The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. AO/VUV Exposure Test
2.3. Sample Characterization
3. Results and Discussion
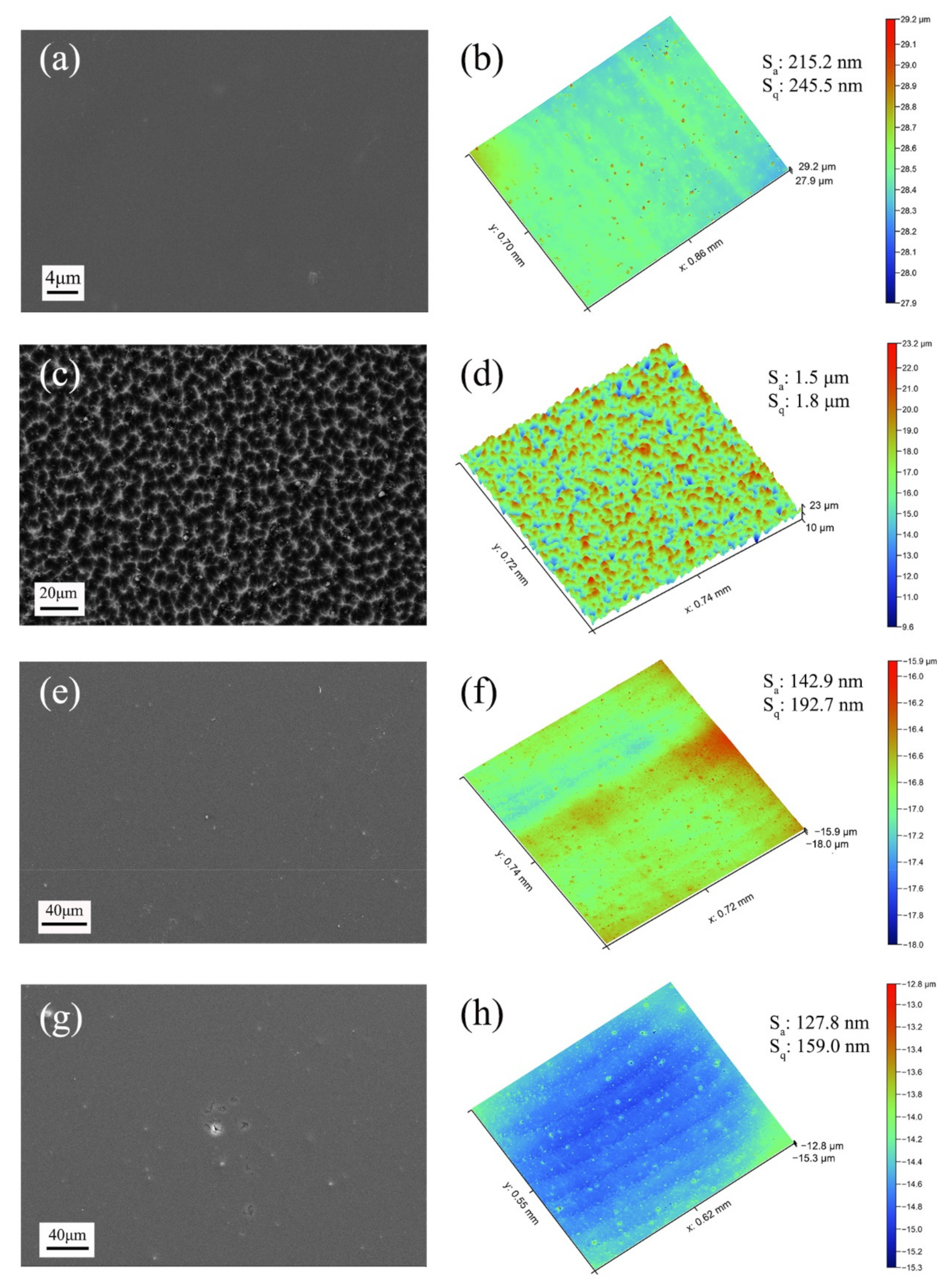
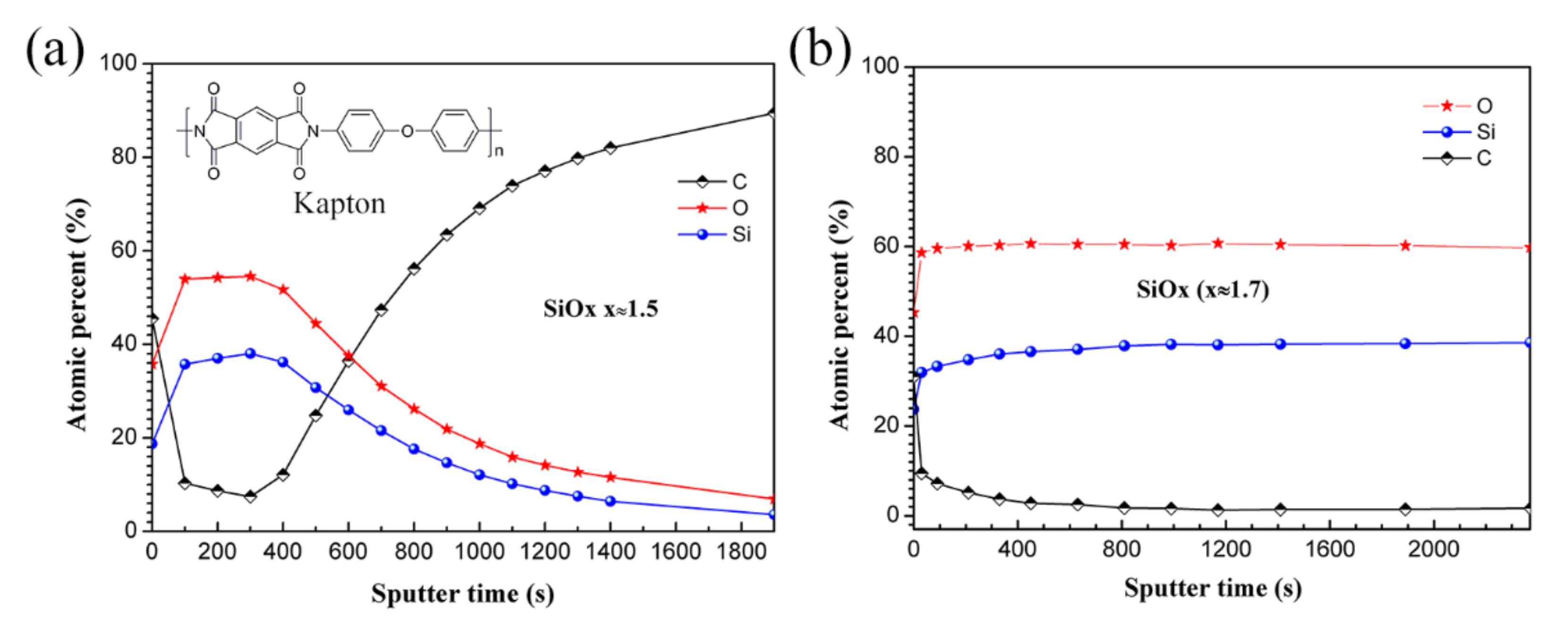
3.1. Morphology and Chemical Composition Characteristics
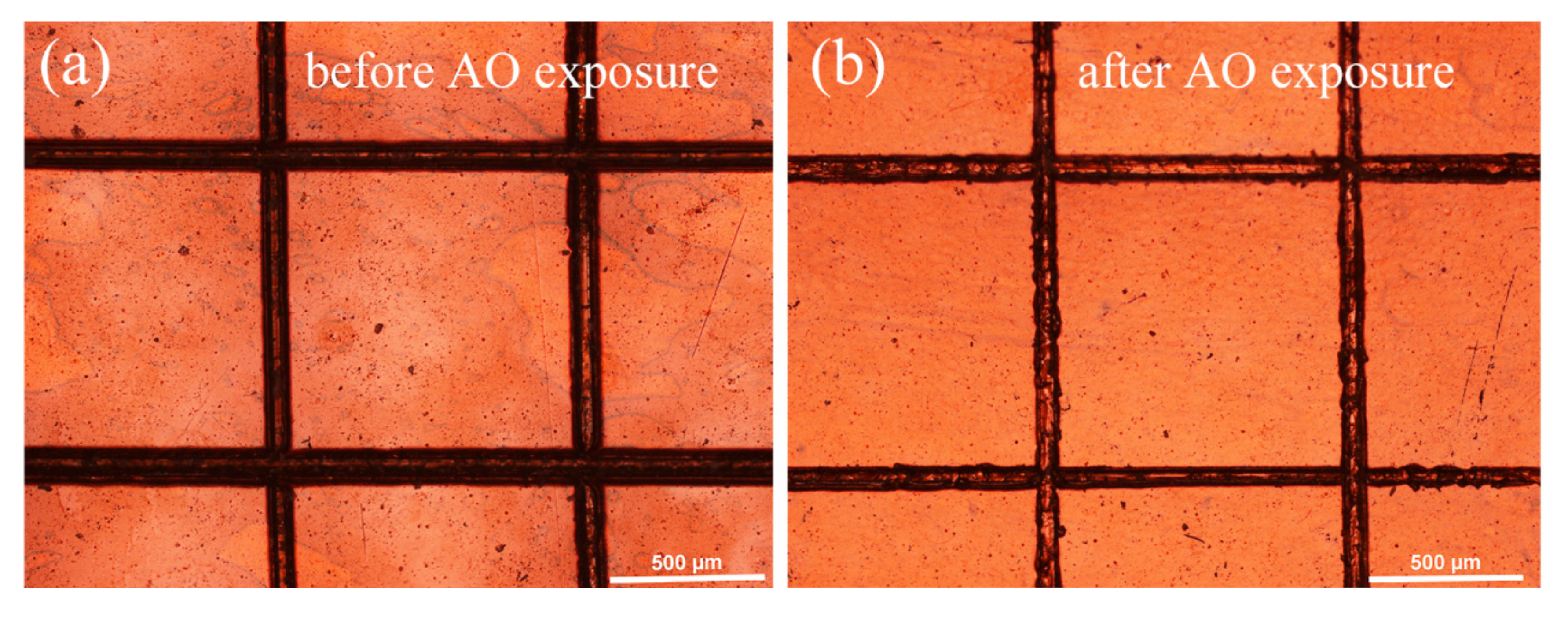
3.2. Adhesion Property of the PHPS Coating
3.3. AO and AO + VUV Erosion
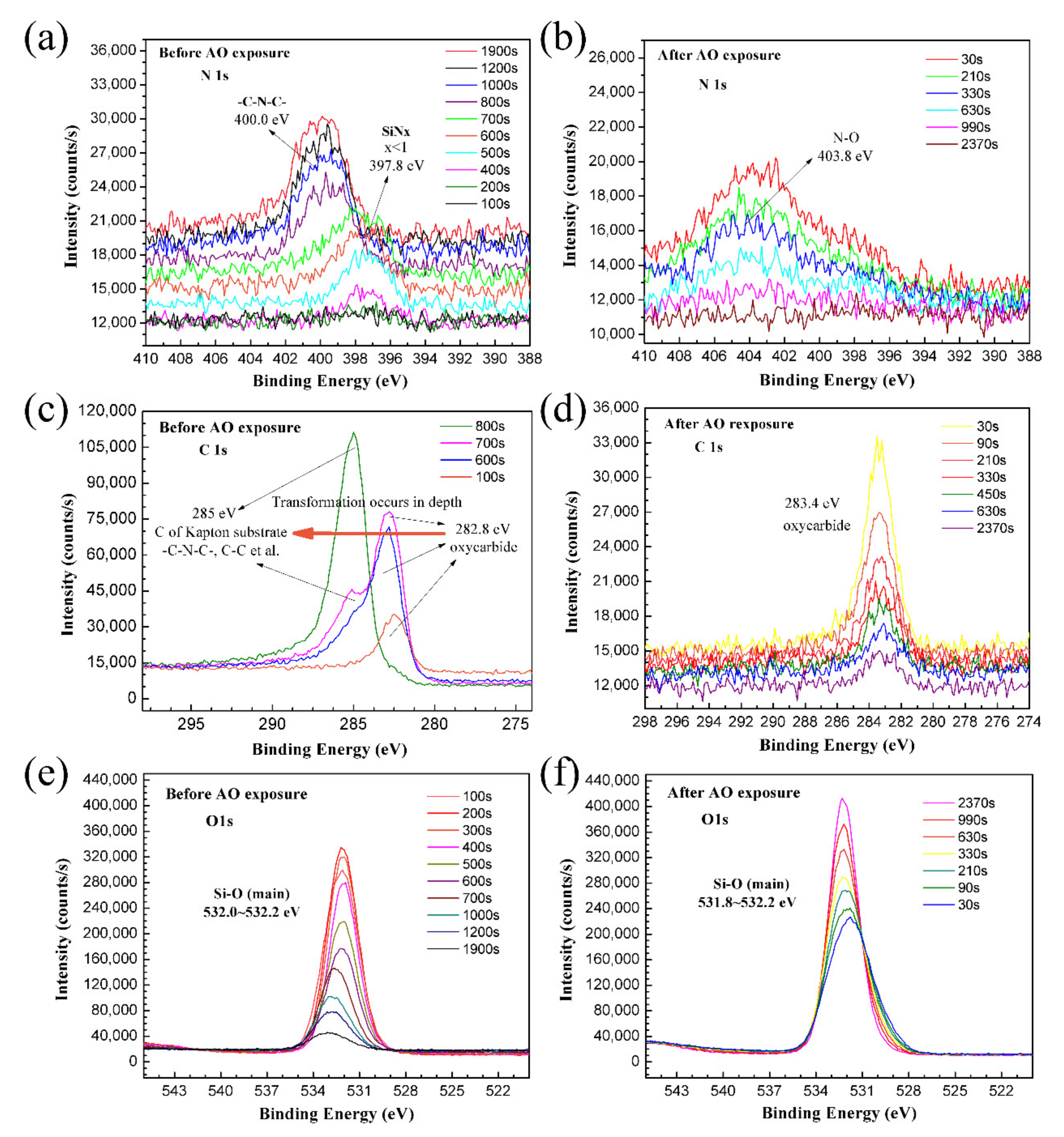
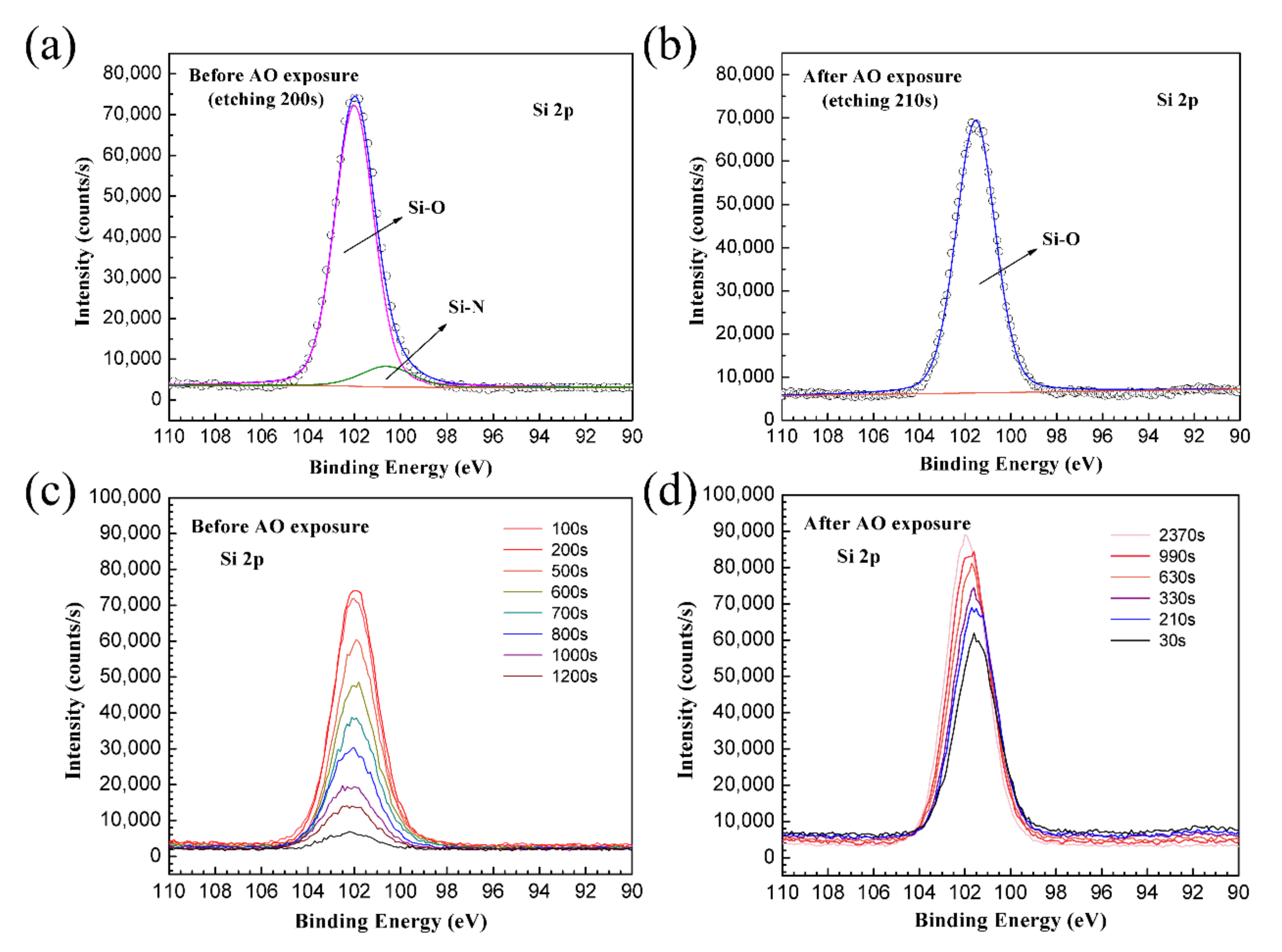
3.4. XPS Analysis Results
3.5. AO Resistance Mechanism of the PHPS Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lian, R.; Lei, X.; Xue, S.; Chen, Y.; Zhang, Q. Janus polyimide films with outstanding AO resistance, good optical transparency and high mechanical strength. Appl. Surf. Sci. 2021, 535, 147654. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K. Atomic oxygen-induced polymer degradation phenomena in simulated LEO space environments: How do polymers react in a complicated space environment? Acta Astronaut. 2008, 62, 203–211. [Google Scholar] [CrossRef]
- Suliga, A.; Jakubczyk, E.M.; Hamerton, I.; Viquerat, A. Analysis of atomic oxygen and ultraviolet exposure effects on cycloaliphatic epoxy resins reinforced with octa-functional POSS. Acta Astronaut. 2018, 142, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Clausi, M.; Santonicola, M.G.; Schirone, L.; Laurenzi, S. Analysis of ultraviolet exposure effects on the surface properties of epoxy/graphene nanocomposite films on Mylar substrate. Acta Astronaut. 2017, 134, 307–313. [Google Scholar] [CrossRef]
