Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete
Abstract
1. Introduction
2. Materials Characterization and Methodology
2.1. Materials and Chemicals
2.2. Methodology
3. Testing Method
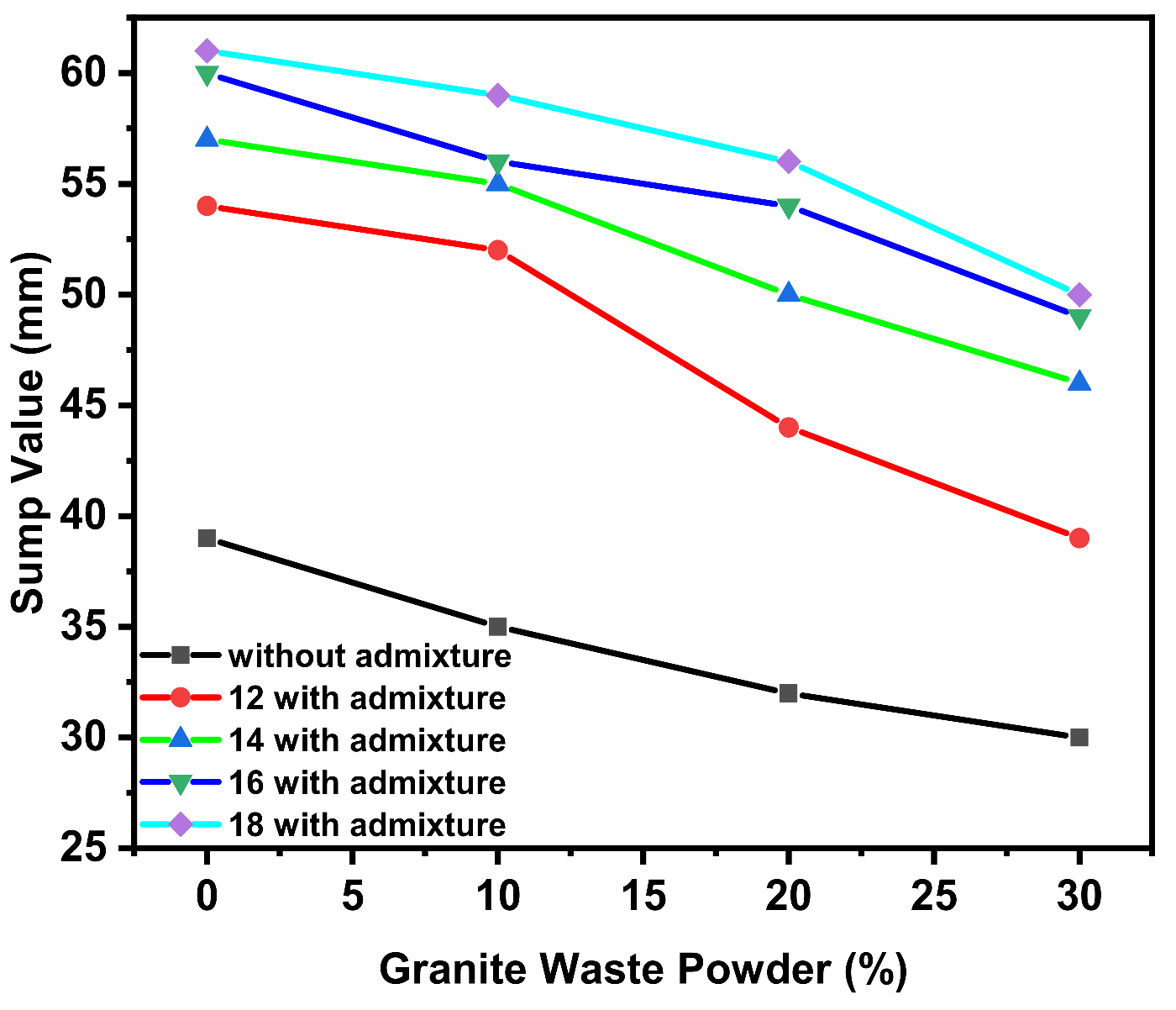
3.1. Workability
3.2. Compressive Strength Test
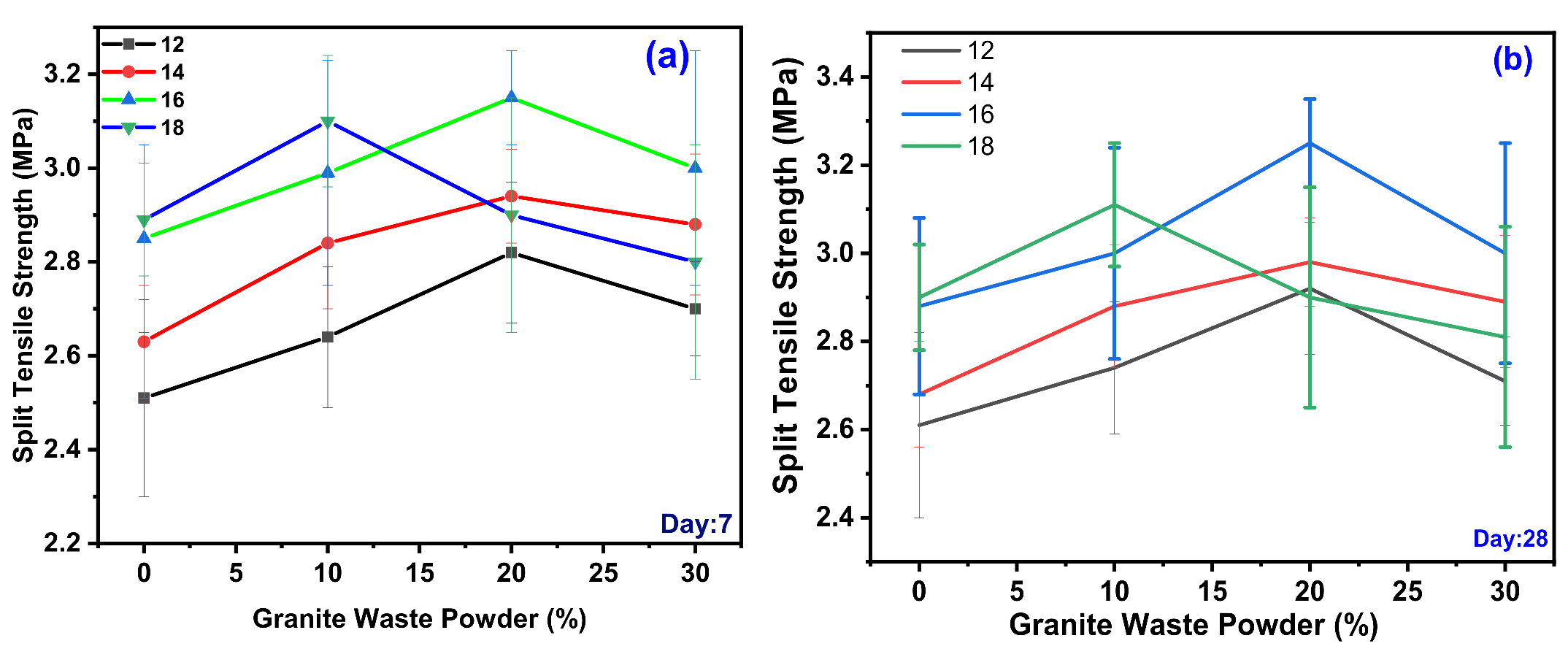
3.3. Split Tensile Test
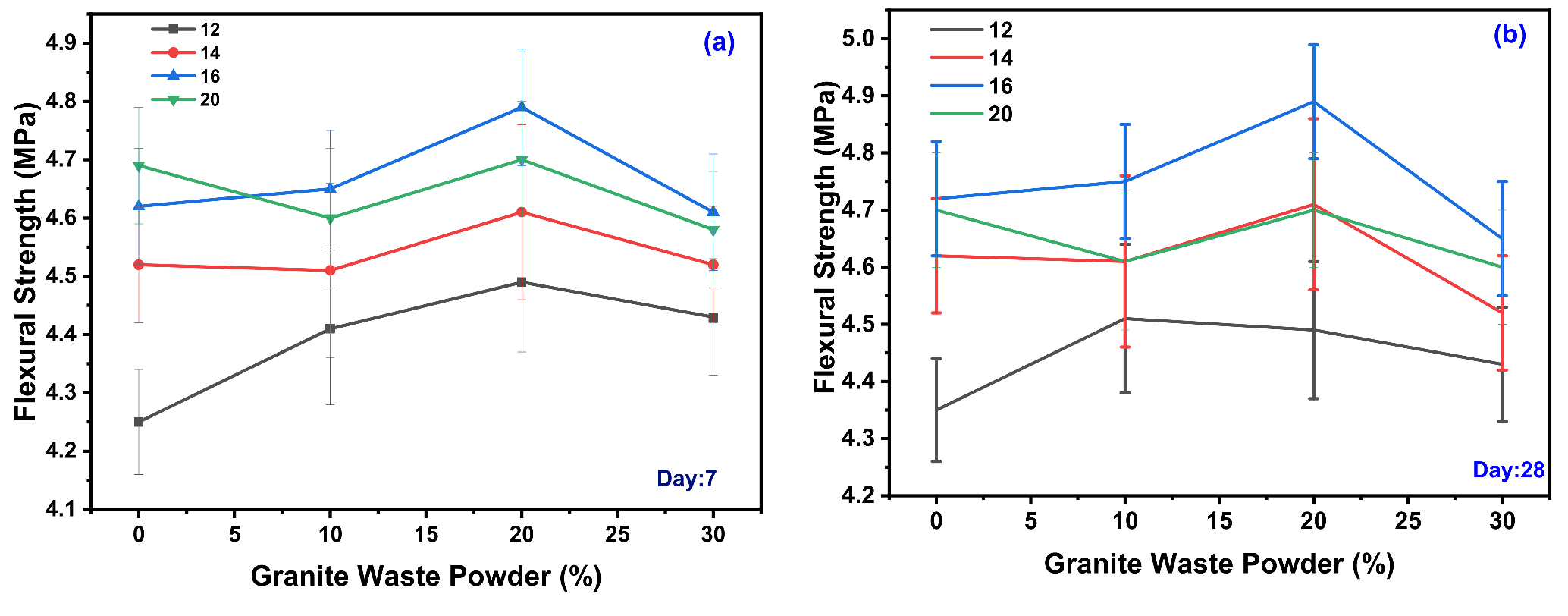
3.4. Flexural Strength Test
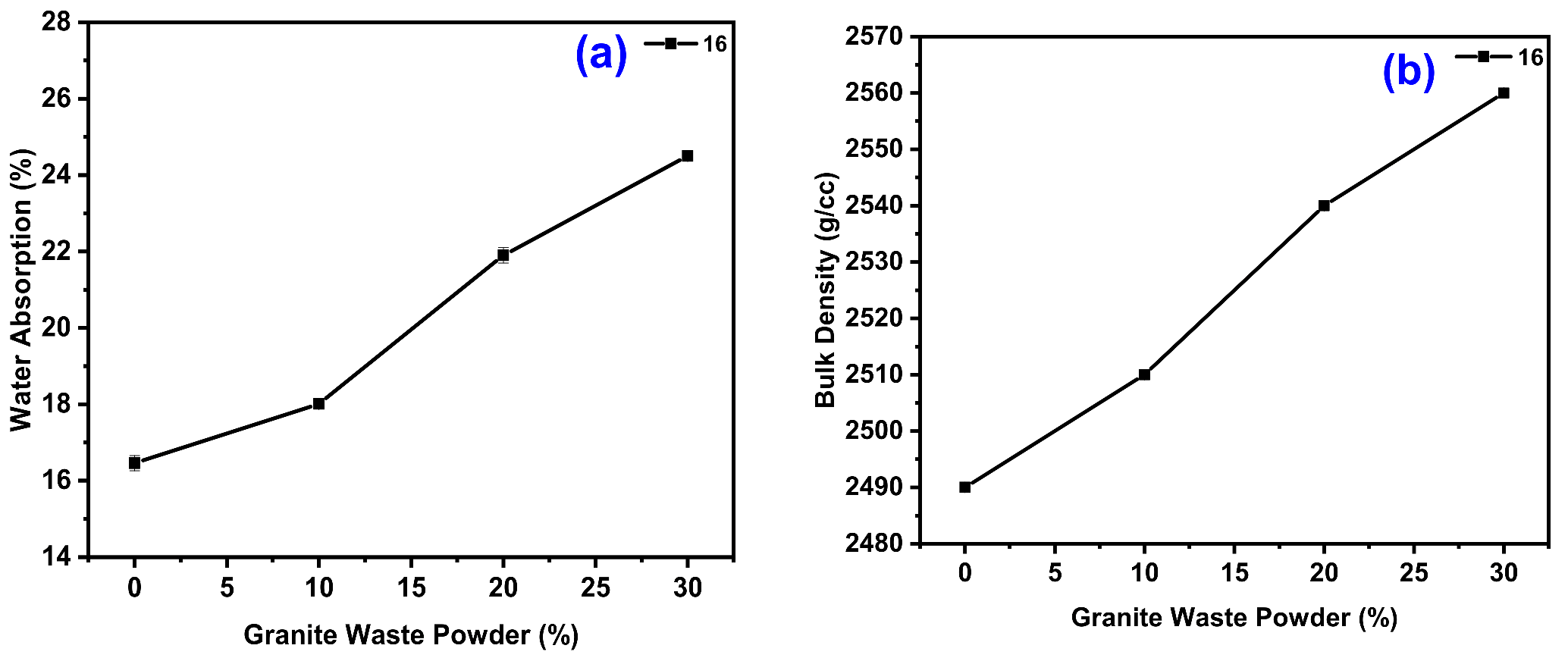
3.5. Water Absorption Test
3.6. Bulk Density Test
4. Results and Discussion
4.1. Effect of GWP on the Fresh State of GPC
4.2. Effect of GWP on the Hardened Properties
4.2.1. Effect of GWP on the Compressive Strength (CS) of GPC
4.2.2. Effect of GWP on the Split Tensile Strength (STS) of GPC
4.2.3. Effect of GWP on the Flexural Strength (FS) of GPC
4.2.4. Effect of GWP on the Water Absorption (WB) and Bulk Density (BD) of GPC
