Fe(III)-Rhamnoxylan—A Novel High Spin Fe(III) Octahedral Complex Having Versatile Physical and Biological Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Fe(III)-Rhamnoxylan
2.2. Characterization
2.2.1. Elemental Analysis
2.2.2. Monosaccharide Analysis
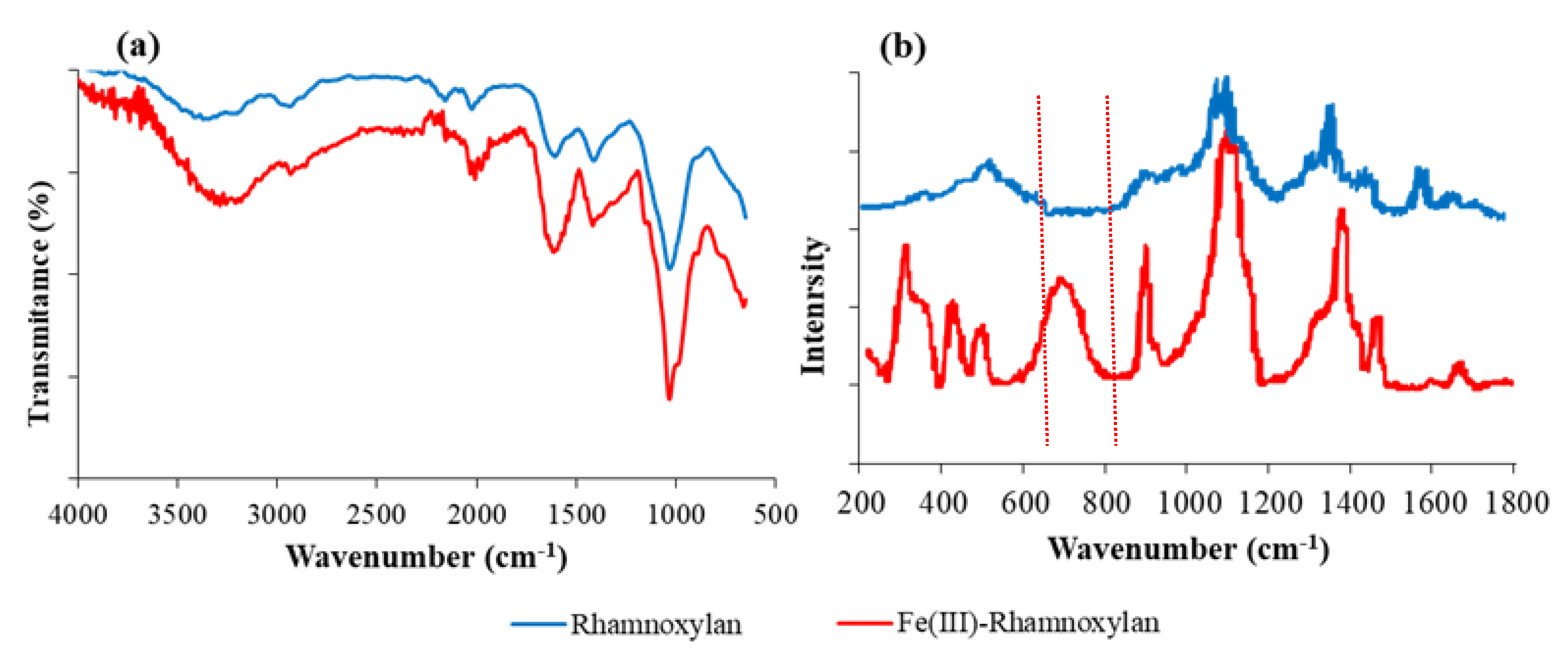
2.2.3. FT-IR and Raman Analysis
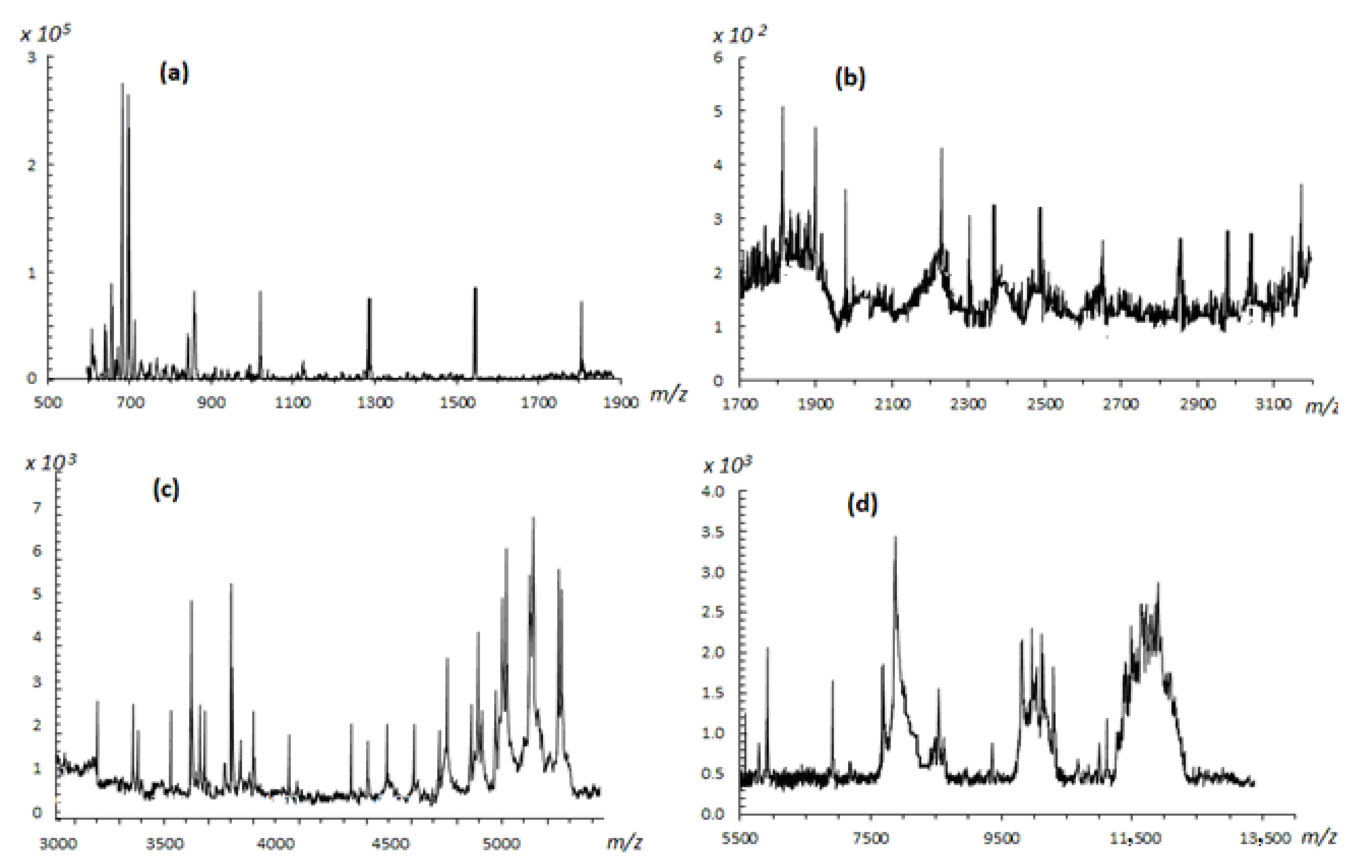
2.2.4. Structural Analysis of Rhamnoxylan by MALDI-ToF Mass Spectroscopy
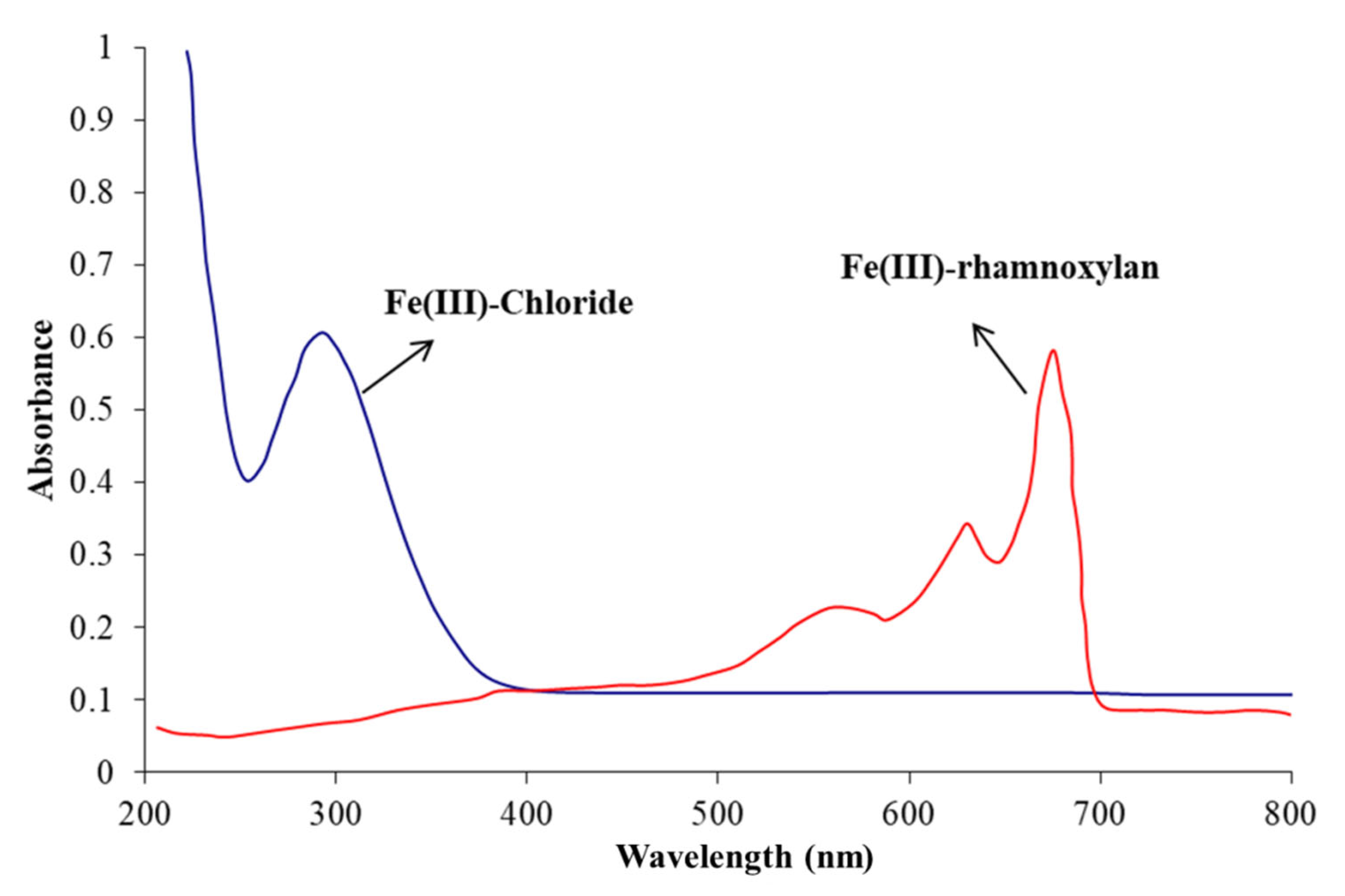
2.2.5. Electronic Absorption Spectrophotometry
2.2.6. Mössbauer Specroscopy
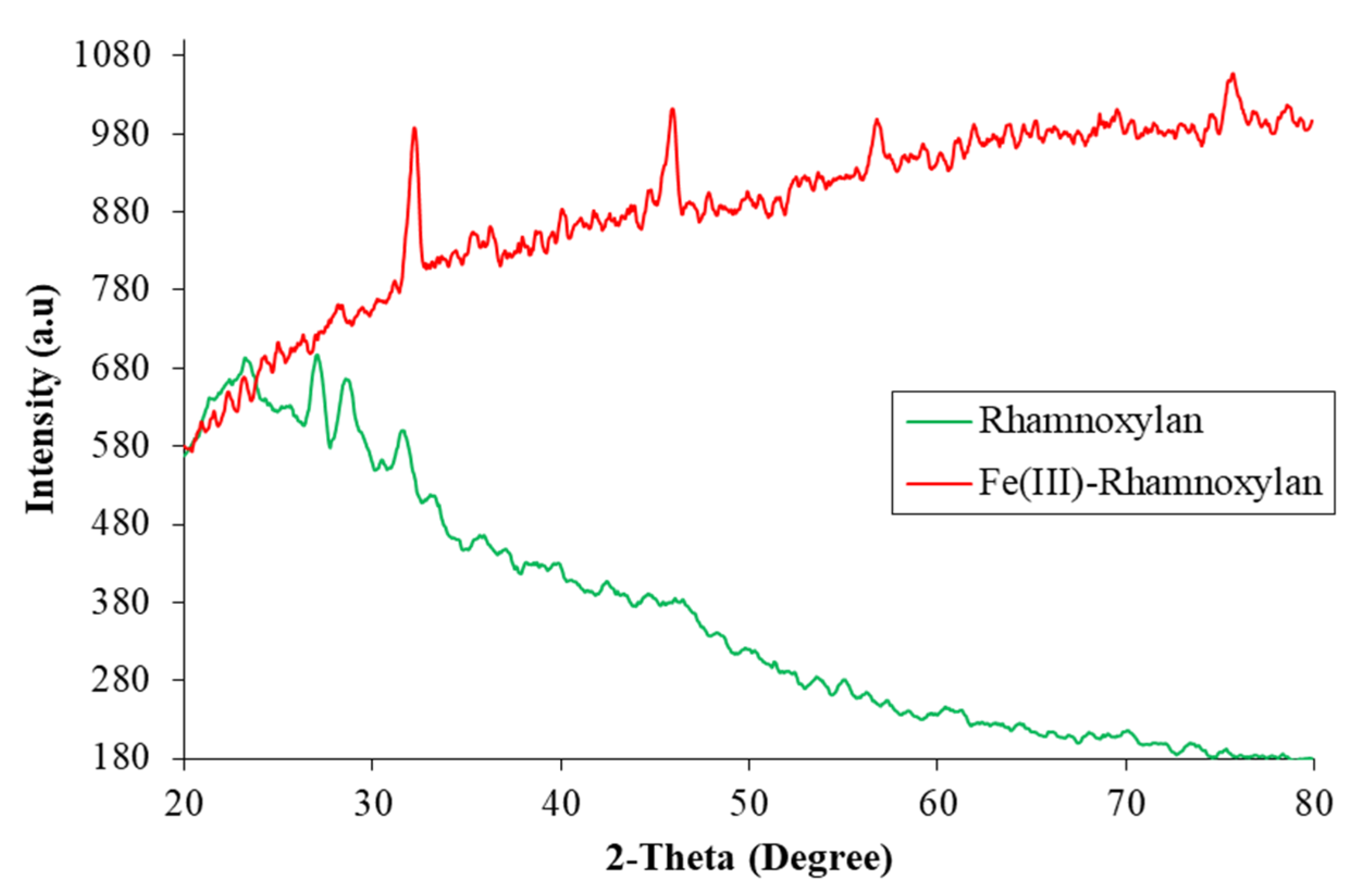
2.2.7. Powder X-ray Diffraction Analysis
2.2.8. Magnetic Properties
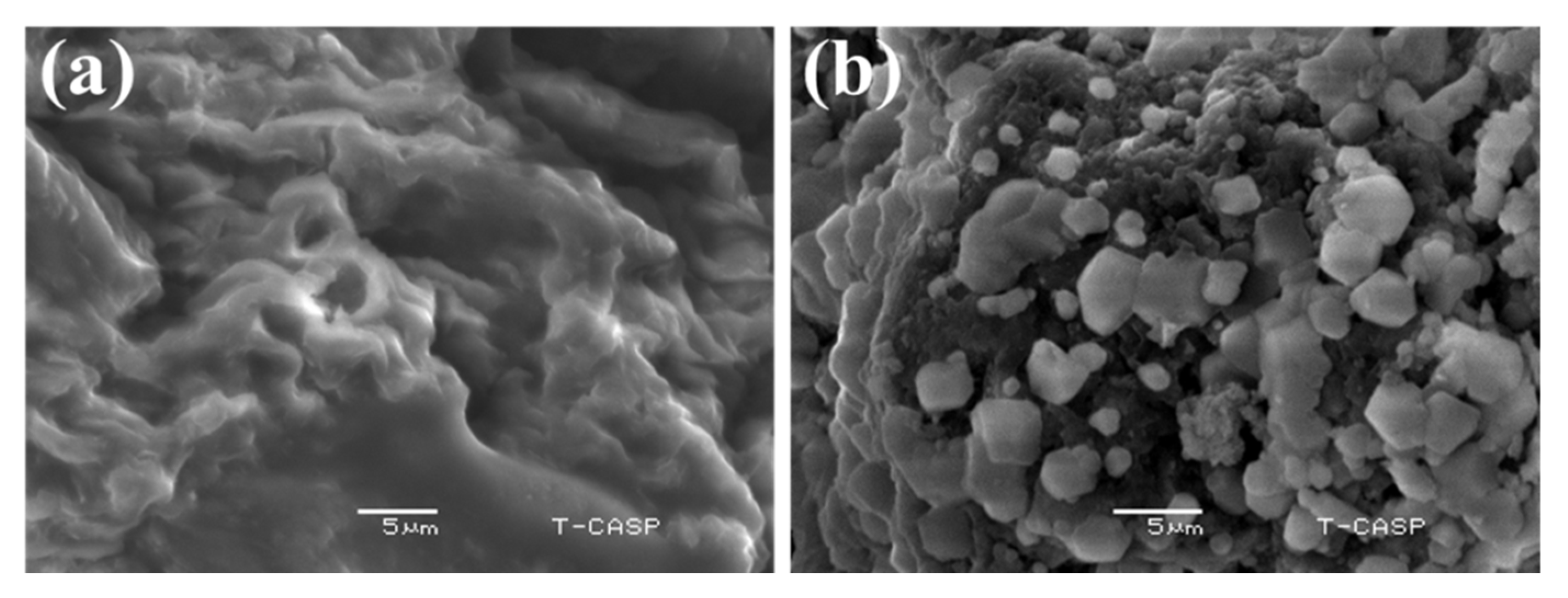
2.2.9. Scanning Electron Microscopy
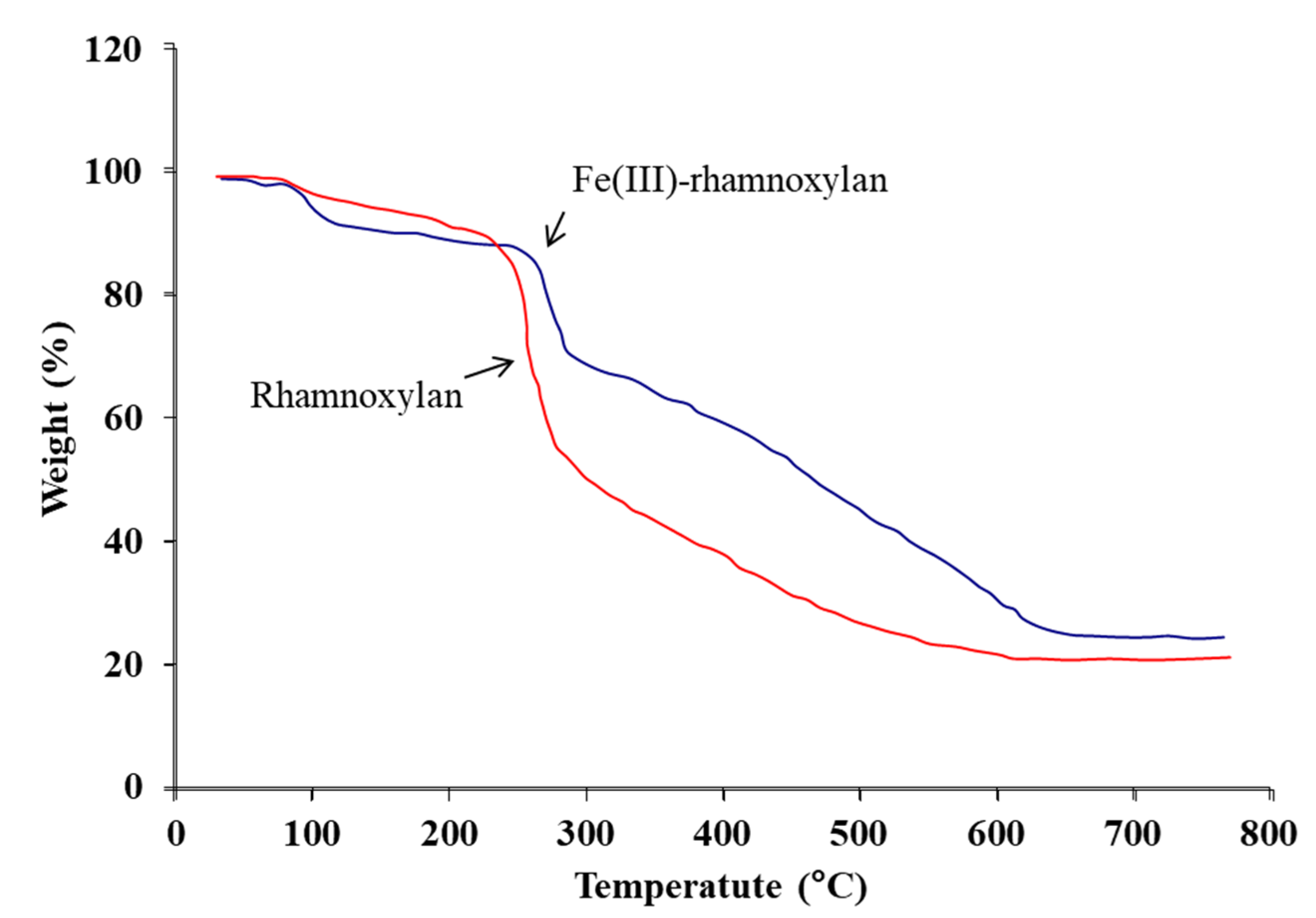
2.2.10. Thermal Analysis
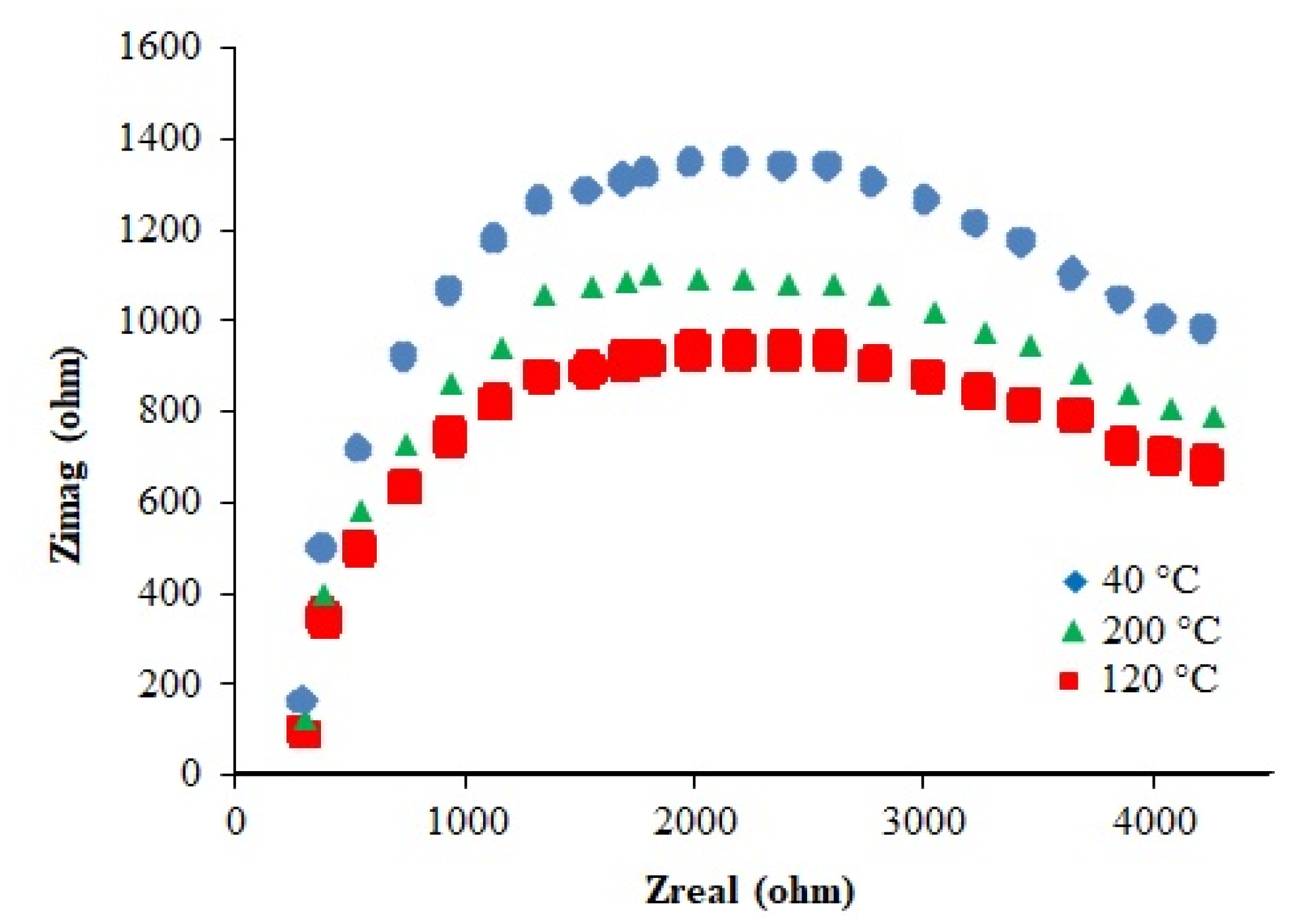
2.2.11. Electrical Properties
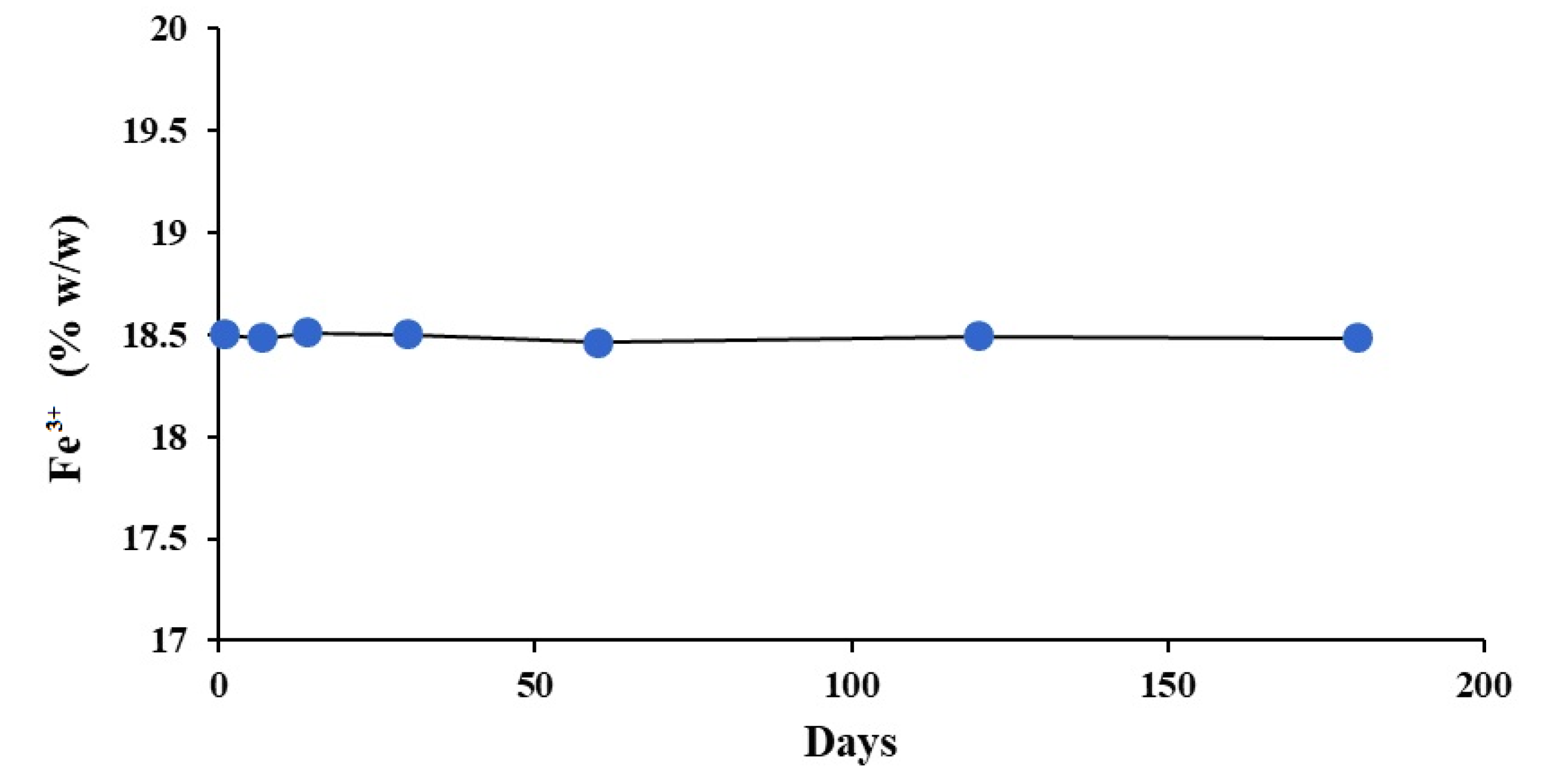
2.3. Stability Study
2.4. Biological Evaluation
2.4.1. Acute Oral Toxicity
2.4.2. In Vivo Absorption and Excretion of Iron
2.4.3. Therapeutic Evaluation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe(III)-Rhamnoxylan Complex
3.3. Characterization
3.4. Biological Evaluation
3.4.1. Acute Oral Toxicity Test
3.4.2. Therapeutic Evaluation
