Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of CNCs
2.2.2. Preparation of PVP- and PAM-Modified CNCs
2.2.3. Preparation of PHBV/PCL/CNC Composite Films
2.3. Characterization
2.4. Computational Details
3. Results and Discussion
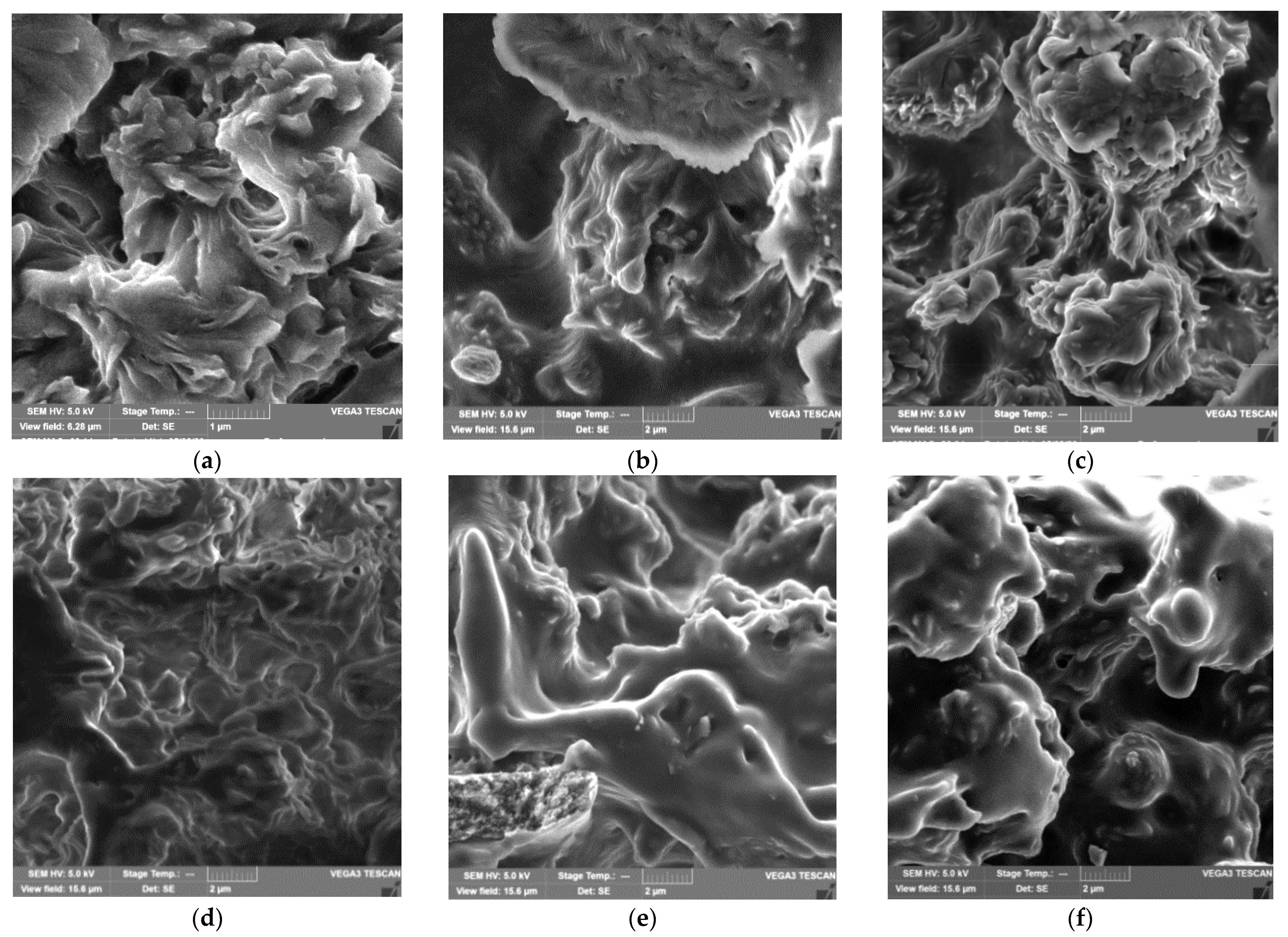
3.1. Morphological and Adsorption Properties of PHBV/PCL/CNC Composites
3.2. FTIR Analysis
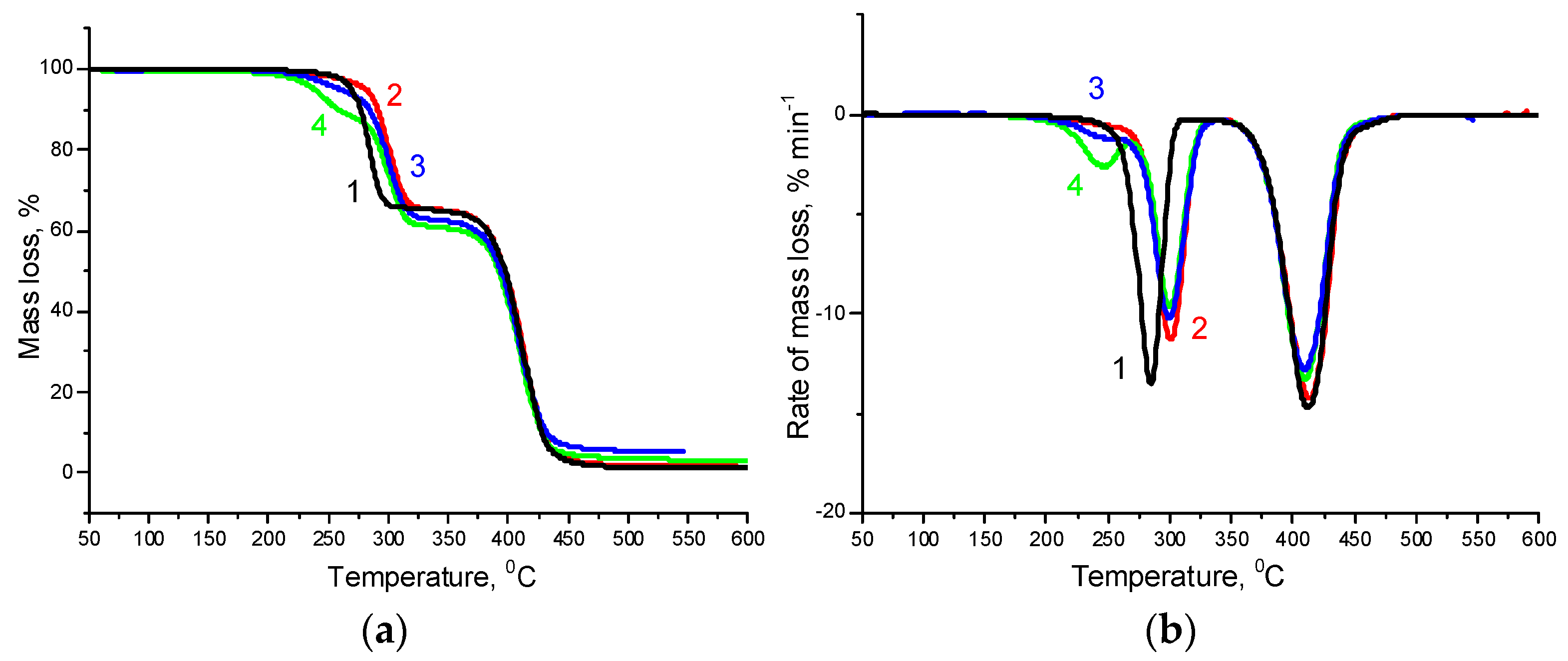
3.3. Thermal Properties
3.4. X-ray Diffraction Analysis
3.5. Tensile Properties
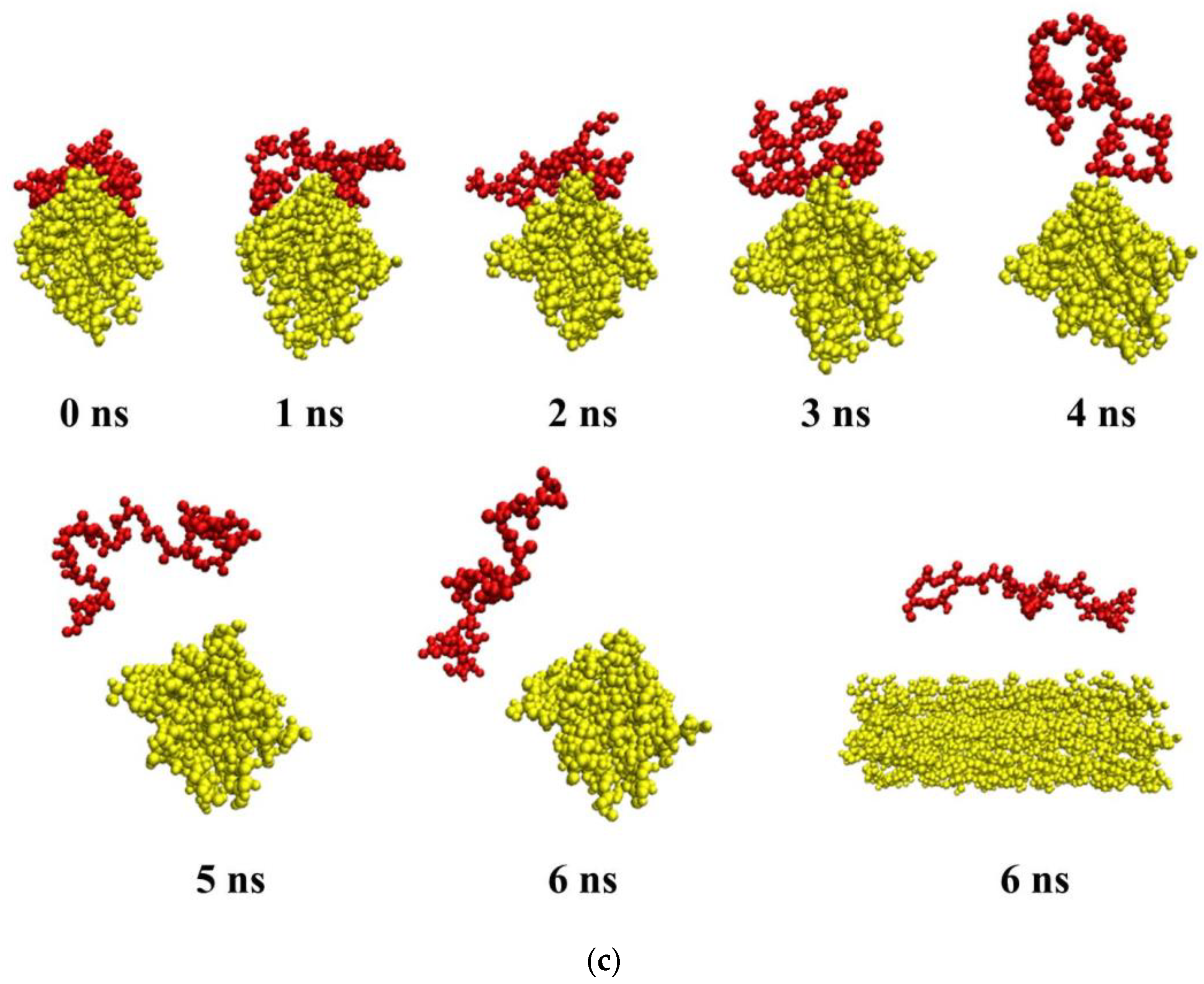
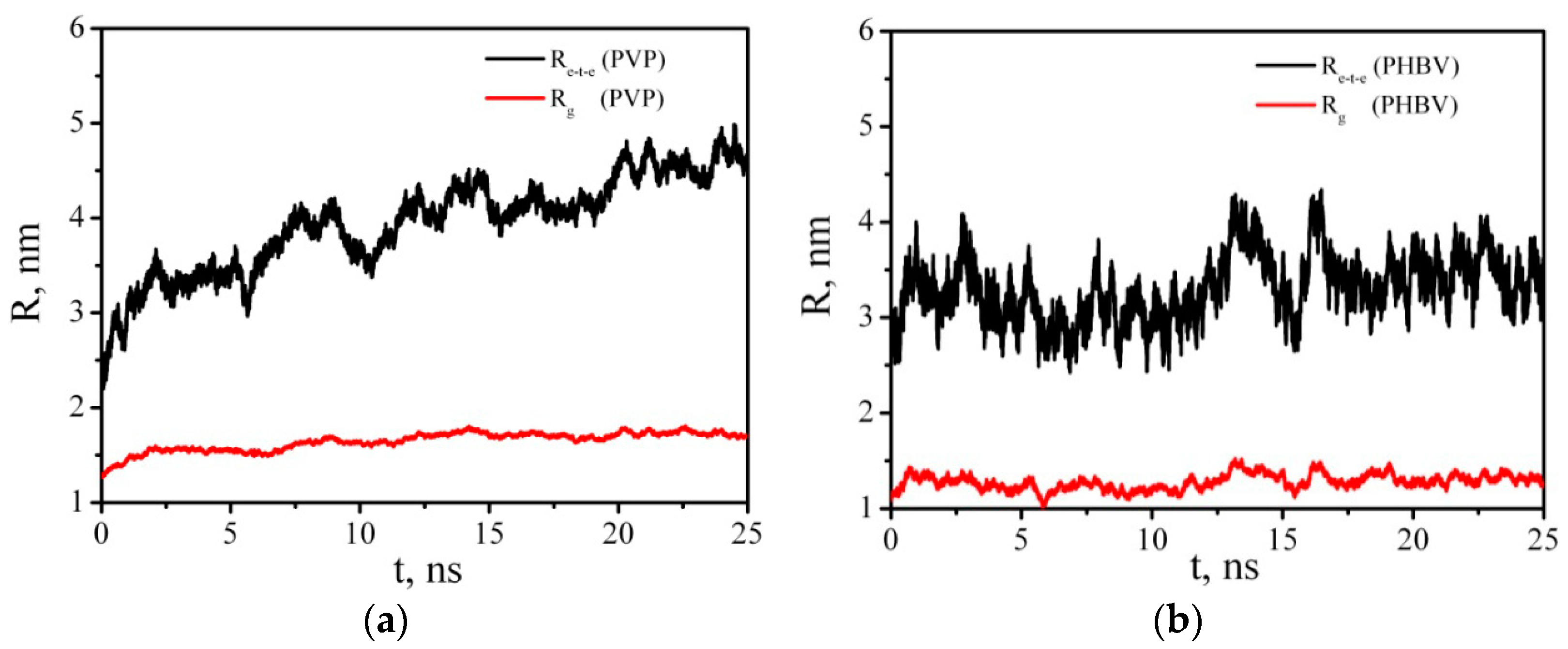
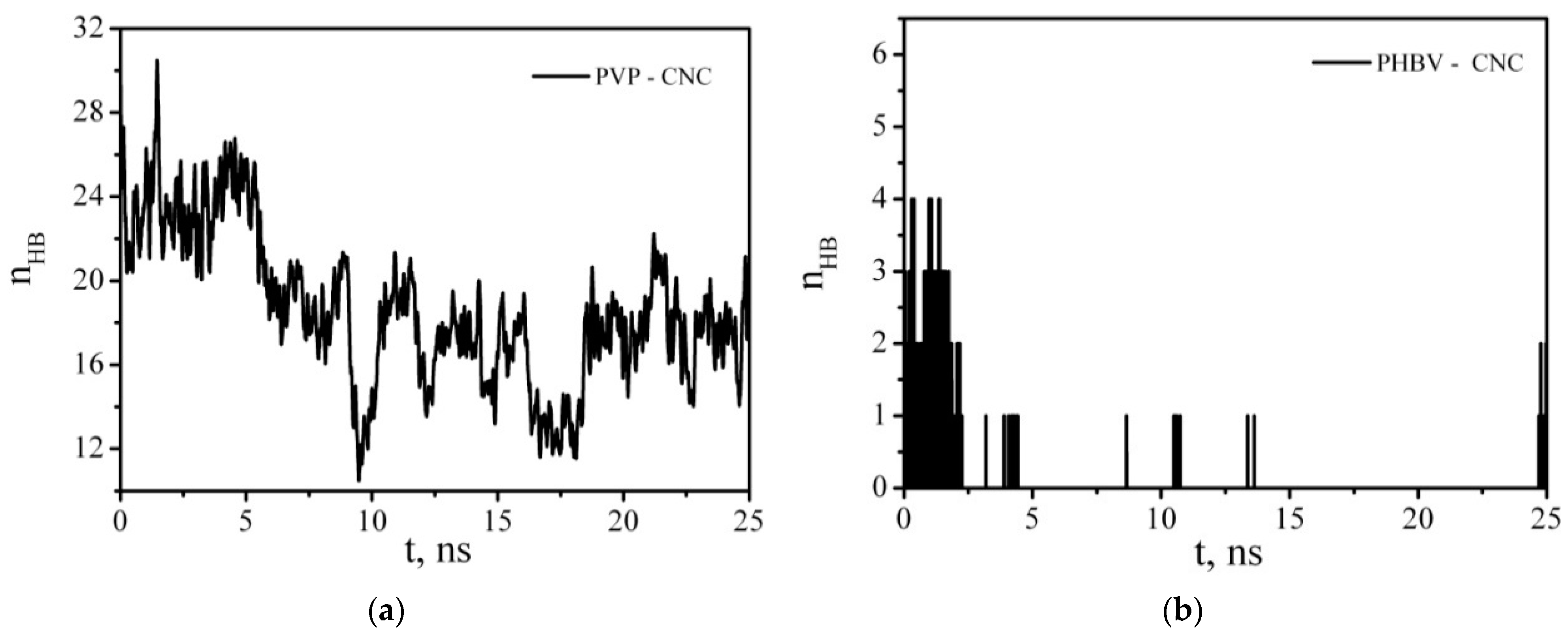
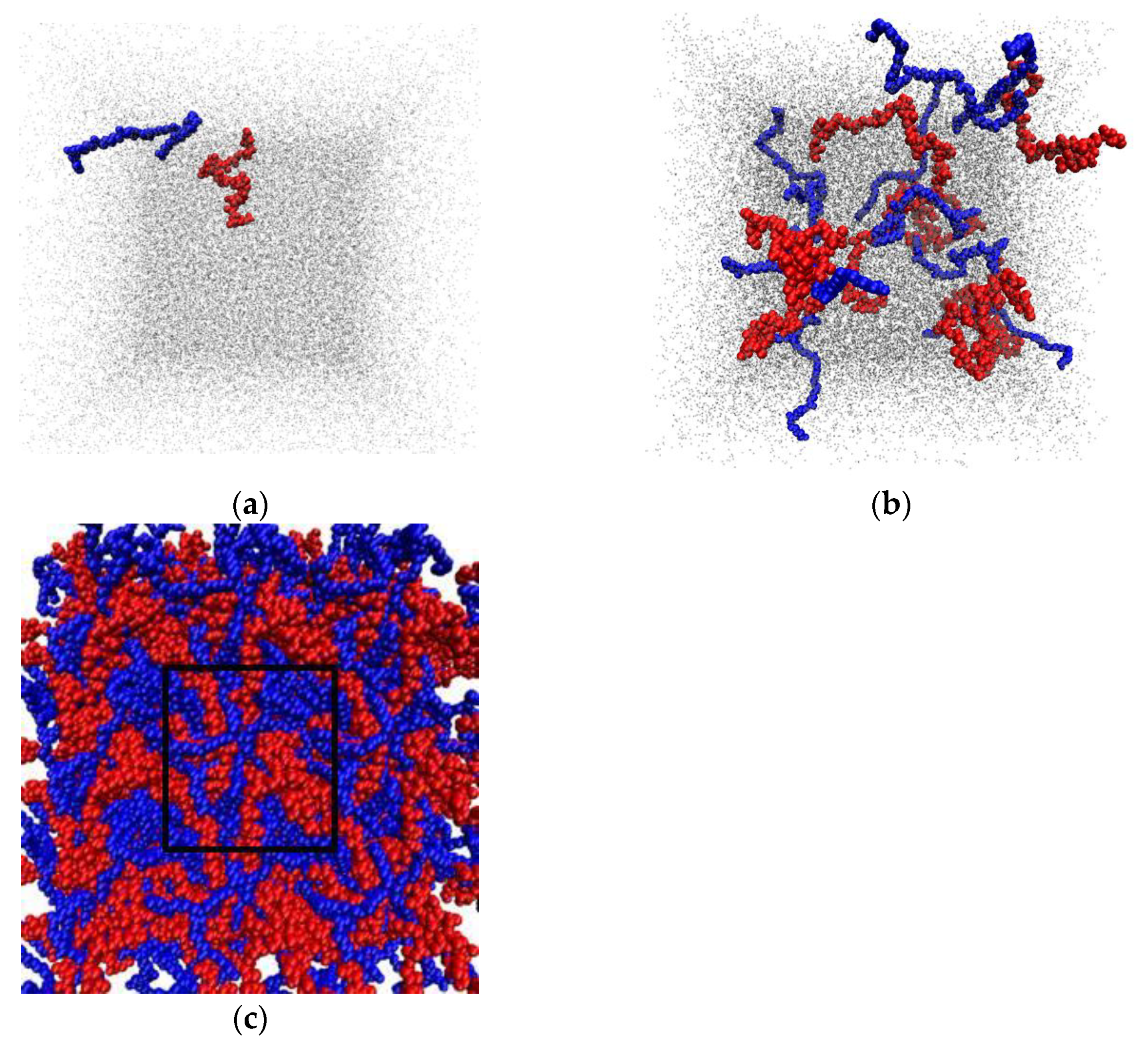
3.6. Molecular Dynamics Simulation
3.6.1. Intermolecular Interactions in a Multicomponent System Containing CNC, PVP, PHBV, and a Solvent (Water, DCM)
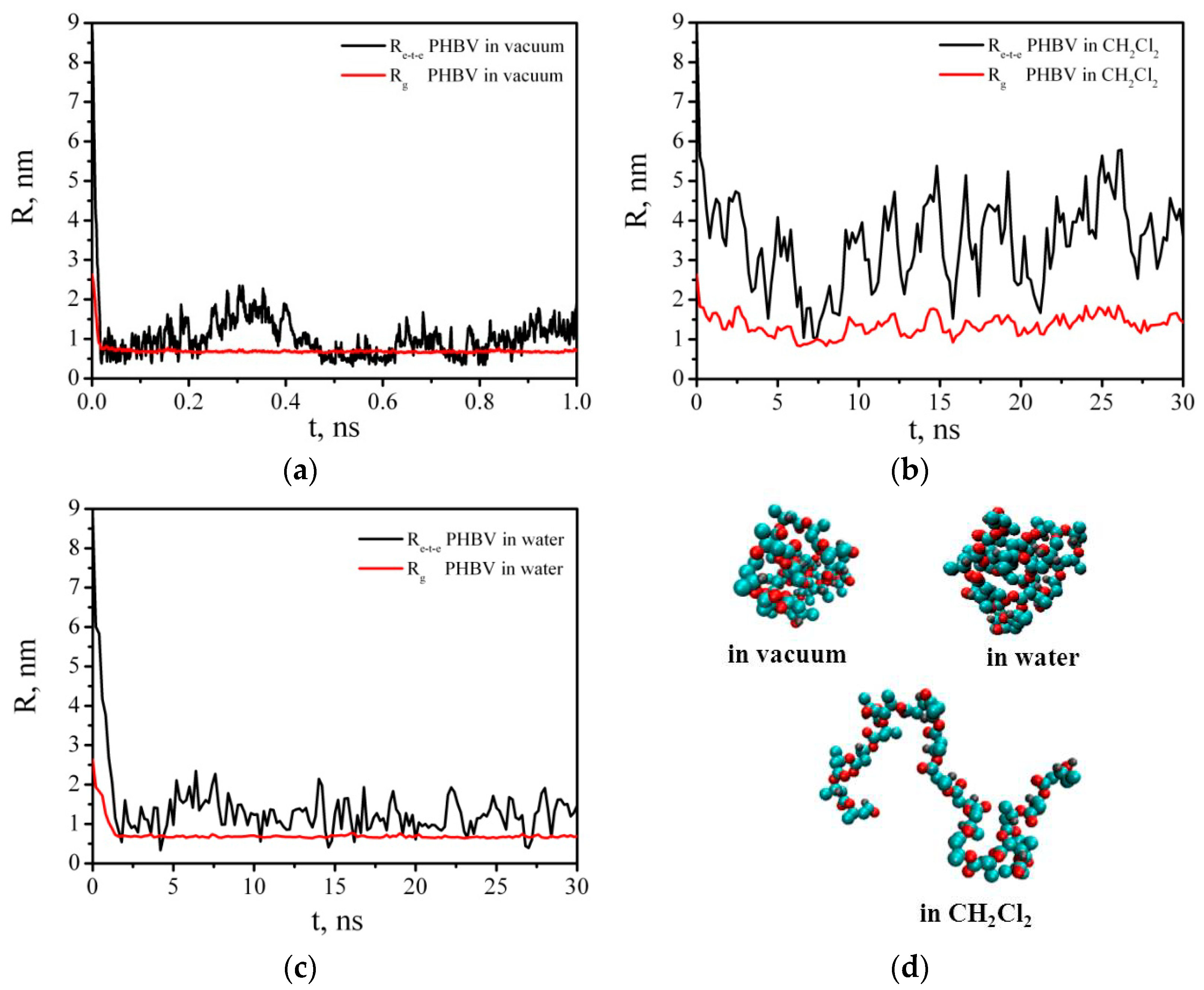
3.6.2. Intermolecular Interactions in Binary PHBV/PCL System in DCM and Vacuum
3.6.3. Intermolecular Interactions in a Multicomponent System Containing CNC, PVP, PHBV, PCL, and a Solvent (Water, DCM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Isolation and characterization of cellulose nanocrystals from Saharan aloe vera cactus fibers. Int. J. Polym. Anal. Charact. 2020, 25, 51–64. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 2017. [Google Scholar]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packaging applications. Curr. Opin. Green Sustain. Chem. 2021, 31, 100512. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Tarafdar, A.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Siqueira, G.; Fraschini, C.; Bras, J.; Dufresne, A.; Prudhomme, R.; Laborie, M. Impact of the nature and shape of cellulosic nanoparticles on the isothermal crystallization kinetics of poly(ε-caprolactone). Eur. Polym. J. 2011, 47, 2216–2227. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Syromotina, D.S.; Shkarina, S.N.; Shkarin, R.; Cecilia, A.; Weinhardt, V.; Baumbach, T.; Saveleva, M.S.; Gorin, D.A.; Douglas, T.E.L.; et al. Effect of low-temperature plasma treatment of electrospun polycaprolactone fibrous scaffolds on calcium carbonate mineralization. RSC Adv. 2018, 8, 39106–39114. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Ghobeira, R.; Cools, P.; Liu, Z.; Yan, K.; De Geyter, N.; Morent, R. Comparative study of different nitrogen-containing plasma modifications applied on 3D porous PCL scaffolds and 2D PCL films. Appl. Surf. Sci. 2020, 516, 146067. [Google Scholar] [CrossRef]