5. Commercial Value and Cost Estimation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacheco-Torgal, F., Chindaprasirt, P., Ozbakkaloglu, T., Eds.; Handbook of Advances in Alkali-Activated Concrete; Elsevier: Amsterdam, The Netherlands, 1st ed. Available online: https://www.elsevier.com/books/handbook-of-advances-in-alkali-activated-concrete/pacheco-torgal/978-0-323-85469-6 (accessed on 26 December 2021).
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability”. Front. Chem. 2021, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Arif, M.; Shariq, M. Age-dependent compressive strength and elastic modulus of fly ash-based geopolymer concrete. Struct. Concr. 2020. [Google Scholar] [CrossRef]
- Nagaraj, V.K.; Venkatesh Babu, D.L. Assessing the performance of molarity and alkaline activator ratio on engineering properties of self-compacting alkaline activated concrete at ambient temperature. J. Build. Eng. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Toprak, M.U.; Uygunoğlu, T. Durability and microstructure characteristics of alkali activated coal bottom ash geopolymer cement. J. Clean. Prod. 2014, 81, 211–217. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Effect of ground granulated blast furnace slag and fly ash ratio and the curing conditions on the mechanical properties of geopolymer concrete. Struct. Concr. 2021. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Liew, M.S. Effect of paste aggregate ratio and curing methods on the performance of one-part alkali-activated concrete. Constr. Build. Mater. 2020, 261, 120024. [Google Scholar] [CrossRef]
- Krishna Rao, A.; Kumar, D.R. Effect of various alkaline binder ratio on geopolymer concrete under ambient curing condition. Mater. Today Proc. 2020, 27, 1768–1773. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Investigation on soil–geopolymer with slag, fly ash and their blending. Arab. J. Sci. Eng. 2016, 41, 393–400. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Koushkbaghi, M.; Alipour, P.; Tahmouresi, B.; Mohseni, E.; Saradar, A.; Sarker, P.K. Influence of different monomer ratios and recycled concrete aggregate on mechanical properties and durability of geopolymer concretes. Constr. Build. Mater. 2019, 205, 519–528. [Google Scholar] [CrossRef]
- Poloju, K.K.; Sinivasu, K. Influence of GGBS and Alkaline Ratio on Compression Strength of Geopolymer Concrete: Influence of GGBS and Alkaline Ratio on Compression Strength of Geopolymer Concrete. SPAST Abstr. 2021, 1. Available online: https://spast.org/techrep/article/view/2900 (accessed on 5 November 2021).