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.H.; Choi, E.K.; Han, K.D.; Lee, S.R.; Cha, M.J.; Oh, S. Impact of hemoglobin levels and their dynamic changes on the risk of atrial fibrillation: A nationwide population-based study. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Funk, F.; Canclini, C.; Geisser, P. Interactions between Iron(III)-hydroxide polymaltose complex and commonly used medications laboratory studies in rats. Arzneim.-Forsch. Drug Res. 2007, 57, 370–375. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Sharma, V.; Singh, A.K. Preparation of succinylated sodium caseinate-iron complex by adopting ultrafiltration technology: A novel food fortificant. Innov. Food Sci. Emerg. Technol. 2015, 32, 165–171. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Akbar, J.; Hussain, M.A.; Saghir, S.; Sher, M. Evaluation of hot-water extracted arabinoxylans from ispaghula seeds as drug carriers. Carbohydr. Polym. 2011, 83, 1218–1225. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and characterization of hemicellulose-based films for antibacterial wound-dressing application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloway, W.D.; Tasman-Jones, C.; Lee, S.P. Digestion of certain fractions of dietary fiber in humans. Am. J. Clin. Nutr. 1978, 31, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Y.; Wan, X.-H.; Niu, F.-J.; Xie, S.-M.; Guo, H.; Yang, Y.-Y.; Guo, L.-Y.; Zhou, C.-Z. Salvia plebeia R. Br.: An overview about its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 121, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Seon, Y.; Lim, C.; Park, E. Anti-Inflammatory, anti-angiogenic and anti-nociceptive activities of an ethanol extract of Salvia Plebeia R. Brown. J. Ethnopharmacol. 