- De Groh, K.K.; Banks, B.A.; Miller, S.K.R.; Dever, J.A. Degradation of Spacecraft Materials, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically robust atomic oxygen-resistant coatings capable of autonomously healing damage in low earth orbit space environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G. Numerical simulation on atomic oxygen undercutting of Kapton film in low earth orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Banks, B.A.; De Groh, K.K.; Miller, S.K. Low earth orbital atomic oxygen interactions with spacecraft materials. Mater. Res. Soc. Symp. Proc. 2005, 851, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, X.; Shen, Z.; Zhang, X. Preparation of two-component hybrid polyimide film for atomic oxygen erosion resistance. Mater. Today Commun. 2021, 27, 102141. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Zuo, J.; Li, M.; Zhang, Z.; Xie, X.; Shi, Q. Fabrication of 60 wt% SiO2 filled hybrid nanocomposite and its application in erosion-corrosion and radiation resistance. Vacuum 2021, 189, 110228. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Li, M. Studies on atomic oxygen erosion resistance of deposited Mg-alloy coating on Kapton. Corros. Sci. 2017, 124, 56–62. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Zuo, J.; Li, M. An AZ31 magnesium alloy coating for protecting polyimide from erosion-corrosion by atomic oxygen. Corros. Sci. 2018, 138, 170–177. [Google Scholar] [CrossRef]