- Kumar, V.V.P.; Prasad, N.; Dey, S. Influence of metakaolin on strength and durability characteristics of ground granulated blast furnace slag based geopolymer concrete. Struct. Concr. 2020, 21, 1040–1050. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of calcium aluminate cement on geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- EL Alouani, S.M.; Alehyen, M.; EL Achouri, A.; Hajjaji, C.; Taibi, M. Influence of the Nature and Rate of Alkaline Activator on the Physicochemical Properties of Fly Ash-Based Geopolymers. Adv. Civ. Eng. 2020, 2020, e8880906. [Google Scholar] [CrossRef]
- Kantarci, F.; Türkmen, İ.; Ekinci, E. Influence of various factors on properties of geopolymer paste: A comparative study. Struct. Concr. 2021, 22, E315–E331. [Google Scholar] [CrossRef]
- Ghannam, S.; Najm, H.; Vasconez, R. Experimental study of concrete made with granite and iron powders as partial replacement of sand. Sustain. Mater. Technol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Ganesan, N.; Abraham, R.; Deepa Raj, S. Durability characteristics of steel fibre reinforced geopolymer concrete. Constr. Build. Mater. 2015, 93, 471–476. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Menezes, R.R.; Ferreira, H.S.; Neves, G.D.A.; Ferreira, H.C. The use of granite wastes as ceramic raw materials. Cerâmica 2002, 48, 92–101. [Google Scholar] [CrossRef][Green Version]
- Gao, X.; Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Characterization and application of municipal solid waste incineration (MSWI) bottom ash and waste granite powder in alkali activated slag. J. Clean. Prod. 2017, 164, 410–419. [Google Scholar] [CrossRef]
- Al Bakri Abdullah, M.M.; Kamarudin, H.; Abdulkareem, O.A.K.A.; Ghazali, C.M.R.; Rafiza, A.R.; Norazian, M.N. Optimization of Alkaline Activator/Fly ASH Ratio on the Compressive Strength of Manufacturing Fly ASH-BASED Geopolymer. Appl. Mech. Mater. 2012, 110–116, 734–739. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix Design and Mechanical Properties of Geopolymer and Alkali Activated Concrete: Review of the state-of-the-art and the Development of a New Unified Approach. Constr. Build. Mater. 2020, 256. [Google Scholar] [CrossRef]
- Thormark, C. A low energy building in a life cycle—Its embodied energy, energy need for operation and recycling potential. Build. Environ. 2002, 37, 429–435. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Sodium hydroxide effect on the mechanical properties of flyash-slag based geopolymer concrete. Struct. Concr. 2021, 22, E368–E379. [Google Scholar] [CrossRef]
- Ikeda, K. Recent Development of Geopolymer Technique in Relevance to Carbon Dioxide and Waste Management Issues. Dev. Porous Biol. Geopolym. Ceram. Eng. Sci. Proc. 2007, 28, 293–308. [Google Scholar]
- Durak, U.; Karahan, O.; Uzal, B.; İlkentapar, S.; Atiş, C.D. Influence of nano SiO2 and nano CaCO3 particles on strength, workability, and microstructural properties of fly ash-based geopolymer. Struct. Concr. 2021, 22, E352–E367. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Jayarajan, G.; Arivalagan, S. An experimental studies of geopolymer concrete incorporated with fly-ash & GGBS. Mater. Today Proc. 2021, 45, 6915–6920. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. Final Setting Time and Compressive Strength of Fly Ash and GGBS-Based Geopolymer Paste and Mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074. [Google Scholar] [CrossRef]
- IS 1199; Methods of Sampling and Analysis of Concrete.; Bureau of Indian Standards: New Delhi, India, 1959; 49p.