2009, 126, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Huang, Y.; He, R.; Zeng, X. Synthesis and characterization of a new soluble soybean polysaccharide-iron(iii) complex using ion exchange column. Int. J. Biol. Macromol. 2018, 108, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.C. In situ synthesis of magnetic field-responsive hemicellulose hydrogels for drug delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Oderinde, O.; Liu, S.; Huang, Q.; Ma, W.; Yao, F.; Fu, G. Characterization of xanthan gum-based hydrogel with Fe3+ ions coordination and its reversible sol-gel conversion. Carbohydr. Polym. 2019, 203, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kačuráková, M.; Wellner, N.; Ebringerová, A.; Hromádková, Z.; Wilson, R.H.; Belton, P.S. Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocoll. 1999, 13, 35–41. [Google Scholar] [CrossRef]
- Peng, P.; She, D. Isolation, Structural characterization, and potential applications of hemicelluloses from bamboo: A Review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Geetha, K.; Raghavan, M.S.S.; Kulshreshtha, S.K.; Sasikala, R.; Rao, C.P. Transition-metal saccharide chemistry: Synthesis, spectroscopy, electrochemistry and magnetic susceptibility studies of iron(iii) complexes of mono- and disaccharides. Carbohydr. Res. 1995, 271, 163–175. [Google Scholar] [CrossRef]