- Shivakumar, R.; Bolker, A.; Tsang, S.H.; Atar, N.; Verker, R.; Gouzman, I.; Hala, M.; Moshe, N.; Jones, A.; Grossman, E.; et al. POSS enhanced 3D graphene-polyimide film for atomic oxygen endurance in low earth orbit space environment. Polymer 2020, 191, 122270. [Google Scholar] [CrossRef]
- Miyazaki, E.; Tagawa, M.; Yokota, K.; Yokota, R.; Kimoto, Y.; Ishizawa, J. Investigation into tolerance of polysiloxane-block-polyimide film against atomic oxygen. Acta Astronaut. 2010, 66, 922–928. [Google Scholar] [CrossRef]
- Chang, Y.C.; Liu, T.Z.; Zhang, H.; Duo, S.W. Protection of kapton from atomic-oxygen erosion using a polysilazane coating. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 651–653, pp. 65–68. [Google Scholar] [CrossRef]
- Xu, C.; Gao, Z.; Guo, Y.; Shu, M.; Gao, Y. Study on in-situ growth of polyhedral oligomeric silsesquioxane (POSS) layer on kapton surface and the properties of SiO2/POSS coatings. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124720. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Li, Z.; Xie, Y.; Gao, Y.; Liu, H. Preparation and atomic oxygen erosion resistance of SiOx coating formed on polyimide film by plasma polymer deposition. Vacuum 2019, 165, 7–11. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, H.; Wang, J.; Zhang, Y.; Shao, Y. (3-Glycidoxypropyl)-terminated silsesquioxane impact on nanomechanical properties of polyimide coatings exposed to atomic oxygen. Mater. Lett. 2011, 65, 821–824. [Google Scholar] [CrossRef]