- Sheng, L.; Jiang, R.; Zhu, Y.; Ji, Y. Electrospun cellulose nanocrystals/polycaprolactone nanocomposite fiber mats. J. Macromol. Sci. B 2014, 53, 820–828. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A.; Siehr, A.; Sahoo, A.; Milan, P.B.; Joghataei, M.T.; Amoupour, M.; Simorgh, S. Cellulose nanocrystal effect on crystallization kinetics and biological properties of electrospun polycaprolactone. Mat. Sci. Eng. C 2021, 121, 111855. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Hameed, N.; Guo, Q. Cellulose/polycaprolactone blends regenerated from ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohyd. Polym. 2012, 90, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Ou, J.; Tang, C.; Xiang, M.; Wu, D.; Tang, J.; Tam, K.C. Functionalized cellulose nanocrystals as the performance regulators of poly (β-hydroxybutyrate-co-valerate) biocomposites. Carbohyd. Polym. 2020, 242, 116399. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.Y.; Wang, D.C.; Yao, Y.M. Electrospun poly(3-hydroxybutyrate-co-3-hydroxy-valerate)/cellulose reinforced nanofibrous membranes with ZnO nanocrystals for antibacterial wound dressings. Cellulose 2017, 24, 2925–2938. [Google Scholar] [CrossRef]
- Li, F.; Yu, H.-Y.; Li, Y.; Abdalkarim, S.Y.H.; Zhu, J.; Zhou, Y. “Soft-rigid” synergistic reinforcement of PHBV composites with functionalized cellulose nanocrystals and amorphous recycled polycarbonate. Compos. Part B-Eng. 2021, 206, 108542. [Google Scholar] [CrossRef]
- Zheng, T.; Clemons, C.M.; Pilla, S. Comparative Study of Direct Compounding, Coupling Agent-Aided and Initiator-Aided Reactive Extrusion to Prepare Cellulose Nanocrystal/PHBV (CNC/PHBV) Nanocomposite. ACS Sustain. Chem. Eng. 2020, 8, 814–822. [Google Scholar] [CrossRef]
- Jun, D.; Zhao, G.M.; Pan, M.Z.; Zhuang, L.L.; Li, D.G.; Zhang, R. Crystallization and mechanical properties of reinforced PHBV composites using melt compounding: Effect of CNCs and CNFs. Carbohyd. Polym. 2017, 168, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Malmir, S.; Barral, L.; Bouza, R.; Esperanza, M.; Seoane, M.; Feijoo-Bandίn, S.; Lago, F. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanocrystal films: Artificial weathering, humidity absorption, water vapor transmission rate, antimicrobial activity and biocompatibility. Cellulose 2019, 26, 2333–2348. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R.; Farrag, Y. PHBV/CNC bionanocomposites processed by extrusion: Structural characterization and properties. Polym. Compos. 2019, 40, E275–E284. [Google Scholar] [CrossRef]
- Li, F.; Yu, H.-Y.; Wang, Y.-Y.; Zhou, Y.; Zhang, H.; Yao, J.-M.; Abdalkarim, S.Y.H.; Tam, K.C. Natural Biodegradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Nanocomposites with Multifunctional Cellulose Nanocrystals/Graphene Oxide Hybrids for High-Performance Food Packaging. J. Agric. Food Chem. 2019, 67, 10954–10967. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.Y.; Song, M.L.; Zhou, Y.; Yao, J.M.; Ni, Q.Q. In vitro degradation and possible hydrolytic mechanism of PHBV nanocomposites by incorporating cellulose nanocrystal-ZnO nanohybrids. Carbohyd. Polym. 2017, 176, 38–49. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. Part A Appl. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Singh, A.A.; Genovese, M.E.; Mancini, G.; Marini, L.; Athanassiou, A. Green Processing Route for Polylactic Acid_Cellulose Fiber Biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4128–4136. [Google Scholar] [CrossRef]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and characterization of polyvinylpyrrolidone/cellulose nanocrystals composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef] [Green Version]
- Voronova, M.I.; Surov, O.V.; Afineevskii, A.V.; Zakharov, A.G. Properties of polyacrylamide composites reinforced by cellulose nanocrystals. Heliyon 2020, 6, e05529. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurina, D.; Surov, O.; Voronova, M.; Zakharov, A. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Cellulose Nanocrystals. Nanomaterials 2020, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Voronova, M.I.; Gurina, D.L.; Surov, O.V.; Zakharov, A.G. Interactions in solvent–polycaprolactone–cellulose nanocrystals–polyvinyl pyrrolidone system: Experiment and molecular dynamics simulation. J. Mol. Liq. 2021, 341, 117409. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose Whiskers versus Microfibrils: Influence of the Nature of the Nanoparticle and its Surface Functionalization on the Thermal and Mechanical Properties of Nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, Z.; Zhou, Z. Cellulose nanocrystals as green fillers to improve crystallization and hydrophilic property of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci.-Mater. 2011, 21, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. Gromacs: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Gurina, D.; Surov, O.; Voronova, M.; Zakharov, A.; Kiselev, M. Water effects on molecular adsorption of poly(N-vinyl-2-pyrrolidone) on cellulose nanocrystals surfaces: Molecular dynamics simulations. Materials 2019, 12, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.; Dormidontova, E.E. Polymer-threaded and polymer-wrapped wormlike micelle solutions: Molecular dynamics simulations. Macromolecules 2019, 52, 7016–7027. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, J.L.; Perin, G.B.; Felisberti, M.I. Plasticization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with an Oligomeric Polyester: Miscibility and Effect of the Microstructure and Plasticizer Distribution on Thermal and Mechanical Properties. ACS Omega 2021, 6, 3278–3290. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated force field Topology Builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Gomes, T.C.F.; Skaf, M.S. Cellulose-Builder: A toolkit for building crystalline structures of cellulose. J. Comput. Chem. 2012, 33, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Nose, S.A. Molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8592. [Google Scholar] [CrossRef] [Green Version]
- Kosorn, W.; Wutticharoenmongkol, P. Poly(ε-caprolactone)/Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Blend from Fused Deposition Modeling as Potential Cartilage Scaffolds. Int. J. Polym. Sci. 2021, 2021, 6689789. [Google Scholar] [CrossRef]
- Zytner, P.; Wu, F.; Misra, M.; Mohanty, A.K. Toughening of Biodegradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(ε-caprolactone) Blends by In Situ Reactive Compatibilization. ACS Omega 2020, 5, 14900–14910. [Google Scholar] [CrossRef] [PubMed]