- Sharma, S.K. Raman Study of ferric chloride hexahydrate and ferric chloride hexadeutrate in crystalline, molten and glassy states. J. Non. Cryst. Solids 1974, 15, 83–95. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of Glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Viseux, N.; De Hoffmann, E.; Domon, B. Structural assignment of permethylated oligosaccharide subunits using sequential tandem mass spectrometry. Anal. Chem. 1998, 70, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Iram, F.; Massey, S.; Iqbal, M.S.; Ward, D.G. Structural investigation of hemicelluloses from plantago ovata, mimosa pudica and lallemantia royleana by MALDI-ToF mass spectrometry. J. Carbohydr. Chem. 2018, 37, 285–301. [Google Scholar] [CrossRef]
- Abderrazak, H.; Dachraoui, M.; Lendl, B. A novel flow injection procedure for determination of phosphate in industrial raw phosphoric acid. Analyst 2000, 125, 1211–1213. [Google Scholar] [CrossRef]
- Kudasheva, D.S.; Lai, J.; Ulman, A.; Cowman, M.K. Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J. Inorg. Biochem. 2004, 98, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Rossman, G.R. The optical spectroscopic comparison of the ferric iron tetrameric clusters in amarantite and leucophosphite. Am. Mineral. 1976, 61, 933–938. [Google Scholar]