- Nyczyk-Malinowska, A.; Niemiec, W.; Smoła, G.; Gaweł, R.; Szuwarzyński, M.; Grzesik, Z. Preparation and characterization of oxidation-resistant black glass (SiCO) coatings obtained by hydrosilylation of polysiloxanes. Surf. Coat. Technol. 2021, 407, 126760. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y.; Zhou, Y. A polysilazane coating protecting polyimide from atomic oxygen and vacuum ultraviolet radiation erosion. Surf. Coat. Technol. 2009, 203, 3338–3343. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y. Perhydropolysilazane derived silica coating protecting Kapton from atomic oxygen attack. Thin Solid Film 2011, 520, 1063–1068. [Google Scholar] [CrossRef]
- Kääriäinen, T.O.; Maydannik, P.; Cameron, D.C.; Lahtinen, K.; Johansson, P.; Kuusipalo, J. Atomic layer deposition on polymer based flexible packaging materials: Growth characteristics and diffusion barrier properties. Thin Solid Film 2011, 519, 3146–3154. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.N.; Yan, W.Q.; Li, Q.; Chen, L.; Ou, Y.X.; Liao, B. Protection of Kapton from atomic oxygen attack by SiOx/NiCr coating. Surf. Coat. Technol. 2021, 423, 127582. [Google Scholar] [CrossRef]
