Methyl Orange-Doped Polypyrrole Promoting Growth of ZIF-8 on Cellulose Fiber with Tunable Tribopolarity for Triboelectric Nanogenerator
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of CelF-Based Composites
2.2.1. Preparation of MO-PPy@CelF Composite
2.2.2. Preparation of ZIF-8@CelF Composite
2.2.3. Preparation of ZIF-8/MO-PPy@CelF Composite
2.3. Calculation of ZIF-8 Deposition Ratio
2.4. Fabrication of C-TENG
2.5. Characterization
3. Results and Discussion
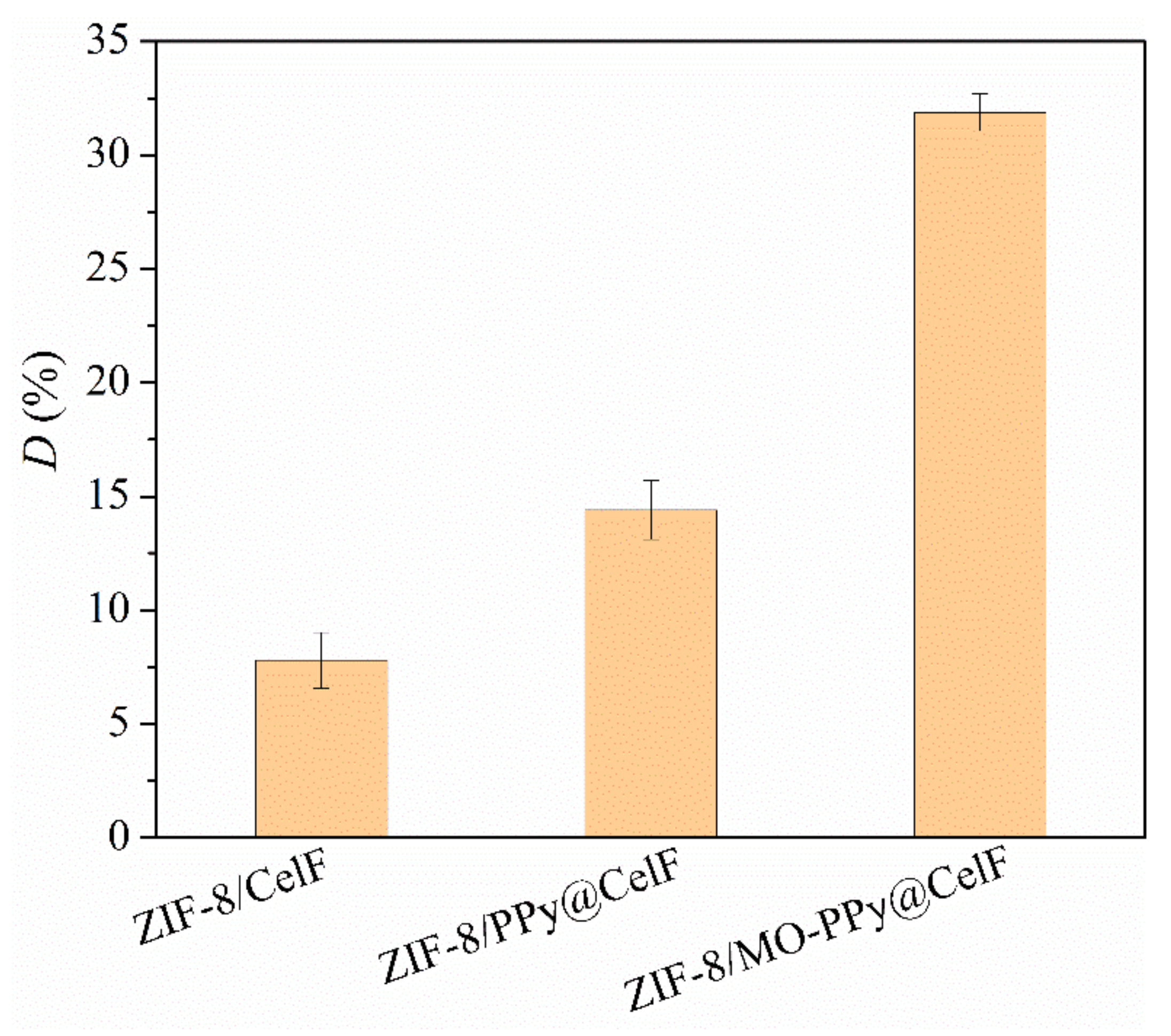
3.1. Promoting Effect of MO-PPy on the Growth of ZIF-8
3.2. Morphology and Structure of Composites
3.2.1. SEM Observation
3.2.2. XRD Analysis
3.2.3. FTIR Analysis
3.2.4. XPS Analysis
3.3. TENG for Energy Harvesting
3.3.1. Working Principle
3.3.2. Output Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guilar, N.J.; Kleeburg, T.J.; Chen, A.; Yankelevich, D.R.; Amirtharajah, R. Integrated solar energy harvesting and storage. IEEE Trans. Very Large Scale Integr. Syst. 2009, 17, 627–637. [Google Scholar] [CrossRef]
- Lin, L.; Wang, S.H.; Niu, S.; Liu, C.; Xie, Y.N.; Wang, Z.L. Noncontact free-rotating disk triboelectric nanogenerator as a sustainable energy harvester and self-powered mechanical sensor. ACS Appl. Mater. Interfaces 2014, 6, 3031–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Z.; Pan, H.; Salman, W.; Yuan, Y.; Liu, Y. A portable high-efficiency electromagnetic energy harvesting system using supercapacitors for renewable energy applications in railroads. Energy Convers. Manag. 2016, 118, 287–294. [Google Scholar] [CrossRef]
- Orrego, S.; Shoele, K.; Ruas, A.; Doran, K.; Caggiano, B.; Mittal, R.; Kang, S.H. Harvesting ambient wind energy with an inverted piezoelectric flag. Appl. Energy 2017, 194, 212–222. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Z.; Shu, C.; Zhang, J.; Zhang, Q.; Li, C. A rotational piezoelectric energy harvester for efficient wind energy harvesting. Sens. Actuators A Phys. 2017, 262, 123–129. [Google Scholar] [CrossRef]
- Xiao, T.X.; Tao, J.; Zhu, J.X.; Xi, L.; Xu, L.; Shao, J.J.; Zhang, L.C.; Wang, J.; Wang, Z.L. Silicone-based triboelectric nanogenerator for water wave energy harvesting. ACS Appl. Mater. Interfaces 2018, 10, 3616–3623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Kuang, S.Y.; Wang, Z.L.; Zhu, G. Highly adaptive solid-liquid interfacing triboelectric nanogenerator for harvesting diverse water wave energy. ACS Nano 2018, 12, 4280–4285. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, S.; Zu, J.; Inman, D. High-performance piezoelectric energy harvesters and their applications. Joule 2018, 2, 642–697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Su, C.; Cui, X.; Li, P.; Wang, Z.; Gu, L.; Tang, Z. Free-standing triboelectric layer-based full fabric wearable nanogenerator for efficient mechanical energy harvesting. ACS Appl. Electro. Mater. 2020, 2, 3366–3372. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenemtors as new energy technology for self-powered systems and as active mechanical and chemical sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Bai, P.; Chen, J.; Jing, Q.; Wang, Z.L. Triboelectric nanogenerators as a new energy technology from fundamentals devices to applications. Nano Energy 2015, 14, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Zhang, Z. Fundamental theories and basic principles of triboelectric effect: A review. Friction 2019, 7, 2–17. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.L.; Yang, Y. Self-powered wireless smart sensor node enabled by an ultrastable, highly efficient, and superhydrophobic-surface-based triboelectric nanogenerator. ACS Nano 2016, 10, 9044–9052. [Google Scholar] [CrossRef] [PubMed]
- Mallineni, S.S.K.; Behlow, H.; Dong, Y.; Bhattacharya, S.; Rao, A.M.; Podila, R. Facile and robust triboelectric nanogenerators assembled using off-the-shelf materials. Nano Energy 2017, 35, 263–270. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, X.; Huo, Z.; Li, X.; Que, M.; Peng, Z.; Wang, H.; Pan, C. A highly stretchable transparent self-powered triboelectric tactile sensor with metallized nanofibers for wearable electronics. Adv. Mater. 2018, 30, 1706738. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xie, Y.; Wang, S.; Wu, W.; Niu, S.; Wen, X.; Wang, Z.L. Triboelectric active sensor array for self-powered static and dynamic pressure detection and tactile imaging. ACS Nano 2013, 7, 8266–8274. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, J.; Jing, Q.; Yang, J.; Wen, X.; Su, Y.; Zhu, G.; Bai, P.; Wang, Z.L. 3D stack integrated triboelectric nanogenerator for harvesting vibration energy. Adv. Funct. Mater. 2014, 24, 4090–4096. [Google Scholar] [CrossRef]
- Fan, X.; Chen, J.; Yang, J.; Bai, P.; Li, Z.; Wang, Z.L. Ultrathin, rollable, paper-based triboelectric nanogenerator for acoustic energy harvesting and self-powered sound recording. ACS Nano 2015, 9, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, X.; Lin, L.; Guo, H.; Wang, Z.L. Paper-based triboelectric nanogenerators made of stretchable interlocking kirigami patterns. ACS Nano 2016, 10, 4652–4659. [Google Scholar] [CrossRef]
- Yao, C.; Hernandez, A.; Yu, Y.; Cai, Z.; Wang, X. Triboelectric nanogenerators and power-boards from cellulose nanofibrils and recycled materials. Nano Energy 2016, 30, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Yeh, M.-H.; Zi, Y.; Wen, Z.; Chen, J.; Liu, G.; Hu, C.; Wang, Z.L. Ultralight cut-paper-based self-charging power unit for self-powered portable electronic and medical systems. ACS Nano 2017, 11, 4475–4482. [Google Scholar] [CrossRef]
- Kim, I.; Jeon, H.; Kim, D.; You, J.; Kim, D. All-in-one cellulose based triboelectric nanogenerator for electronic paper using simple filtration process. Nano Energy 2018, 53, 975–981. [Google Scholar] [CrossRef]