- IS 516; Method of Tests for Strength of Concrete; Bureau of Indian Standards: New Delhi, India, 1959; 30p.
- IS 5816; Method of Test Splitting Tensile Strength of Concrete; Bureau of Indian Standards: New Delhi, India, 1999; 14p.
- ASTM C1585-20. Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. Available online: https://www.techstreet.com/standards/astm-c1585-20?product_id=2189851 (accessed on 26 December 2021).
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Bakthavatchalam, K.; Rajendran, M. An experimental investigation on potassium activator based geopolymer concrete incorporated with hybrid fibers. Mater. Today Proc. 2021, 46. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Li, L.; Jaya, N.A.; Abdullah, M.M.A.B.; Tan, S.J.; Hussin, K. Formation of one-part-mixing geopolymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Padmakar, M.; Barhmaiah, B.; Leela Priyanka, M. Characteristic compressive strength of a geo polymer concrete. Mater. Today Proc. 2021, 37, 2219–2222. [Google Scholar] [CrossRef]
- Kumar, R.; Das, P.; Beulah, M.; Arjun, H.R.; Ignatius, G. Utilization of Iron Ore Tailings for the Production of Fly Ash—GGBS-Based Geopolymer Bricks. J. Adv. Manuf. Syst. 2017, 16, 275–290. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Chemical interactions between siliceous aggregates and low-Ca alkali-activated cements. Cem. Concr. Res. 2007, 37, 844–855. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete—A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. 2—An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 19–47. [Google Scholar]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Samadi, M.; Huseien, G.F.; Lim, N.H.A.S.; Mohammadhosseini, H.; Alyousef, R.; Mirza, J.; Rahman, A.B.A. Enhanced performance of nano-palm oil ash-based green mortar against sulphate environment. J. Build. Eng. 2020, 32, 101640. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mohd Mustafa Al-Bakri, A.; Mohamed, B.; Luqman, M.; Khairul Nizar, I.; Liew, Y.M. Effect of alkali concentration on mechanical properties of kaolin geopolymers. Rom. J. Mater. 2012, 42, 179–186. [Google Scholar]
- AlKhatib, A.; Maslehuddin, M.; Al-Dulaijan, S.U. Development of high performance concrete using industrial waste materials and nano-silica. J. Mater. Res. Technol. 2020, 9, 6696–6711. [Google Scholar] [CrossRef]
- Xie, J.; Chen, W.; Wang, J.; Fang, C.; Zhang, B.; Liu, F. Coupling effects of recycled aggregate and GGBS/metakaolin on physicochemical properties of geopolymer concrete. Constr. Build. Mater. 2019, 226, 345–359. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Liu, T.; Qin, Z.; Tan, H.; Zhang, H.; Chen, Z.; Ni, H. Mechanical and microstructural characterization of geopolymers synthesized from FCC waste catalyst and silica fume. Ceram. Int. 2021, 47, 15186–15194. [Google Scholar] [CrossRef]
- Their, J.M.; Özakça, M. Developing geopolymer concrete by using cold-bonded fly ash aggregate, nano-silica, and steel fiber. Constr. Build. Mater. 2018, 180, 12–22. [Google Scholar] [CrossRef]