- Bauer, F.; Decker, U.; Dierdorf, A.; Ernst, H.; Heller, R.; Liebe, H.; Mehnert, R. Preparation of moisture curable polysilazane coatings: Part I. Elucidation of low temperature curing kinetics by FT-IR spectroscopy. Prog. Org. Coat. 2005, 53, 183–190. [Google Scholar] [CrossRef]
- Wang, K.; Günthner, M.; Motz, G.; Bordia, R.K. High performance environmental barrier coatings, Part II: Active filler loaded SiOC system for superalloys. J. Eur. Ceram. Soc. 2011, 31, 3011–3020. [Google Scholar] [CrossRef]
- Dargere, N.; Bounor-Legaré, V.; Boisson, F.; Cassagnau, P.; Martin, G.; Sonntag, P.; Garois, N. Hydridosilazanes hydrolysis-condensation reactions studied by 1H and 29Si liquid NMR spectroscopy. J. Sol-Gel Sci. Technol. 2012, 62, 389–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Z.; Luo, Y.; An, P.; Zhang, M.; Xu, C. Hydrophobic, transparent and hard silicon oxynitride coating from perhydropolysilazane. Polym. Int. 2015, 64, 971–978. [Google Scholar] [CrossRef]
- Song, J.; Wang, D.; Hu, L.; Huang, X.; Chen, Y. Superhydrophobic surface fabricated by nanosecond laser and perhydropolysilazane. Appl. Surf. Sci. 2018, 455, 771–779. [Google Scholar] [CrossRef]
- Naganuma, Y.; Horiuchi, T.; Kato, C.; Tanaka, S. Low-temperature synthesis of silica coating on a poly(ethylene terephthalate) film from perhydropolysilazane using vacuum ultraviolet light irradiation. Surf. Coat. Technol. 2013, 225, 40–46. [Google Scholar] [CrossRef]
