Controlled Structure of Polyester/Hydroxyapatite Microparticles Fabricated via Pickering Emulsion Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
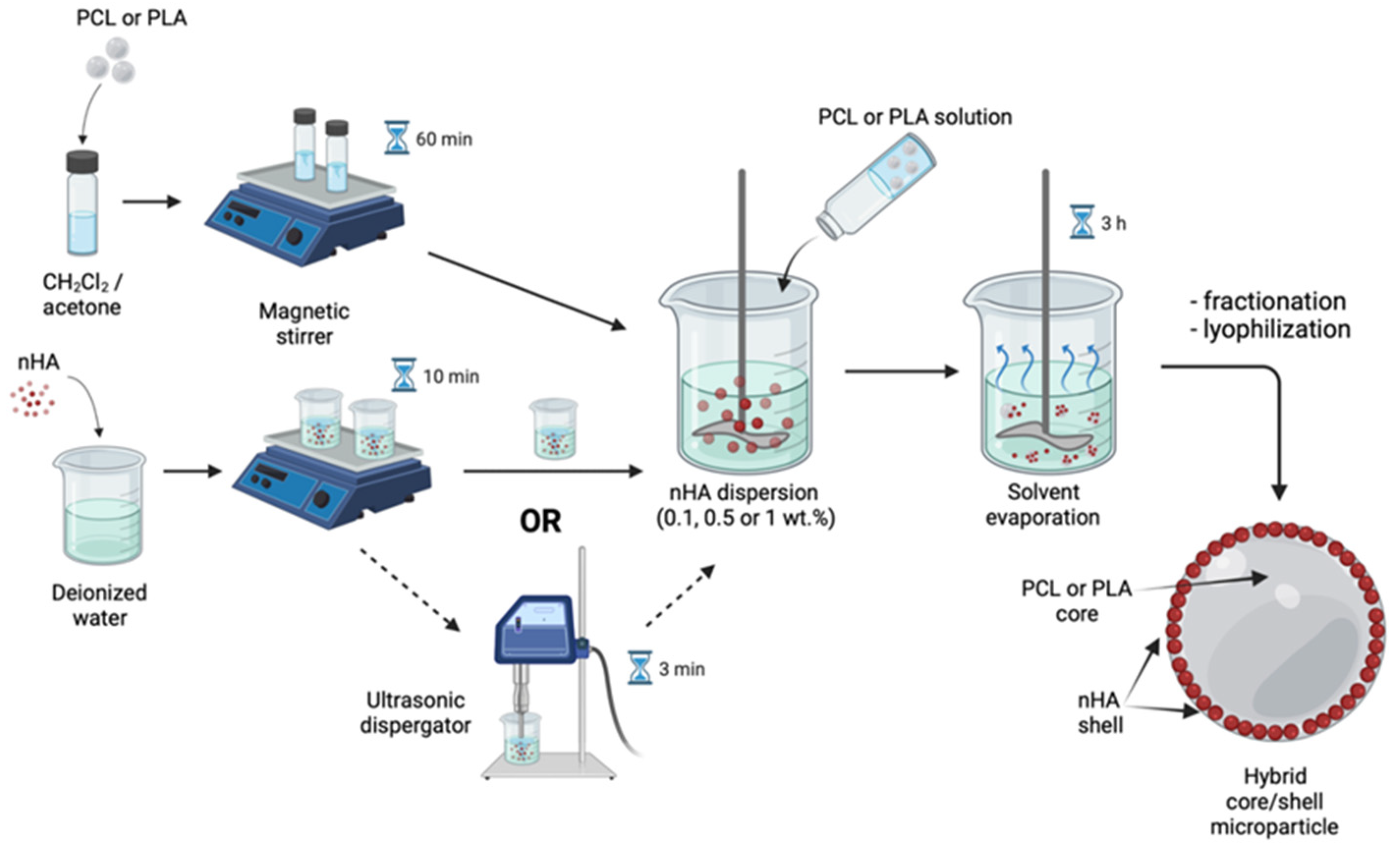
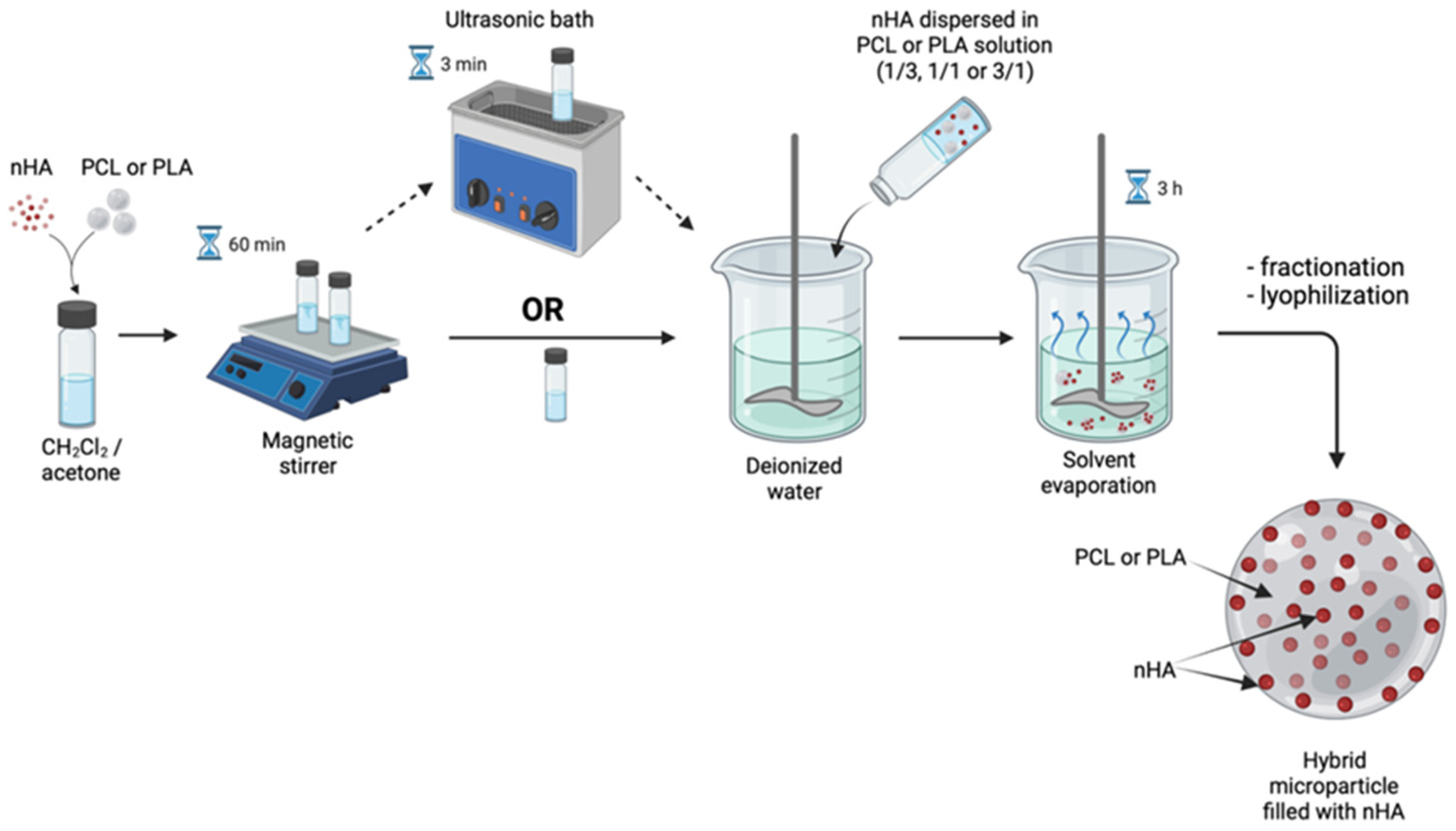
2.2. Hybrid Microparticle Fabrication