- Ganesh, A.C.; Muthukannan, M. Development of high performance sustainable optimized fiber reinforced geopolymer concrete and prediction of compressive strength. J. Clean. Prod. 2021, 282, 124543. [Google Scholar] [CrossRef]
- Luhar, S.; Chaudhary, S.; Luhar, I. Development of rubberized geopolymer concrete: Strength and durability studies. Constr. Build. Mater. 2019, 204, 740–753. [Google Scholar] [CrossRef]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Sethi, H.; Bansal, P.P.; Sharma, R. Effect of Addition of GGBS and Glass Powder on the Properties of Geopolymer Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2019, 43, 607–617. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejía de Gutiérrez, R.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M.; Adak, D. Effect of parameters on the compressive strength of fly ash based geopolymer concrete. Struct. Concr. 2018, 19, 1202–1209. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chalee, W. Effect of sodium hydroxide concentration on chloride penetration and steel corrosion of fly ash-based geopolymer concrete under marine site. Constr. Build. Mater. 2014, 63, 303–310. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Malkawi, A.B.; Nuruddin, M.F.; Fauzi, A.; Almattarneh, H.; Mohammed, B.S. Effects of Alkaline Solution on Properties of the HCFA Geopolymer Mortars. Procedia Eng. 2016, 148, 710–717. [Google Scholar] [CrossRef]
- Mousavinejad, S.H.G.; Gashti, M.F. Effects of alkaline solution/binder and Na2SiO3/NaOH ratios on fracture properties and ductility of ambient-cured GGBFS based heavyweight geopolymer concrete. Structures 2021, 32, 2118–2129. [Google Scholar] [CrossRef]
- Nuaklong, P.; Jongvivatsakul, P.; Pothisiri, T.; Sata, V.; Chindaprasirt, P. Influence of rice husk ash on mechanical properties and fire resistance of recycled aggregate high-calcium fly ash geopolymer concrete. J. Clean. Prod. 2020, 252, 119797. [Google Scholar] [CrossRef]
- Nuaklong, P.; Wongsa, A.; Boonserm, K.; Ngohpok, C.; Jongvivatsakul, P.; Sata, V.; Sukontasukkul, P.; Chindaprasirt, P. Enhancement of mechanical properties of fly ash geopolymer containing fine recycled concrete aggregate with micro carbon fiber. J. Build. Eng. 2021, 41, 102403. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Prinsse, S.; Hordijk, D.A.; Ye, G.; Lagendijk, P.; Luković, M. Time-dependent material properties and reinforced beams behavior of two alkali-activated types of concrete. Struct. Concr. 2020, 21, 642–658. [Google Scholar] [CrossRef]
- Bouaissi, A.; Li, L.; Al Bakri Abdullah, M.M.; Bui, Q.-B. Mechanical properties and microstructure analysis of FA-GGBS-HMNS based geopolymer concrete. Constr. Build. Mater. 2019, 210, 198–209. [Google Scholar] [CrossRef]

| Characteristics | GGBS (wt. %) | GWS (wt. %) |
|---|---|---|
| Chemical Composition | ||
| Silica | 27–38 | 72.04% |
| Aluminum oxide | 7–12 | 14.42% |
| Iron oxide | 0.2–1.6 | 1.68% |
| Calcium oxide | 34–43 | 1.82% |
| Magnesium oxide | 0.15–0.76 | 0.71% |
| Titanium oxide | - | 0.30% |
| Phosphorous | - | 0.12% |
| Sulfates | 1.0–1.9 | - |
| Alkali oxide | - | - |
| Loss of ignition | 1.9 | 0.29 |
| Physical Properties | ||
| Specific Gravity | 2.91 | 2.59 |
| Specific Surface Area | 400 m2/kg | 370 m2/kg |