- Lee, J.Y.; Takeichi, T.; Saito, R. Study on synthesis and the reaction mechanism of polybenzoxazine-silica nanocomposites provided from perhydropolysilazane. Polymer 2016, 99, 536–543. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.P.; Alberola, N. Thin gas-barrier silica layers from perhydropolysilazane obtained through low temperature curings: A comparative study. Thin Solid Film 2012, 524, 62–66. [Google Scholar] [CrossRef]
- Blankenburg, L.; Schrödner, M. Perhydropolysilazane derived silica for flexible transparent barrier foils using a reel-to-reel wet coating technique: Single- and multilayer structures. Surf. Coat. Technol. 2015, 275, 193–206. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Li, P.; Wang, D.; Zhang, Z.; Guo, Y.; Jiang, L.; Xu, C. Room-temperature, solution-processed SiOx via photochemistry approach for highly flexible resistive switching memory. ACS Appl. Mater. Interfaces 2020, 12, 56186–56194. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Wang, F. Experimental study of thermal diffusion enhanced vapor transfer performance with perhydropolysilazane-derived silica (PDS) coating membranes in air dehumidification process. Int. J. Refrig. 2021, 122, 21–32. [Google Scholar] [CrossRef]
- Park, S.; Cho, J.; Jeong, D.; Jo, J.; Nam, M.; Rhee, H.; Han, J.S.; Cho, Y.J.; Ju, B.K.; Ko, D.H.; et al. Simultaneous enhancement of luminescence and stability of CsPbBr3 perovskite nanocrystals via formation of perhydropolysilazane-derived nanopatterned film. Chem. Eng. J. 2020, 393, 124767. [Google Scholar] [CrossRef]