- He, X.; Zou, H.; Geng, Z.; Wang, X.; Ding, W.; Hu, F.; Zi, Y.; Xu, C.; Zhang, S.L.; Yu, H.; et al. A hierarchically nanostructured cellulose fiber-based triboelectric nanogenerator for self-powered healthcare products. Adv. Funct. Mater. 2018, 28, 1805540. [Google Scholar] [CrossRef]
- Li, M.; Jie, Y.; Shao, L.H.; Guo, Y.; Cao, X.; Wang, N.; Wang, Z.L. All-in-one cellulose based hybrid tribo/piezoelectric nanogenerator. Nano Res. 2019, 12, 1831–1835. [Google Scholar] [CrossRef]
- Roy, S.; Ko, H.U.; Maji, P.K.; Hai, L.V.; Kim, J. Large amplification of triboelectric property by allicin to develop high performance cellulosic triboelectric nanogenerator. Chem. Eng. J. 2019, 385, 123723. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, J.; Xu, F.; Gong, S. Crepe cellulose paper and nitrocellulose membrane-based triboelectric nanogenerators for energy harvesting and self-powered human-machine interaction. Nano Energy 2019, 61, 69–77. [Google Scholar] [CrossRef]
- Oh, H.; Kwak, S.S.; Kim, B.; Han, E.; Lim, G.; Kim, S.; Lim, B. Highly conductive ferroelectric cellulose composite papers for efficient triboelectric nanogenerators. Adv. Funct. Mater. 2019, 29, 1904066. [Google Scholar] [CrossRef]
- Shi, K.; Zou, H.; Sun, B.; Jiang, P.; He, J.; Huang, X. Dielectric modulated cellulose paper/PDMS-based triboelectric nanogenerators for wireless transmission and electropolymerization applications. Adv. Funct. Mater. 2020, 30, 1904536. [Google Scholar] [CrossRef]
- Rajabi-Abhari, A.; Kim, J.-N.; Lee, J.; Tabassian, R.; Mahato, M.; Youn, H.J.; Lee, H.; Oh, I.-K. Diatom bio-silica and cellulose nanofibril for bio-triboelectric nanogenerators and self-powered breath monitoring masks. ACS Appl. Mater. Interfaces 2020, 13, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, C.; Ying, Y.; Ping, J. Biotriboelectric nanogenerators: Materials, structures, and applications. Adv. Energy Mater. 2020, 10, 2002001. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, J.; Fu, Q.; Liu, Y.; Wang, S.; Nie, S. Wood-cellulose-fiber-based functional materials for triboelectric nanogenerators. Nano Energy 2021, 81, 105637. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Polysaccharide-based triboelectric nanogenerators: A review. Carbohydr. Polym. 2021, 251, 117055. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.X.; Cheng, W.L.; Cao, M.L.; Wang, D.; Wang, Q.X.; Han, J.Q.; Long, Y.Z.; Han, G.P. Recent advances in cellulose-based flexible triboelectric nanogenerators. Nano Energy 2021, 87, 106175. [Google Scholar] [CrossRef]
- Khandelwal, G.; Chandrasekhar, A.; Raj, N.P.M.J.; Kim, S.-J. Metal–organic framework: A novel material for triboelectric nanogenerator–based self-powered sensors and systems. Adv. Energy Mater. 2019, 9, 1803581. [Google Scholar] [CrossRef]
- Tu, K.K.; Puértolas, B.; Adobes-Vidal, M.; Wang, Y.; Sun, J.G.; Traber, J.; Burgert, I.; Pérez-Ramírez, J.; Keplinger, T. Green synthesis of hierarchical metal–organic framework/wood functional composites with superior mechanical properties. Adv. Sci. 2020, 7, 1902897. [Google Scholar] [CrossRef] [Green Version]