| Mix ID | GGBS % | GWP % | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | NaOH (kg/m3) | Na2SiO3 (kg/m3) | Molarity (M) | Alkali/Binder Ratio | Admixture Dosage (kg/m3) |
|---|---|---|---|---|---|---|---|---|---|
| Standard | 100 | 0 | 556 | 1296 | 14.9 | 52.4 | 12 | 0.30 | 0.0 |
| 12G0 | 100 | 0 | 556 | 1296 | 14.9 | 52.4 | 12 | 0.30 | 8.5 |
| 12G10 | 90 | 10 | 556 | 1296 | 14.9 | 52.4 | 12 | 0.30 | 8.5 |
| 12G20 | 80 | 20 | 556 | 1296 | 14.9 | 52.4 | 12 | 0.30 | 8.5 |
| 12G30 | 70 | 30 | 556 | 1296 | 14.9 | 52.4 | 12 | 0.30 | 8.5 |
| 14G0 | 100 | 0 | 556 | 1296 | 14.9 | 52.4 | 14 | 0.30 | 8.5 |
| 14G10 | 90 | 10 | 556 | 1296 | 14.9 | 52.4 | 14 | 0.30 | 8.5 |
| 14G20 | 80 | 20 | 556 | 1296 | 14.9 | 52.4 | 14 | 0.30 | 8.5 |
| 14G30 | 70 | 30 | 556 | 1296 | 14.9 | 52.4 | 14 | 0.30 | 8.5 |
| 16G0 | 100 | 0 | 556 | 1296 | 14.9 | 52.4 | 16 | 0.30 | 8.5 |
| 16G10 | 90 | 10 | 556 | 1296 | 14.9 | 52.4 | 16 | 0.30 | 8.5 |
| 16G20 | 80 | 20 | 556 | 1296 | 14.9 | 52.4 | 16 | 0.30 | 8.5 |
| 16G30 | 70 | 30 | 556 | 1296 | 14.9 | 52.4 | 16 | 0.30 | 8.5 |
| 18G0 | 100 | 0 | 556 | 1296 | 14.9 | 52.4 | 18 | 0.30 | 8.5 |
| 18G10 | 90 | 10 | 556 | 1296 | 14.9 | 52.4 | 18 | 0.30 | 8.5 |
| 18G20 | 80 | 20 | 556 | 1296 | 14.9 | 52.4 | 18 | 0.30 | 8.5 |
| 18G30 | 70 | 30 | 556 | 1296 | 14.9 | 52.4 | 18 | 0.30 | 8.5 |
| Sl. No. | Materials | Quantity | Rates (INR) | Cost (USD) |
|---|---|---|---|---|
| Geopolymer Concrete | ||||
| 1 | GGBS | 281.8 kg/m3 | 3000/t | 845.4 |
| 2 | Granite waste powder | 122.7 kg/m3 | 100/t | 12.27 |
| 3 | Fine aggregate | 554 kg/m3 | 3800/t | 2105.2 |
| 4 | Coarse aggregate | 1294 kg/m3 | 1000/t | 1294.0 |
| 5 | NaOH | 14.66 kg/m3 | 95/kg | 1392.7 |
| 6 | Na2SiO3 | 52.4 l/m3 | 25/kg | 1310.0 |
| 7 | Distilled water | 43.09 l/m3 | 20/liter | 861.8 |
| 8 | Admixture–SP 430 | 4.9 l/m3 | 100/liter | 491 |
| Total | 7804 ₹ m−3 | 111.65 $ m−3 | ||
| Conventional Concrete | ||||
| 10 | Cement-43 grade OPC | 420 kg/m3 | 350/Bags | 2940 |
| 11 | Fine aggregate | 700 kg/m3 | 3800/t | 2660.0 |
| 12 | Coarse aggregate | 1200 kg/m3 | 1000/t | 1200.0 |
| 13 | Drinking water | 150 l/m3 | 2/liter | 300.0 |
| 14 | Admixture–SP 430 | 1.89 l/m3 | 100/liter | 189.0 |
| Total | 7289 ₹ m−3 | 97.95 $ m−3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Nisar, K.S.; Abdel-Aty, A.-H.; Yahia, I.S. Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete. Polymers 2022, 14, 306. https://doi.org/10.3390/polym14020306
Shilar FA, Ganachari SV, Patil VB, Nisar KS, Abdel-Aty A-H, Yahia IS. Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete. Polymers. 2022; 14(2):306. https://doi.org/10.3390/polym14020306
Chicago/Turabian StyleShilar, Fatheali A., Sharanabasava V. Ganachari, Veerabhadragouda B. Patil, Kottakkaran Sooppy Nisar, Abdel-Haleem Abdel-Aty, and I. S. Yahia. 2022. "Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete" Polymers 14, no. 2: 306. https://doi.org/10.3390/polym14020306
APA StyleShilar, F. A., Ganachari, S. V., Patil, V. B., Nisar, K. S., Abdel-Aty, A.-H., & Yahia, I. S. (2022). Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete. Polymers, 14(2), 306. https://doi.org/10.3390/polym14020306