- Kozuka, H.; Nakajima, K.; Uchiyama, H. Superior properties of silica thin films prepared from perhydropolysilazane solutions at room temperature in comparison with conventional alkoxide-derived silica gel films. ACS Appl. Mater. Interfaces 2013, 5, 8329–8336. [Google Scholar] [CrossRef]
- Yang, N.; Wang, W.; Cai, W.; Lu, K. Corrosion and tribocorrosion mitigation of perhydropolysilazane-derived coatings on low carbon steel. Corros. Sci. 2020, 177, 108946. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, C.; Zhu, J.; Wang, W. Passivation Characteristics of New Silicon Oxide. IEEE J. Photovolt. 2019, 9, 1873–1879. [Google Scholar] [CrossRef] [Green Version]
- Duo, L.; Zhang, Z.; Zheng, K.; Wang, D.; Xu, C.; Xia, Y. Perhydropolysilazane derived SiON interfacial layer for Cu/epoxy molding compound composite. Surf. Coat. Technol. 2020, 391, 125703. [Google Scholar] [CrossRef]
- Nakajima, K.; Uchiyama, H.; Kitano, T.; Kozuka, H. Conversion of solution-derived perhydropolysilazane thin films into silica in basic humid atmosphere at room temperature. J. Am. Ceram. Soc. 2013, 96, 2806–2816. [Google Scholar] [CrossRef]
- Duo, S.; Li, M.; Wang, Y. A simulator for producing of high flux atomic oxygen beam by using ECR plasma source. J. Mater. Sci. Technol. 2004, 20, 759–762. [Google Scholar]
- Hu, L.; Li, M.; Zhou, Y. Effects of vacuum ultraviolet radiation on atomic oxygen erosion of polysiloxane/SiO2 hybrid coatings. J. Mater. Sci. Technol. 2009, 25, 483–488. [Google Scholar]