- Nahanmoghadam, A.; Asemani, M.; Goodarzi, V.; Ebrahimi-Barough, S. Design and fabrication of bone tissue scaffolds based on PCL/PHBV containing hydroxyapatite nanoparticles: Dual-leaching Technique. J. Biomed. Mater. Res. 2020, 109, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Nartker, S.; Hassan, M.; Stogsdill, M. Electrospun cellulose nitrate and polycaprolactone blended nanofibers. Mater. Res. Express 2015, 2, 035401. [Google Scholar] [CrossRef]
- Surov, O.V.; Voronova, M.I.; Rubleva, N.V.; Afineevskii, A.V.; Zakharov, A.G. Cellulose nanocrystals as a compatibilizer for improved miscibility of water-soluble polymer binary blends. J. Appl. Polym. Sci. 2020, 137, 48662. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Guseinov, S.S.; Zakharov, A.G. Thermal stability of composites of polyvinyl alcohol with nanocrystallinecellulose in its acid and neutralized forms. Compos. Commun. 2016, 2, 15–18. [Google Scholar] [CrossRef]
- Ahmed, F.; Saleemi, S.; Khatri, Z.; Abro, M.I.; Kim, I.-S. Co-electrospun poly(ε-caprolactone)/cellulose nanofibers-fabrication and characterization. Carbohyd. Polym. 2015, 115, 388–393. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, A. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
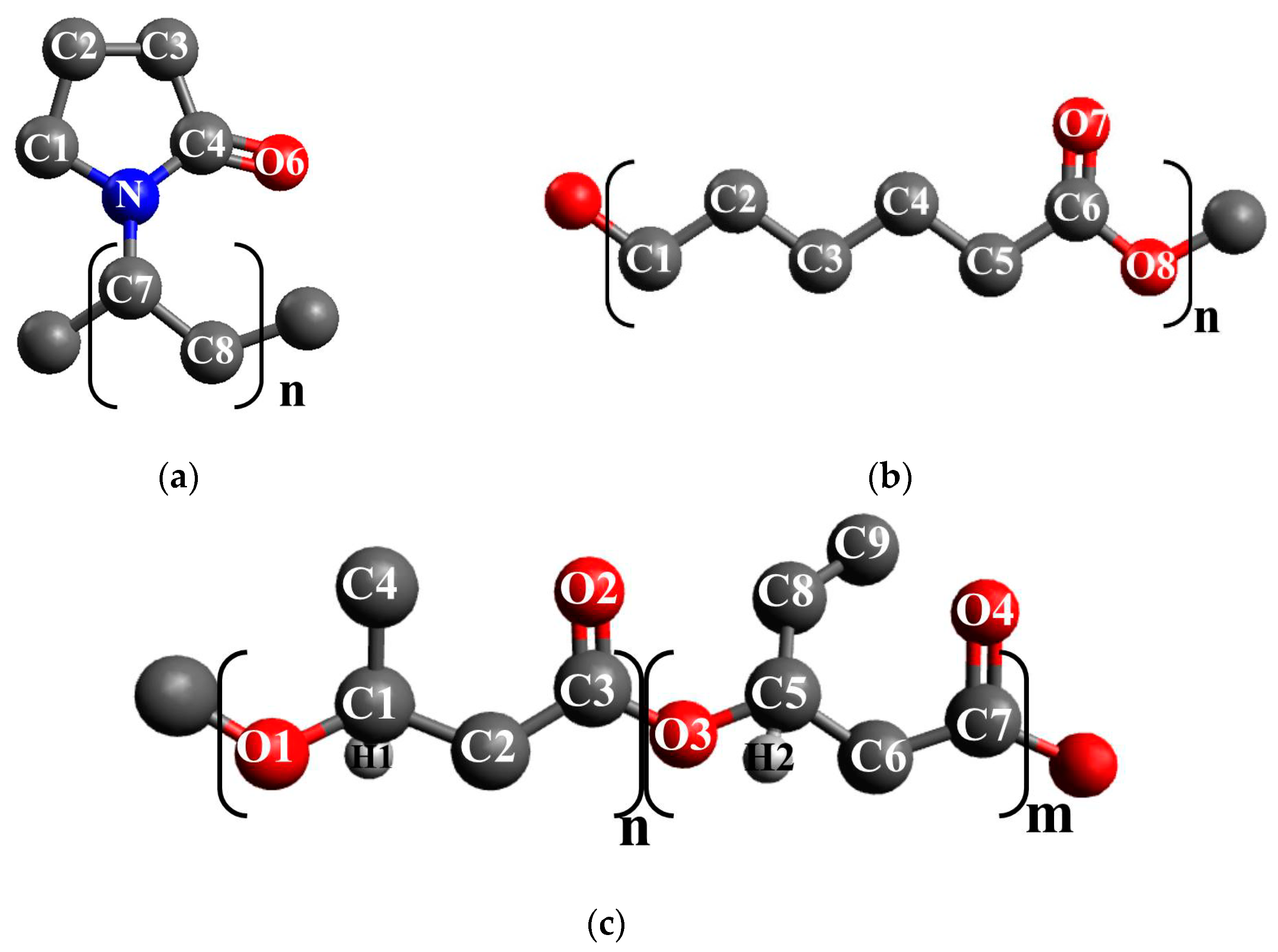



















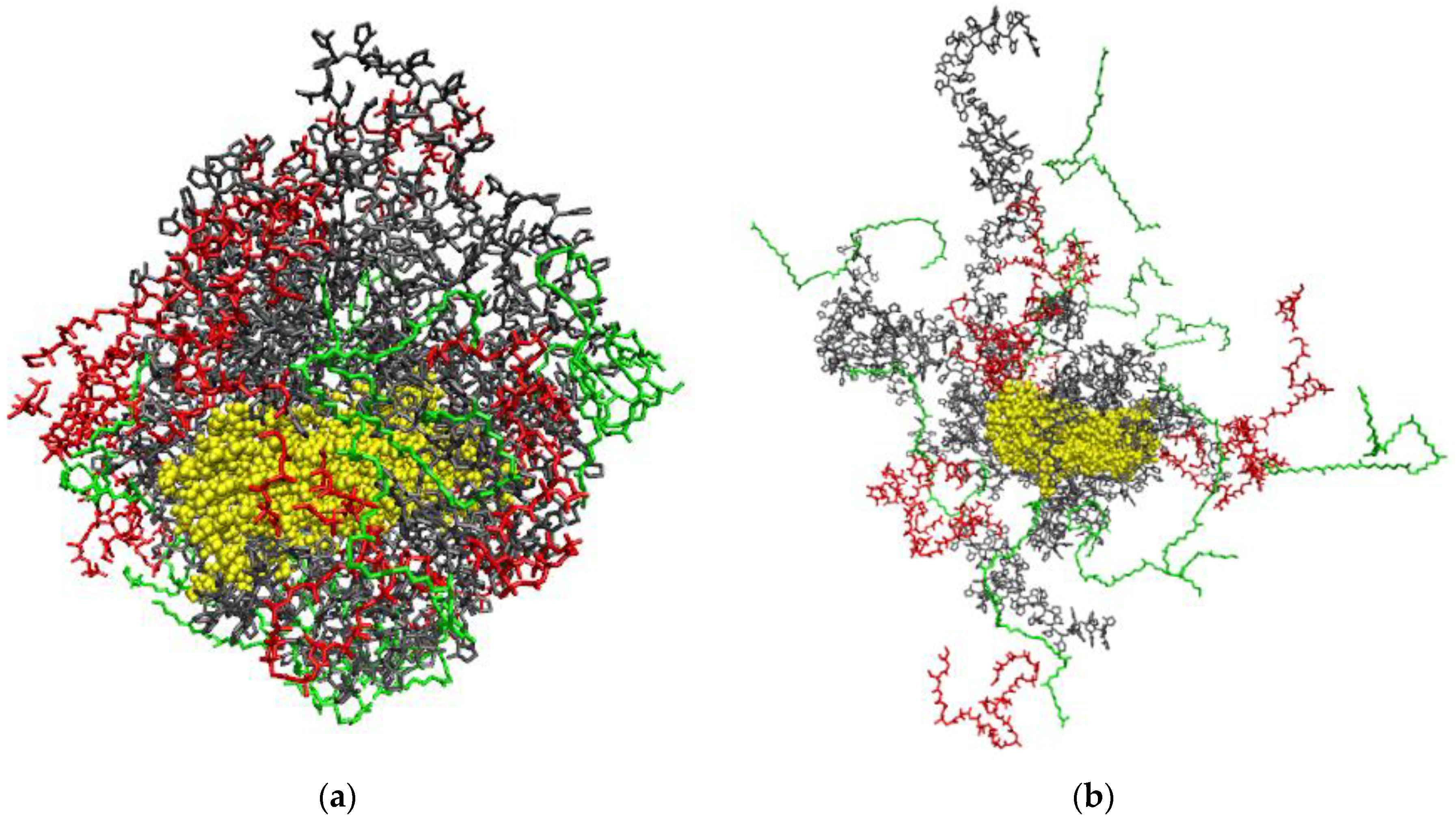

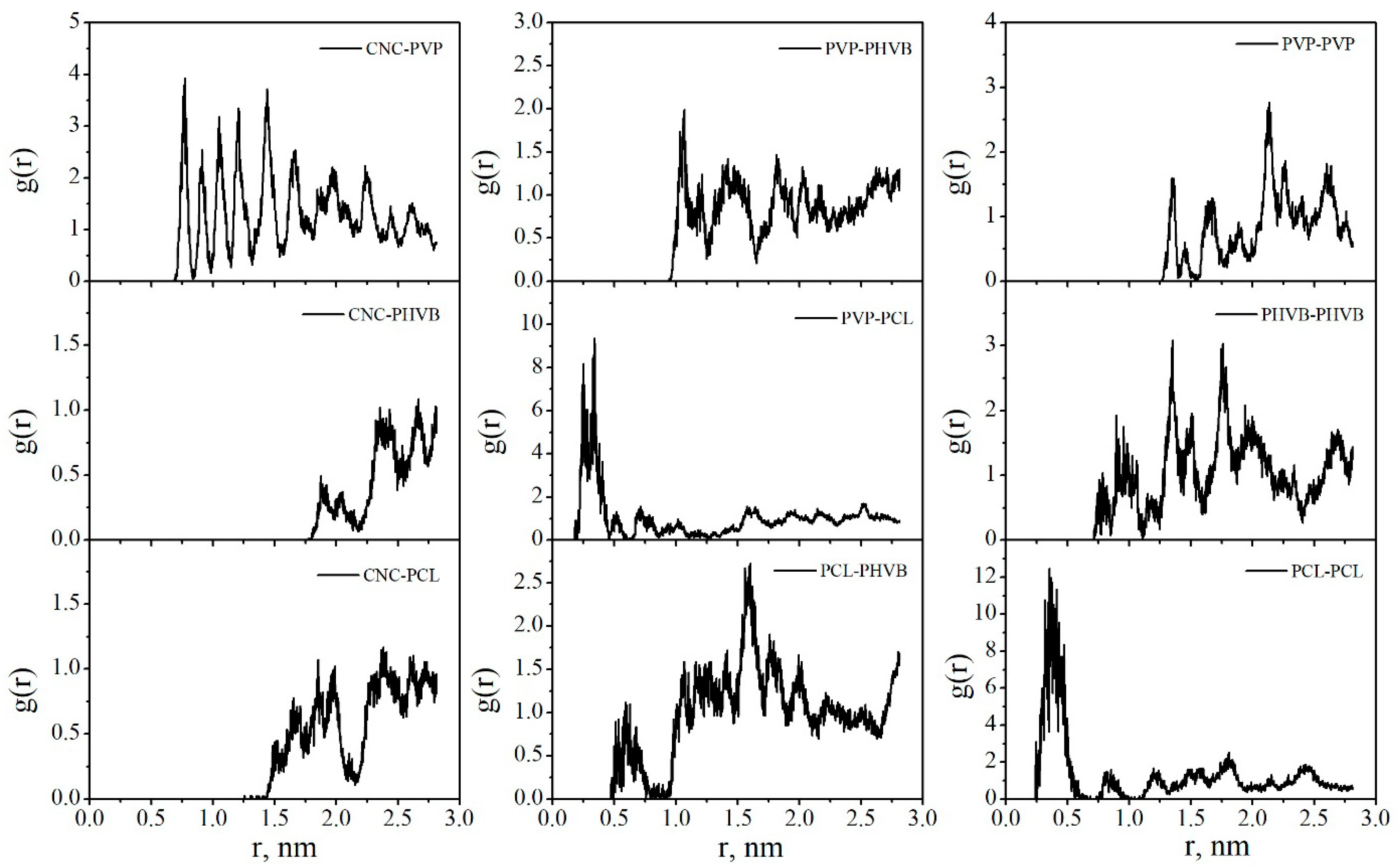
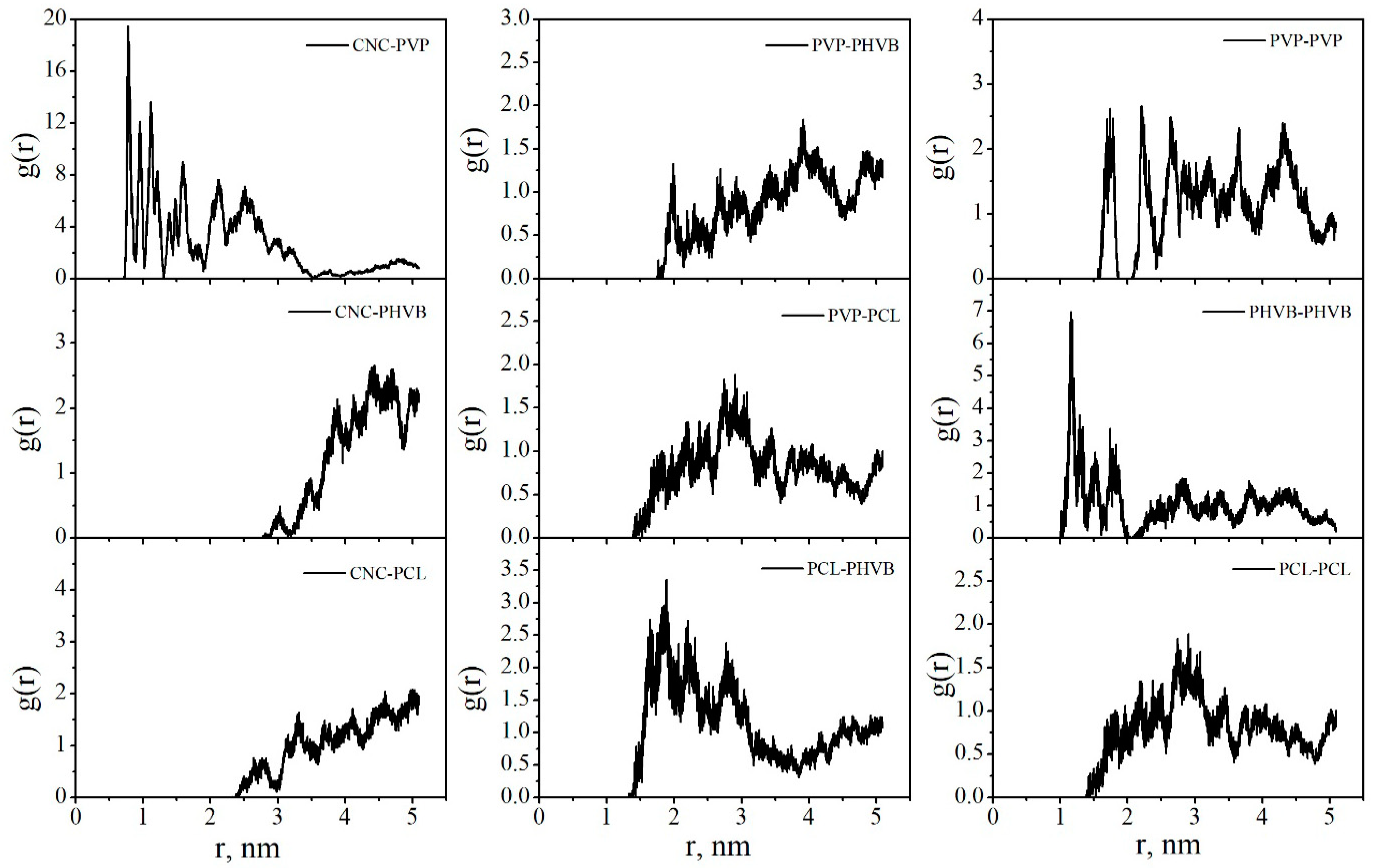
| Sample | BET Specific Surface Area, m2 g−1 | Maximum Water Vapor Sorption, % |
|---|---|---|
| PHBV/PCL(1:1) | 4.9 | 2.6 |
| PHBV/PCL(1:1)/CNC(PVP)–5 | 7.8 | 10.7 |
| PHBV/PCL(1:1)/CNC(PVP)–10 | 8.5 | 11.3 |
| PHBV/PCL(1:1)/CNC(PVP)–15 | 18.7 | 13.7 |
| PHBV/PCL(1:1)/CNC(PAM)–5 | 7.2 | 13.1 |
| PHBV/PCL(1:1)/CNC(PAM)–10 | 11.4 | 13.3 |
| PHBV/PCL(1:1)/CNC(PAM)–15 | 19.0 | 13.8 |
| PHBV/PCL(1:2) | 3.8 | 2.5 |
| PHBV/PCL(1:2)/CNC(PVP)–5 | 7.3 | 5.3 |
| PHBV/PCL(1:2)/CNC(PVP)–10 | 7.7 | 9.5 |
| PHBV/PCL(1:2)/CNC(PVP)–15 | 12.7 | 11.2 |
| PHBV/PCL(1:2)/CNC(PAM)–5 | 11.6 | 11.7 |
| PHBV/PCL(1:2)/CNC(PAM)–10 | 17.7 | 12.3 |
| PHBV/PCL(1:2)/CNC(PAM)–15 | 24.2 | 12.3 |
| Sample | Tm PCL, °C | Tm1 PHBV, °C | Tm2 PHBV, °C | Tcryst PCL, °C | Tcryst PHBV, °C | ΔHm PCL, J g−1 | ΔHm PHBV, J g−1 | χc PCL, % | χc PHBV, % |
|---|---|---|---|---|---|---|---|---|---|
| PHBV | - | 145.7 | 156.5 | - | 88.6 | - | 46.5 | - | 31.7 |
| PCL | 55.6 | - | - | 24.1 | - | 76.0 | - | 48.4 | - |
| PHBV/PCL (1:1) | 54.3 | 144.0 | 155.8 | 29.2 | 84.9 | 20.9 | 39.7 | 26.6 | 54.1 |