- Silva Pinto, M.D.; Sierra-Avila, C.A.; Hinestroza, J.P. In situ synthesis of a Cu-BTC metal-organic framework (MOF 199) onto cellulosic fibrous substrates: Cotton. Cellulose 2012, 19, 1771–1779. [Google Scholar] [CrossRef]
- Zhao, J.; Losego, M.D.; Lemaire, P.C.; Williams, P.S.; Gong, B.; Atanasov, S.E.; Blevins, T.M.; Oldham, C.J.; Walls, H.J.; Shepherd, S.D.; et al. Highly adsorptive, MOF-functionalized nonwoven fiber mats for hazardous gas capture enabled by atomic layer deposition. Adv. Mater. Interfaces 2014, 1, 1400040. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Kamel, O.M.H.M.; Amr, A.; Rocha, J.; Silva, A.M.S. Antimosquito activity of a titanium-organic framework supported on fabrics. ACS Appl. Mater. Interfaces 2017, 9, 22112–22120. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Meng, J.; Wang, X.; Meng, X.; Sun, X.; Xu, Y.; Zhao, W.; Ni, Y. Synthesis of novel cellulose-based antibacterial composites of Ag nanoparticles@ metal-organic frameworks@ carboxymethylated fibers. Carbohydr. Polym. 2018, 193, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Au-Duong, A.N.; Lee, C.K. Flexible metal-organic framework-bacterial cellulose nanocomposite for iodine capture. Cryst. Growth. Des. 2018, 18, 356–363. [Google Scholar] [CrossRef]
- Bao, T.; Su, Y.; Zhang, N.; Gao, Y.; Wang, S. Hydrophilic carboxyl cotton for in situ growth of UiO-66 and its application as adsorbents. Ind. Eng. Chem. Res. 2019, 58, 20331–20339. [Google Scholar] [CrossRef]
- Zha, J.; Yin, X.; Baltzegar, J.R.; Zhang, X. Coordinatively unsaturated metal site-promoted selective adsorption of organic molecules on supported metal–organic framework nanosheets. Langmuir 2019, 35, 12908–12913. [Google Scholar] [CrossRef]
- Shen, C.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Wang, B.; Feng, X.; Tao, C.; Sui, X. Catalytic MOF-loaded cellulose sponge for rapid degradation of chemical warfare agents simulant. Carbohydr. Polym. 2019, 213, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, Z.; Smith, L.M.; Hong, G.; Song, W.; Zhang, S. Polypyrrole-modified bamboo fiber/polylactic acid with enhanced mechanical, the antistatic properties and thermal stability. Ind. Crop. Prod. 2021, 162, 113227. [Google Scholar] [CrossRef]
- Weidlich, C.; Mangold, K.-M.; Jüttner, K. Conducting polymers as ion-exchangers for water purification. Electrochim. Acta 2001, 47, 741–745. [Google Scholar] [CrossRef]
- Deng, B.; Qiu, D.; Wang, J.; Liao, L.; Huang, H.; Lei, G. Molecular design and synthesis of azo compounds of metal ion complexing agent. J. Huazhong Normal Univ. Nat. Sci. Ed. 2014, 48, 206–209, 227. [Google Scholar]