- Rossman, G.R. Spectrosopic and magnetic studies of ferric iron hydroxy sulfates: Intensification of color in ferric iron clusters bridged by a single hydroxide ion. Am. Mineral. 1975, 60, 698–704. [Google Scholar]
- Fontana, I.; Lauria, A.; Spinolo, G. Optical absorption spectra of Fe2+ and Fe3+ in aqueous solutions and hydrated crystals. Phys. Status Solidi Basic Res. 2007, 244, 4669–4677. [Google Scholar] [CrossRef]
- Pansuriya, P.; Patel, M.N. Iron(III) Complexes: Preparation, characterization, antibacterial activity and DNA-Binding. J. Enzyme Inhib. Med. Chem. 2008, 23, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Coe, E.M.; Bowen, L.H.; Speer, J.A.; Wang, Z.; Sayers, D.E.; Bereman, R.D. The recharacterization of a polysaccharide iron complex (Niferex). J. Inorg. Biochem. 1995, 58, 269–278. [Google Scholar] [CrossRef]
- Murad, E.; Schwertmann, U. The Mössbauer spectrum of ferrihydrite and its relations to those of other iron oxides. Am. Mineral. 1980, 65, 1044–1049. [Google Scholar]
- Cui, J.; Li, Y.; Yu, P.; Zhan, Q.; Wang, J.; Chi, Y.; Wang, P. A novel low molecular weight enteromorpha polysaccharide-iron (iii) complex and its effect on rats with Iron Deficiency Anemia (IDA). Int. J. Biol. Macromol. 2018, 108, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, M.A.; Ummineni, D.; Sasaki, T.; Nishio-Hamane, D.; Bhanage, B.M. Magnetically separable γ-Fe2O3 nanoparticles: An efficient catalyst for acylation of alcohols, phenols and amines using sonication energy under solvent free condition. J. Mol. Catal. A Chem. 2015, 404, 8–17. [Google Scholar] [CrossRef]