| PHBV/PCL (1:1)/CNC(PVP)–5 | 54.9 | 145.3 | 156.9 | 28.2 | 87.6 | 15.8 | 28.5 | 21.2 | 40.9 |
| PHBV/PCL (1:1)/CNC(PVP)–10 | 55.3 | 145.3 | 156.9 | 30.0 | 86.5 | 21.5 | 18.9 | 30.4 | 28.6 |
| PHBV/PCL (1:1)/CNC(PVP)–15 | 55.1 | 144.3 | 156.3 | 31.4 | 84.4 | 22.3 | 22.2 | 33.4 | 35.6 |
| PHBV/PCL (1:1)/CNC(PAM)–5 | 55.6 | 144.5 | 156.7 | 31.4 | 82.8 | 18.0 | 18.1 | 24.1 | 26.0 |
| PHBV/PCL (1:1)/CNC(PAM)–10 | 55.1 | 143.9 | 156.3 | 32.0 | 81.8 | 20.8 | 24.6 | 29.4 | 37.3 |
| PHBV/PCL (1:1)/CNC(PAM)–15 | 54.7 | 143.6 | 155.8 | 31.9 | 84.7 | 23.2 | 22.6 | 34.8 | 36.3 |
| PHBV/PCL (1:2) | 55.7 | 143.9 | 156.1 | 31.1 | 78.7 | 30.3 | 19.3 | 29.2 | 39.5 |
| PHBV/PCL (1:2)/CNC(PVP)–5 | 55.7 | 144.9 | 156.8 | 29.7 | 84.8 | 32.2 | 13.9 | 32.4 | 29.9 |
| PHBV/PCL (1:2)/CNC(PVP)–10 | 56.0 | 145.2 | 157.0 | 30.7 | 84.5 | 30.0 | 11.9 | 31.8 | 27.1 |
| PHBV/PCL (1:2)/CNC(PVP)–15 | 55.8 | 143.9 | 156.3 | 30.9 | 80.0 | 29.5 | 13.2 | 33.1 | 31.8 |
| PHBV/PCL (1:2)/CNC(PAM)–5 | 55.9 | 143.9 | 156.1 | 31.7 | 79.8 | 30.8 | 14.9 | 31.0 | 32.1 |
| PHBV/PCL (1:2)/CNC(PAM)–10 | 55.6 | 142.9 | 155.8 | 31.6 | 76.4 | 33.3 | 13.5 | 35.4 | 30.7 |
| PHBV/PCL (1:2)/CNC(PAM)–15 | 56.3 | 144.4 | 156.4 | 30.7 | 79.9 | 27.8 | 12.9 | 31.2 | 31.1 |
| Sample | The First Stage of Thermal Decomposition | The Second Stage of Thermal Decomposition | Total Mass Loss, % | ||||
|---|---|---|---|---|---|---|---|
| 1Ton, °C | Tmax, °C | Mass Loss, % | 1Ton, °C | 2Tmax, °C | Mass Loss, % | ||
| PHBV | 261.6 | 275.2 | 98.6 | - | - | - | 98.6 |
| PCL | - | - | - | 343.7 | 412.0 | 96.5 | 96.5 |
| PHBV/PCL(1:1) | 265.0 | 279.9 | 60.7 | 384.8 | 410.0 | 38.3 | 99.0 |
| PHBV/PCL(1:1)/CNC(PVP)–5 | 278.1 | 296.4 | 52.5 | 386.5 | 409.7 | 45.0 | 97.5 |
| PHBV/PCL(1:1)/CNC(PVP)–10 | 286.2 | 298.0 | 40.7 | 386.9 | 407.9 | 45.3 | 96.3 |
| PHBV/PCL(1:1)/CNC(PVP)–15 | 276.2 | 295.0 | 50.7 | 386.7 | 410.8 | 44.0 | 94.7 |
| PHBV/PCL(1:1)/CNC(PAM)–5 | 278.0 | 296.6 | 50.1 | 387.5 | 409.0 | 45.9 | 96.0 |
| PHBV/PCL(1:1)/CNC(PAM)–10 | 279.6 | 297.9 | 52.9 | 386.8 | 408.1 | 41.6 | 94.5 |
| PHBV/PCL(1:1)/CNC(PAM)–15 | 280.0 | 298.0 | 53.0 | 387.0 | 408.0 | 42.0 | 95.0 |
| PHBV/PCL(1:2) | 270.0 | 284.7 | 34.5 | 389.5 | 412.0 | 64.4 | 98.9 |
| PHBV/PCL(1:2)/CNC(PVP)–5 | 283.4 | 300.8 | 34.9 | 389.6 | 413.3 | 63.1 | 98.0 |
| PHBV/PCL(1:2)/CNC(PVP)–10 | 288.6 | 299.9 | 27.2 | 387.7 | 410.1 | 58.1 | 97.1 |
