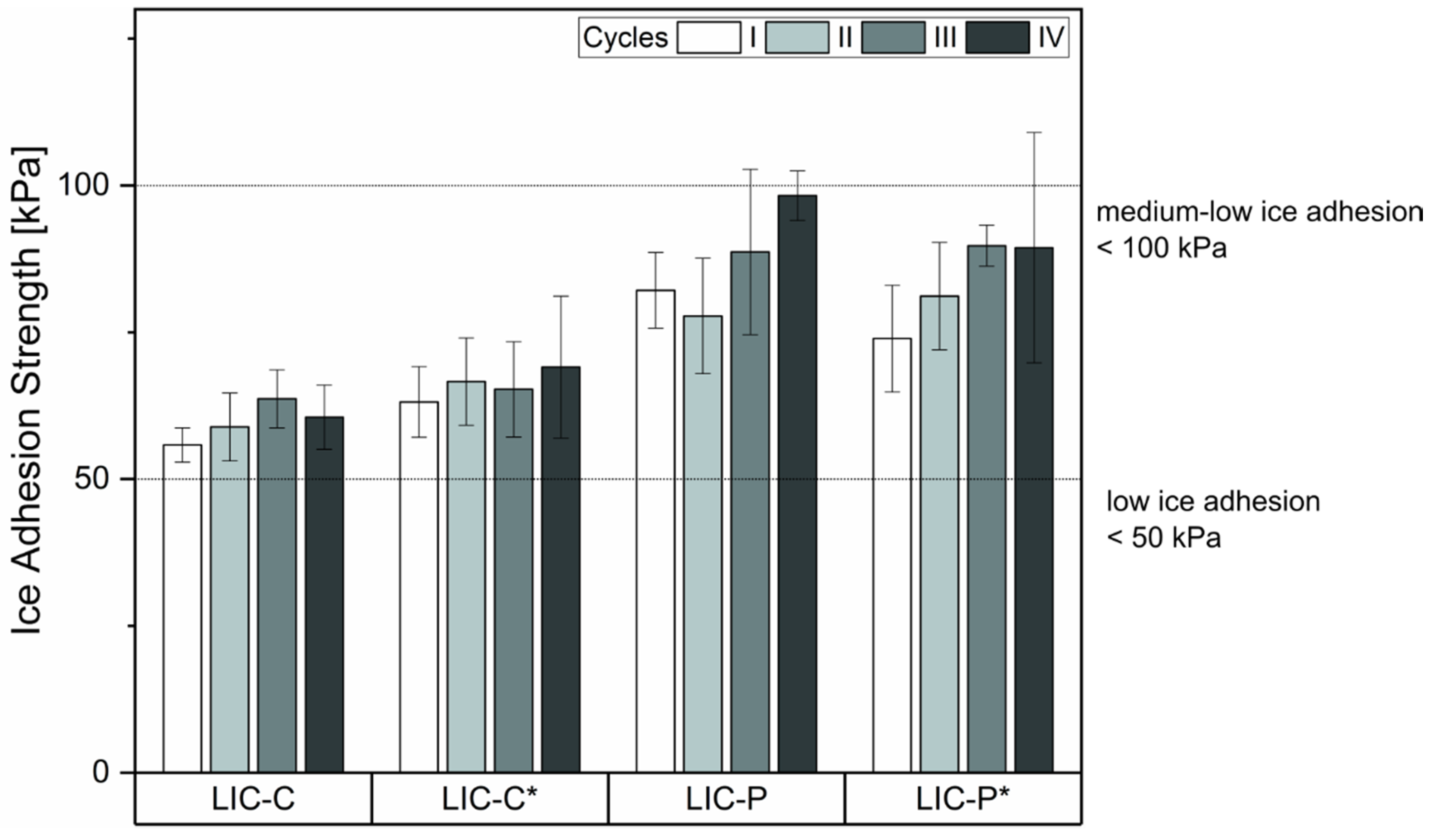

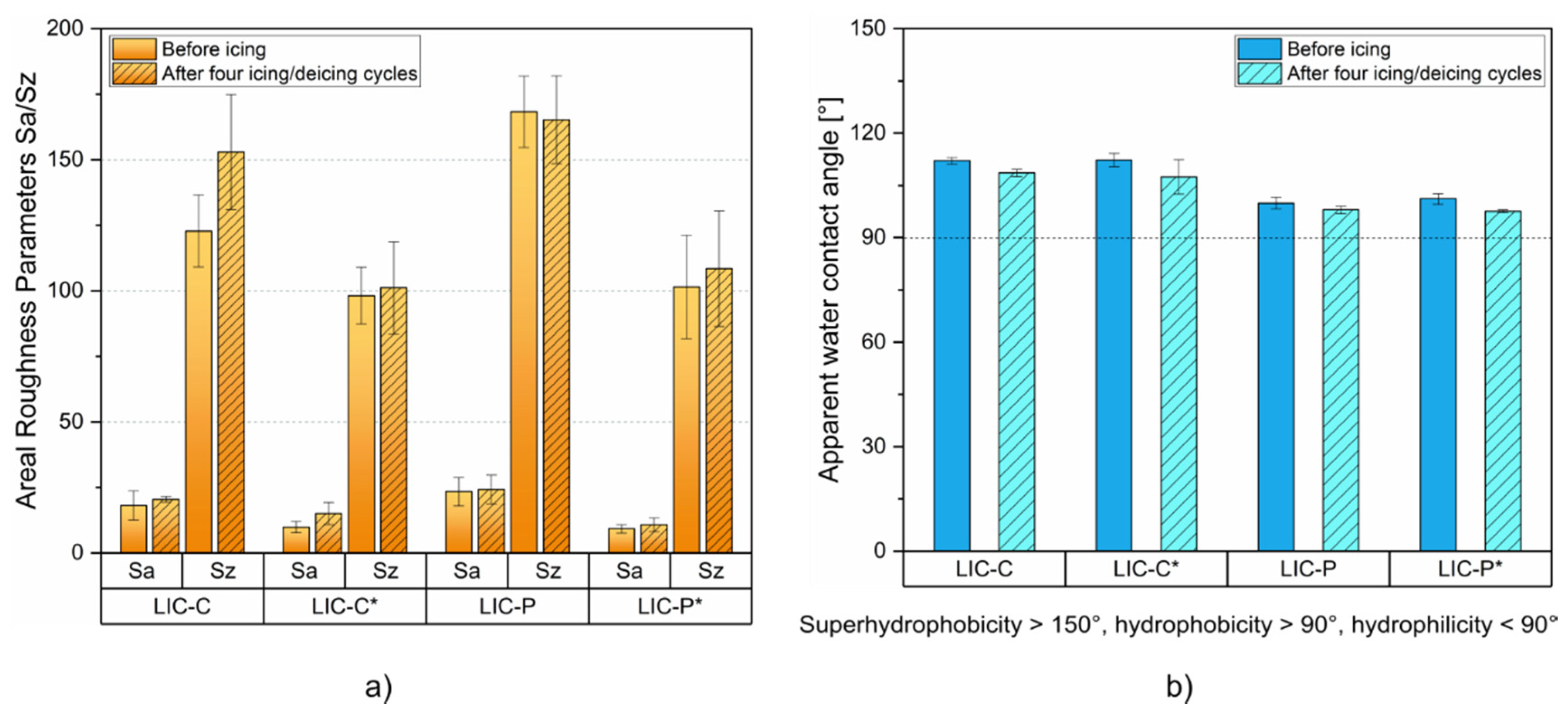




| Process Parameters | Value |
|---|---|
| CastoDyn DS 8000—flame spray gun | |
| Nozzle model | SSM10 |
| Combustion gasses | |
| Oxygen pressure [bar] | 4.0 |
| Acetylene pressure [bar] | 0.7 |
| Gun spray distance [mm] | 250 |
| Step size [mm] | 5 |
| Sulzer Metco 4MP-850D dual powder feeder—matrix powder | |
| Matrix material feed rate [g/min] | 26 |
| Carrier gas (nitrogen) flow rate [L/min] | 10 |
| Carrier gas (nitrogen) input pressure [bar] | 5 |
| PT-10 twin powder feeder—additive powder | |
| Lubricating additive material feed rate [g/min] | 8 |
| Carrier gas (argon) flow rate [L/min] | 6 |
| Sample | Materials | Gun Traverse Speed [mm/s] | Gun Air Pressure [bar] | Post-Heating [Y/N] |
|---|---|---|---|---|
| FS-LDPE | LDPE | 500 | 0 | Y |
| LIC-C | LDPE + C | 500 | 2 | N |
| LIC-C * | Y | |||
| LIC-P | LDPE + P | N | ||
| LIC-P * | Y | |||
| FS-C 1 | C | 900 | 4 | N |
| FS-P 1 | P | N |