- Balasubramanian, C.; Joseph, B.; Gupta, P.; Saini, N.L.; Mukherjee, S.; Di Gioacchino, D.; Marcelli, A. X-ray absorption spectroscopy characterization of iron-oxide nanoparticles synthesized by high temperature plasma processing. J. Electron Spectros. Relat. Phenomena 2014, 196, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Structural and optical properties of pure iron and iron oxide nanoparticles prepared via pulsed Nd:YAG laser ablation in liquid. Procedia Mater. Sci. 2015, 11, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Rafi, M.M.; Ahmed, S.Z.; PremNazeer, K.; Kumar, D. Antibacterial Activity of iron oxide nanoparticles on polysaccharide templates: Synthesis, characterization and magnetic Studies. Malays. Polym. J. 2015, 10, 16–22. [Google Scholar]
- Shinkai, M. Functional magnetic particles for medical application. J. Biosci. Bioeng. 2002, 94, 606–613. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X.; Anderson, D.P. Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles. Carbohydr. Polym. 2011, 83, 640–644. [Google Scholar] [CrossRef]
- Yagi, K.; Tokuda, M.; Kobayashi, T.; Kishita, T. Design and synthesis of magnetic ultra fine polysaccharide particles. In Proceedings of the International Symposium on Micro Machine and Human Science; IEEE: Piscataway, NJ, USA, 1999; pp. 157–162. [Google Scholar]