| PHBV/PCL(1:2)/CNC(PVP)–15 | 278.4 | 299.5 | 37.6 | 387.1 | 409.5 | 57.0 | 94.6 |
| PHBV/PCL(1:2)/CNC(PAM)–5 | 280.1 | 298.7 | 36.2 | 387.3 | 409.5 | 59.5 | 95.7 |
| PHBV/PCL(1:2)/CNC(PAM)–10 | 282.1 | 299.8 | 37.2 | 388.5 | 411.4 | 59.6 | 96.8 |
| PHBV/PCL(1:2)/CNC(PAM)–15 | 282.0 | 300.0 | 37.0 | 389.0 | 411.0 | 60.0 | 97.0 |
| Sample | The Total Crystallinity of PHBV and PCL, % |
|---|---|
| PHBV/PCL(1:1) | 43.8 |
| PHBV/PCL(1:1)/CNC(PVP)–5 | 35.3 |
| PHBV/PCL(1:1)/CNC(PVP)–10 | 35.1 |
| PHBV/PCL(1:1)/CNC(PVP)–15 | 37.6 |
| PHBV/PCL(1:1)/CNC(PAM)–5 | 33.3 |
| PHBV/PCL(1:1)/CNC(PAM)–10 | 22.6 |
| PHBV/PCL(1:1)/CNC(PAM)–15 | 41.4 |
| PHBV/PCL(1:2) | 47.2 |
| PHBV/PCL(1:2)/CNC(PVP)–5 | 49.0 |
| PHBV/PCL(1:2)/CNC(PVP)–10 | 51.8 |
| PHBV/PCL(1:2)/CNC(PVP)–15 | 49.1 |
| PHBV/PCL(1:2)/CNC(PAM)–5 | 39.5 |
| PHBV/PCL(1:2)/CNC(PAM)–10 | 38.6 |
| PHBV/PCL(1:2)/CNC(PAM)–15 | 38.9 |
| Sample | Ultimate Tensile Strength (σmax), MPa | Tensile Strength at Break (σb), MPa | Elongation at Break (ε), % | Young’s Modulus (E), MPa |
|---|---|---|---|---|
| PHBV/PCL(1:1) | 11.7 | 11.7 | 8.7 | 350 |
| PHBV/PCL(1:1)/CNC(PVP)–5 | 11.7 | 10.7 | 82.0 | 330 |
| PHBV/PCL(1:1)/CNC(PVP)–10 | 7.1 | 7.1 | 2.0 | 460 |
| PHBV/PCL(1:1)/CNC(PVP)–15 | 3.2 | 3.2 | 3.0 | 340 |
| PHBV/PCL(1:1)/CNC(PAM)–5 | 3.7 | 3.7 | 4.0 | 280 |
| PHBV/PCL(1:1)/CNC(PAM)–10 | 4.2 | 4.2 | 5.6 | 280 |
| PHBV/PCL(1:1)/CNC(PAM)–15 | 4.3 | 4.3 | 7.0 | 220 |
| PHBV/PCL(1:2) | 17.3 | 17.0 | 340 | 260 |
| PHBV/PCL(1:2)/CNC(PVP)–5 | 11.5 | 11.5 | 16.4 | 250 |
| PHBV/PCL(1:2)/CNC(PVP)–10 | 10.1 | 9.4 | 3.4 | 480 |
| PHBV/PCL(1:2)/CNC(PVP)–15 | 7.9 | 7.9 | 8.9 | 360 |
| PHBV/PCL(1:2)/CNC(PAM)–5 | 9.5 | 9.5 | 5.3 | 430 |
| PHBV/PCL(1:2)/CNC(PAM)–10 | 10.4 | 10.4 | 7.6 | 510 |
| PHBV/PCL(1:2)/CNC(PAM)–15 | 10.0 | 10.0 | 9.3 | 360 |
| System | N (CNC) | N (PVP) | N (PHBV) | N (DCM) | N (Water) | t, ns | L, nm |
|---|---|---|---|---|---|---|---|
| 1 | - | 1 | - | 10,000 | - | 30 | 9.75 |
| 2 | - | - | 1 | 10,000 | - | 30 | 9.72 |
| 3 | - | - | 1 | - | 24,000 | 30 | 8.48 |
| 4 | - | 1 | 1 | 10,000 | - | 50 | 9.73 |
| 5 | 1 | 1 | - | 18,000 | - | 20 | 11.85 |
| 6 | 1 | - | 1 | - | - | 20 | 11.95 |
| 7 | 1 | 13 | - | - | - | 30 | 20.00 |
| 8 | 1 | 13 | 8 | 10,000 | - | 20 | 20.00 |