- Hou, X.; Sun, L.; Hu, Y.; An, X.; Qian, X. De-doped polyaniline as a mediating layer promoting in-situ growth of metal–organic frameworks on cellulose fiber and enhancing adsorptive-photocatalytic removal of ciprofloxacin. Polymers 2021, 13, 3298. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.G.; Ren, W.J.; Lei, C.; Xie, Y.B.; Cai, Y.R.; Wang, S.L.; Gao, J.K.; Ni, Q.Q.; Yao, J.M. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Jia, M.M.; Zhang, X.F.; Feng, Y.; Zhou, Y.C.; Yao, J.F. In-situ growing ZIF-8 on cellulose nanofibers to form gas separation membrane for CO2 separation. J. Membran. Sci. 2020, 595, 117579. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathewa, A.P. In-situ growth of zeolitic imidazolate frameworks into a cellulosic filter paper for the reduction of 4-nitrophenol. Carbohydr. Polym. 2021, 274, 118657. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, L.; Zhong, Y.; Sui, X.; Wang, B.; Chen, Z.; Feng, X.; Xu, H.; Mao, Z. High-performance polypyrrole coated knitted cotton fabric electrodes for wearable energy storage. Org. Electron. 2019, 74, 59–68. [Google Scholar] [CrossRef]
- Yang, Q.X.; Lu, R.; Ren, S.S.; Chen, C.T.; Chen, Z.J.; Yang, X.Y. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Mao, H.; Guo, X.; Sun, D.Y.; Sun, Z.Y.; Wang, B.X.; Zhang, Y.; Song, X.M. Hierarchical Ni(OH)2/polypyrrole/graphene oxide nanosheets as excellent electrocatalysts for the oxidation of urea. ACS Sustain. Chem. Eng. 2018, 6, 15570–15581. [Google Scholar] [CrossRef]
- Hou, P.C.; Xing, G.J.; Han, D.; Wang, H.; Yu, C.N.; Li, Y.L. Preparation of zeolite imidazolate framework/graphene hybrid aerogels and their application as highly efficient adsorbent. J. Solid State Chem. 2018, 265, 184–192. [Google Scholar] [CrossRef]
- Sun, J.; Tu, K.; Büchele, S.; Koch, S.M.; Ding, Y.; Ramakrishna, S.N.; Stucki, S.; Guo, H.; Wu, C.; Keplinger, T.; et al. Functionalized wood with tunable tribopolarity for efficient triboelectric nanogenerators. Matter 2021, 4, 3049–3066. [Google Scholar] [CrossRef]
- Liu, Y.H.; Fu, Q.; Mo, J.L.; Lu, Y.X.; Cai, C.C.; Luo, B.; Nie, S.X. Chemically tailored molecular surface modification of cellulose nanofibrils for manipulating the charge density of triboelectric nanogenerators. Nano Energy 2021, 89, 106369. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Kim, D.; Yang, Y.; Lin, L.; Lin, Z.H.; Hwang, W.; Wang, Z.L. Triboelectric nanogenerator for harvesting pendulum oscillation energy. Nano Energy 2013, 2, 1113–1120. [Google Scholar] [CrossRef]
- Chen, S.-N.; Huang, M.-Z.; Lin, Z.-H.; Liu, C.-P. Enhancing charge transfer for ZnO nanorods based triboelectric nanogenerators through Ga doping. Nano Energy 2019, 65, 104069. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chang, C.-Y. Enhanced output performance and stability of triboelectric nanogenerators by employing silane-based self-assembled monolayers. J. Mater. Chem. C 2020, 8, 4542–4548. [Google Scholar] [CrossRef]
- Cui, P.; Parida, K.; Lin, M.F.; Xiong, J.; Cai, G.; Lee, P.S. Transparent, flexible cellulose nanofibril-phosphorene hybrid paper as triboelectric nanogenerator. Adv. Mater. Interfaces 2017, 4, 1700651. [Google Scholar] [CrossRef]