| Reference Liquid | Chemical Formula | Surface Tension at RT [mN/m] | Dispersive Component [mN/m] | Polar Component [mN/m] |
|---|---|---|---|---|
| water | H2O | 72.8 | 21.8 | 51 |
| diiodomethane | CH2I2 | 50.8 | 50.8 | 0 |
| ethylene glycol | C2H6O2 | 47.7 | 26.4 | 21.3 |
| Sample | Tmelting peak [°C] | Tonset [°C] | Tmax degradation [°C] |
|---|---|---|---|
| LDPE | 111.9 ± 0.6 | 458.2 ± 1.3 | 481.1 ± 1.9 |
| C | 55.6 ± 0.5 | 402.5 ± 4.6 | 437.1 ± 1.7 |
| 68.9 ± 2.3 | |||
| P | 100.3 ± 0.2 | 435.0 ± 7.0 | 480.2 ± 1.7 |
| 118.8 ± 0.6 |
| Feedstock Material | SFE [mN/m] | Dispersive Component [mN/m] | Polar Component [mN/m] |
|---|---|---|---|
| LDPE | 30.41 ± 1.25 | 29.14 ± 0.64 | 1.27 ± 0.61 |
| C | 19.77 ± 1.48 | 19.15 ± 1.19 | 0.62 ± 0.28 |
| P | 22.84 ± 0.04 | 22.19 ± 0.03 | 0.64 ± 0.01 |
| Sample | Sa [µm] ± SD [µm] | Sz [μm] ± SD [μm] |
|---|---|---|
| LIC-C | 18.1 ± 5.5 | 122.8 ± 13.8 |
| LIC-C* | 9.8 ± 2.1 | 98.1 ± 10.8 |
| LIC-P | 23.4 ± 5.4 | 168.3 ± 13.5 |