- Maciel, C.B.; Youn, T.S.; Barden, M.M.; Dhakar, M.B.; Zhou, S.E.; Pontes-Neto, O.M.; Silva, G.S.; Theriot, J.J.; Greer, D.M. Corneal Reflex testing in the evaluation of a comatose patient: An ode to precise semiology and examination skills. Neurocrit. Care 2020, 33, 399–404. [Google Scholar] [CrossRef]
- Acute Oral Toxicity-Fixed Dose Procedure; OECD guidelines for the testing of chemicals, Section 4; OECD: Paris, France, 2002; ISBN 9789264070943.
- Lancker, J.L.V. Iron and bile pigment metabolism. In Molecular and Cellular Mechanisms in Disease; Springer: Berlin/Heidelberg, Germany, 1976; pp. 361–395. [Google Scholar]
- Massey, S.; Iqbal, M.S.; Wolf, B.; Mariam, I.; Rao, S. Comparative Drug loading and release study on some carbohydrate polymers. Lat. Am. J. Pharm. 2016, 35, 146–155. [Google Scholar]
- Montgomery, K.O.; Jhaveri, C.R. Process for preparing an iron-saccharide complex. U.S. Patent 3821192, 28 June 1974. [Google Scholar]
- Weitzhandler, M.; Barreto, V.; Pohl, C.; Jandik, P.; Cheng, J.; Avdalovic, N. CarboPac k PA20: A new monosaccharide separator column with electrochemical detection with disposable gold electrodes. J. Biochem. Biophys. Methods 2004, 60, 309–317. [Google Scholar] [CrossRef]
- Annex 2. In Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products; WHO Technical Report Series, No. 953; WHO: Geneva, Switzerland, 2009; Available online: https://www.qlaboratories.com/wp-content/uploads/2019/10/World-Health-Org-WHO-Stability-testing-guidelines.pdf.