- Andropova, U.; Serenko, O.; Tebeneva, N.; Tarasenkov, A.; Buzin, M.; Afanasyev, E.; Sapozhnikov, D.; Bukalov, S.; Leites, L.; Aysin, R.; et al. Atomic oxygen erosion resistance of polyimides filled hybrid nanoparticles. Polym. Test. 2020, 84, 106404. [Google Scholar] [CrossRef]
- Zeng, D.W.; Yung, K.C.; Xie, C.S. XPS investigation of the chemical characteristics of Kapton films ablated by a pulsed TEA CO2 laser. Surf. Coat. Technol. 2002, 153, 210–216. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Shi, Q.; Qian, Y.; Li, Y.; Xu, J.; Xu, C.; Zhang, Z.; Xie, X. The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment. Polymers 2022, 14, 322. https://doi.org/10.3390/polym14020322
Qi H, Shi Q, Qian Y, Li Y, Xu J, Xu C, Zhang Z, Xie X. The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment. Polymers. 2022; 14(2):322. https://doi.org/10.3390/polym14020322
Chicago/Turabian StyleQi, Hong, Qingshan Shi, Yuhai Qian, Yueming Li, Jingjun Xu, Caihong Xu, Zheng Zhang, and Xiaobao Xie. 2022. "The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment" Polymers 14, no. 2: 322. https://doi.org/10.3390/polym14020322
APA StyleQi, H., Shi, Q., Qian, Y., Li, Y., Xu, J., Xu, C., Zhang, Z., & Xie, X. (2022). The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment. Polymers, 14(2), 322. https://doi.org/10.3390/polym14020322