| LIC-P* | 9.2 ± 1.6 | 101.5 ± 19.8 |
| FS-C | 0.9 ± 0.4 | 64.2 ± 10.6 |
| FS-P | 1.6 ± 0.1 | 74.1 ± 6.3 |
| Sample | Water Contact Angle[°] | Water Roll-Off Angle[°] |
| LIC-C | 112.1 ± 1.0 | 73.2 ± 3.9 |
| LIC-C* | 112.3 ± 1.9 | 48.6 ± 5.2 |
| LIC-P | 99.9 ± 1.7 | >90 |
| LIC-P* | 101.2 ± 1.5 | >90 |
| FS-C | 146.3 ± 3.0 | 31.4 ± 1.4 |
| FS-P | 112.6 ± 1.7 | >90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Durability of Lubricated Icephobic Coatings under Various Environmental Stresses. Polymers 2022, 14, 303. https://doi.org/10.3390/polym14020303
Donadei V, Koivuluoto H, Sarlin E, Vuoristo P. Durability of Lubricated Icephobic Coatings under Various Environmental Stresses. Polymers. 2022; 14(2):303. https://doi.org/10.3390/polym14020303
Chicago/Turabian StyleDonadei, Valentina, Heli Koivuluoto, Essi Sarlin, and Petri Vuoristo. 2022. "Durability of Lubricated Icephobic Coatings under Various Environmental Stresses" Polymers 14, no. 2: 303. https://doi.org/10.3390/polym14020303