- Yao, C.; Yin, X.; Yu, Y.; Cai, Z.; Wang, X. Chemically functionalized natural cellulose materials for effective triboelectric nanogenerator development. Adv. Funct. Mater. 2017, 27, 1700794. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, L.; Guo, H.; Yang, K.; Cai, Z.; Meador, M.A.B.; Gong, S. Highly porous polymer aerogel film-based triboelectric nanogenerators. Adv. Funct. Mater. 2018, 28, 1706365. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Zheng, Q.; Fang, L.; Huang, H.-X.; Turng, L.-S.; Gong, S. High-performance flexible triboelectric nanogenerator based on porous aerogels and electrospun nanofibers for energy harvesting and sensitive self-powered sensing. Nano Energy 2018, 48, 327–336. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, X.; Zhang, N.; Lu, Y.; Wu, Z.; Liu, G.; Nie, S. Chemically functionalized cellulose nanofibrils-based gear-like triboelectric nanogenerator for energy harvesting and sensing. Nano Energy 2019, 66, 104126–104136. [Google Scholar] [CrossRef]
- Nie, S.; Fu, Q.; Lin, X.; Zhang, C.; Wang, S. Enhanced performance of a cellulose nanofibrils-based triboelectric nanogenerator by tuning the surface polarizability and hydrophobicity. Chem. Eng. J. 2021, 404, 126512. [Google Scholar] [CrossRef]
- Yang, P.-K.; Lin, Z.-H.; Pradel, K.C.; Lin, L.; Li, X.; Wen, X.; He, J.-H.; Wang, Z.L. Paper-based origami triboelectric nanogenerators and self-powered pressure sensors. ACS Nano 2015, 9, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef]
- Xia, K.; Du, C.; Zhu, Z.; Wang, R.; Zhang, H.; Xu, Z. Sliding-mode triboelectric nanogenerator based on paper and as a self-powered velocity and force sensor. Appl. Mater. Today 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Yang, W.; Lu, X. Triboelectric power generation from heterostructured air-1aid paper for breathable and wearable self-charging power system. Adv. Mater. Technol. 2019, 4, 1900745. [Google Scholar] [CrossRef]
- Shi, X.; Chen, S.; Zhang, H.; Jiang, J.; Ma, Z.; Gong, S. Portable self-charging power system via integration of a flexible paper-based triboelectric nanogenerator and supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 18657–18666. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; An, X.; Qian, X. Methyl Orange-Doped Polypyrrole Promoting Growth of ZIF-8 on Cellulose Fiber with Tunable Tribopolarity for Triboelectric Nanogenerator. Polymers 2022, 14, 332. https://doi.org/10.3390/polym14020332
Li Q, An X, Qian X. Methyl Orange-Doped Polypyrrole Promoting Growth of ZIF-8 on Cellulose Fiber with Tunable Tribopolarity for Triboelectric Nanogenerator. Polymers. 2022; 14(2):332. https://doi.org/10.3390/polym14020332
Chicago/Turabian StyleLi, Qiang, Xianhui An, and Xueren Qian. 2022. "Methyl Orange-Doped Polypyrrole Promoting Growth of ZIF-8 on Cellulose Fiber with Tunable Tribopolarity for Triboelectric Nanogenerator" Polymers 14, no. 2: 332. https://doi.org/10.3390/polym14020332