| System | N (PCL) | N (PHBV) | N (DCM) | t, ns | L, nm |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 10,000 | 10 | 9.73 |
| 2 | 10 | 10 | 10,000 | 10 | 9.75 |
| 3 | 10 | 10 | - | 10 | 3.58 |
| System | PCL | PHBV | ||
|---|---|---|---|---|
| Re-t-e, nm | Rg, nm | Re-t-e, nm | Rg, nm | |
| 1 | 5.33 ± 1.02 | 1.79 ± 0.23 | 3.70 ± 0.96 | 1.42 ± 0.19 |
| 2 | 4.34 ± 0.30 | 1.57 ± 0.06 | 3.26 ± 0.37 | 1.28 ± 0.07 |
| 3 | 3.28 ± 0.06 | 1.29 ± 0.02 | 2.85 ± 0.06 | 1.15 ± 0.01 |
| System | NC (PCL–PHBV) | NC (PCL–PCL) | NC (PHBV–PHBV) |
|---|---|---|---|
| 1 | 0 | 656 ± 16 | 2397 ± 118 |
| 2 | 167 ± 88 | 6742 ± 88 | 24,319 ± 451 |
| 3 | 6477 ± 145 | 10,943 ± 149 | 33,450 ± 284 |
| Medium | PCL | PHBV | PVP | |
|---|---|---|---|---|
| Vacuum (individual molecule) | Re-t-e, nm | 0.63 ± 0.10 | 1.01 ± 0.43 | 1.23 ± 0.18 |
| Rg, nm | 0.69 ± 0.02 | 0.69 ± 0.04 | 1.10 ± 0.01 | |
| Water (individual molecule) | Re-t-e, nm | 1.55 ± 0.63 | 1.24 ± 0.40 | 1.74 ± 0.20 |
| Rg, nm | 0.71 ± 0.08 | 0.68 ± 0.03 | 1.11 ± 0.01 | |
| DCM (individual molecule) | Re-t-e, nm | 3.78 ± 1.34 | 3.49 ± 1.05 | 5.61 ± 1.07 |
| Rg, nm | 1.46 ± 0.26 | 1.35 ± 0.25 | 1.97 ± 0.23 | |
| Vacuum (in the composite) | Re-t-e, nm | 2.59 ± 0.19 | 3.06 ± 0.14 | 2.53 ± 0.06 |
| Rg, nm | 1.06 ± 0.5 | 1.16 ± 0.03 | 1.33 ± 0.01 | |
| DCM (in the composite) | Re-t-e, nm | 3.73 ± 0.19 | 2.99 ± 0.21 | 4.65 ± 0.19 |
| Rg, nm | 1.45 ± 0.03 | 1.20 ± 0.03 | 1.73 ± 0.02 |
| <nHB> | Vacuum | DCM |
|---|---|---|
| CNC–PCL | 0.63 ± 0.12 | 0.12 ± 0.39 |
| CNC–PHVB | 2.9 ± 0.3 | 0.53 ± 0.67 |
| CNC–PVP | 38.9 ± 0.5 | 34.7 ± 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronova, M.I.; Gurina, D.L.; Surov, O.V. Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies. Polymers 2022, 14, 340. https://doi.org/10.3390/polym14020340
Voronova MI, Gurina DL, Surov OV. Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies. Polymers. 2022; 14(2):340. https://doi.org/10.3390/polym14020340
Chicago/Turabian StyleVoronova, Marina I., Darya L. Gurina, and Oleg V. Surov. 2022. "Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies" Polymers 14, no. 2: 340. https://doi.org/10.3390/polym14020340
APA StyleVoronova, M. I., Gurina, D. L., & Surov, O. V. (2022). Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies. Polymers, 14(2), 340. https://doi.org/10.3390/polym14020340