- Krishnamurti, G.S.R.; Huang, P.M. Spectrophotometric Determination of Fe(II) with 2,4,6-Tri(2′-Pyridyl)-1,3,5-Triazine in the presence of large quantities of Fe(III) and complexing ions. Talanta 1990, 37, 745–748. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Iqbal, M.S. Optimization of phenylhydrazine induced hyperbilirubinemia in experimental rabbit. Exp. Anim. 2016, 65, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Kheiri, R.; Koohi, M.K.; Sadeghi-Hashjin, G.; Nouri, H.; Khezli, N.; Hassan, M.A.; Hoomani, F.; Shams, G.; Rasouli, A.; Motaghinejad, M. Comparison of the Effects of Iron Oxide, as a New Form of Iron Supplement, and Ferrous Sulfate on the Blood Levels of Iron and Total Iron-Binding Globulin in the Rabbit. Iran. J. Med. Sci. 2017, 42, 79–84. [Google Scholar] [PubMed]
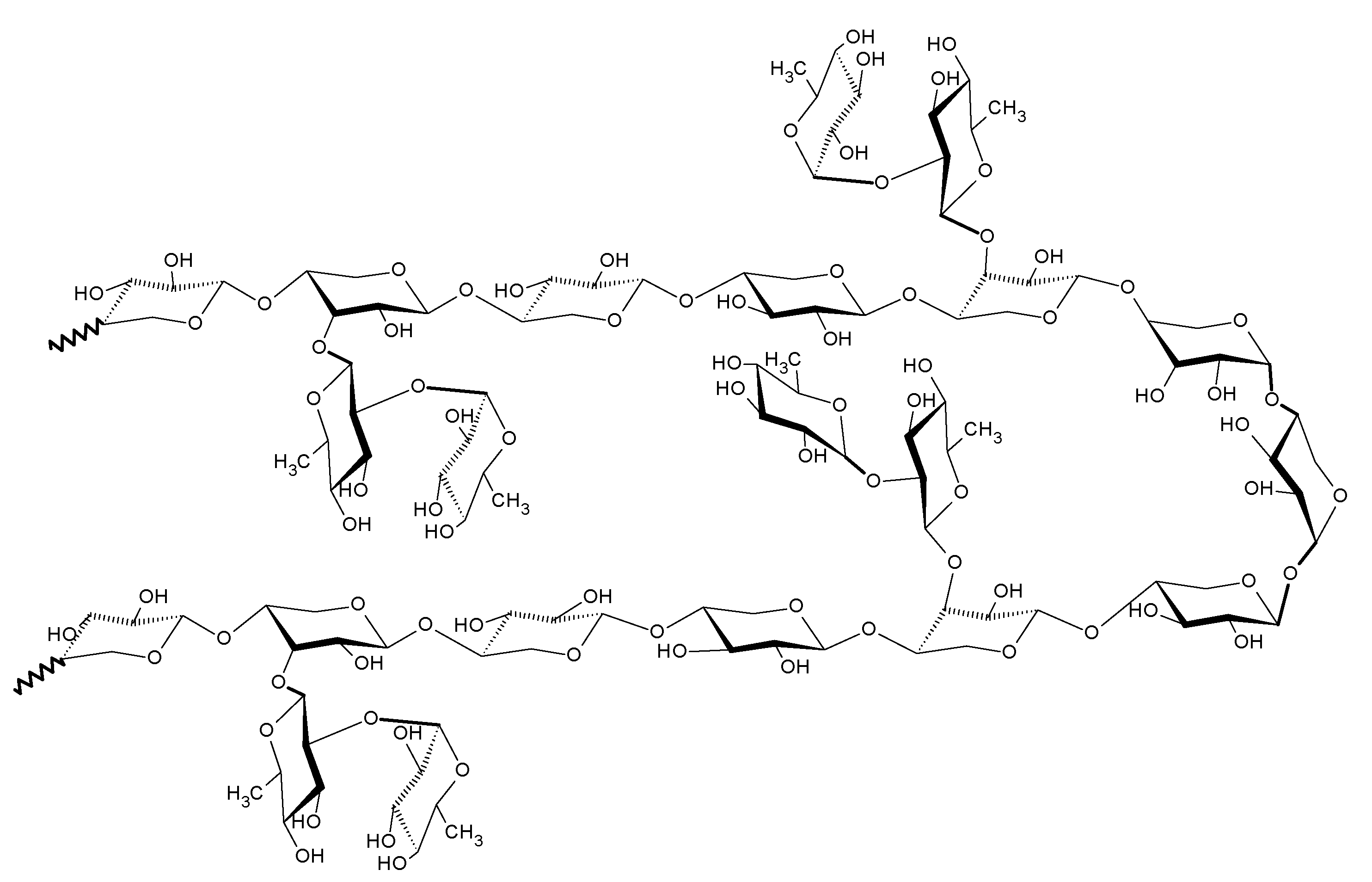




| Compound | Carbon | Hydrogen | Iron | Moisture | Rhap | Xylp | Mw (Dalton) |
|---|---|---|---|---|---|---|---|
| Rhamnoxylan | 46.73 ± 0.04 | 6.45 ± 0.02 | - | 5.98 ± 0.01 | 0.68 ± 0.02 | 99.32 ± 0.05 | 2.47 × 106 |
| Fe(III)-rhamnoxylan | 31.1 ± 0.03 | 5.15 ± 0.01 | 18.40 ± 0.02 | 11.0 ± 0.01 | 0.61 ± 0.01 | 89.12 ± 0.04 | 2.52 × 106 |
| Temperature (°C) | Rg (Ω) | Rgb (Ω) | Rt (Ω) | σ (S cm−1) |
|---|---|---|---|---|
| 40 | 205.35 | 1650.46 | 1845.64 | 2.51 × 10−4 |
| 120 | 100.86 | 456.24 | 507.21 | 1.01 × 10−3 |
| 200 | 36.32 | 65.89 | 107.21 | 4.99 × 10−3 |
| Treatment | Amount of Fe(III) in Control Animals after 14 Days (mg) | Amount of Fe(III) in Blood after 14 Days (mg) | Bioavailability (% Increase) |
|---|---|---|---|
| IPS (33 mg equivalent of iron) | 6.4 | 7.02 ± 0.3 | ~9.78 |
| Fe(III)-rhamnoxylan (33 mg equivalent of iron) | 6.4 | 7.26 ± 0.2 | ~13.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, A.; Iqbal, M.S.; Ahmad, N.; Alruwaili, N.K.; Rehman, A.u. Fe(III)-Rhamnoxylan—A Novel High Spin Fe(III) Octahedral Complex Having Versatile Physical and Biological Properties. Polymers 2022, 14, 4290. https://doi.org/10.3390/polym14204290
Hayat A, Iqbal MS, Ahmad N, Alruwaili NK, Rehman Au. Fe(III)-Rhamnoxylan—A Novel High Spin Fe(III) Octahedral Complex Having Versatile Physical and Biological Properties. Polymers. 2022; 14(20):4290. https://doi.org/10.3390/polym14204290
Chicago/Turabian StyleHayat, Anum, Mohammad Saeed Iqbal, Naveed Ahmad, Nabil K. Alruwaili, and Atta ur Rehman. 2022. "Fe(III)-Rhamnoxylan—A Novel High Spin Fe(III) Octahedral Complex Having Versatile Physical and Biological Properties" Polymers 14, no. 20: 4290. https://doi.org/10.3390/polym14204290