via Oil/Water Solvent Evaporation Technique
2.3. Methods of Microparticle Characterization
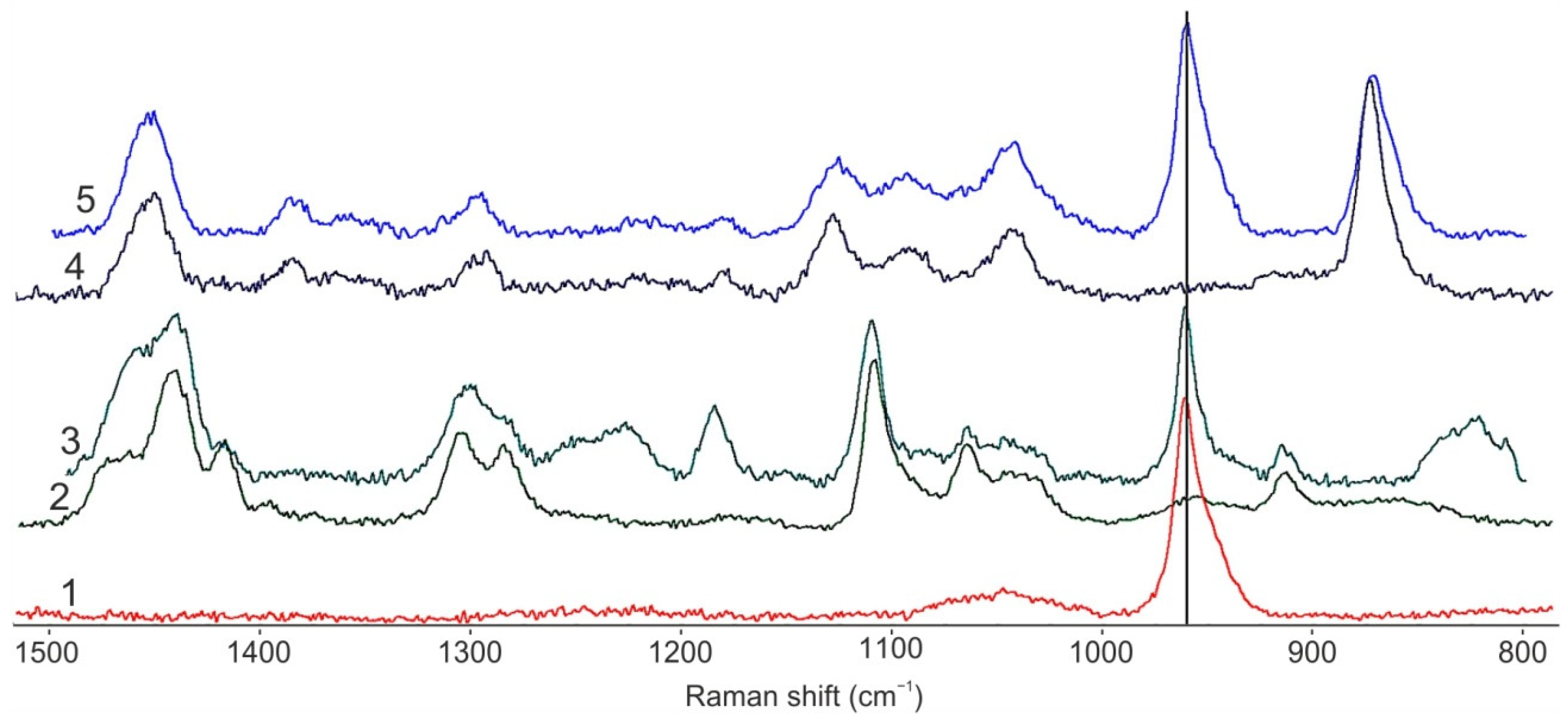
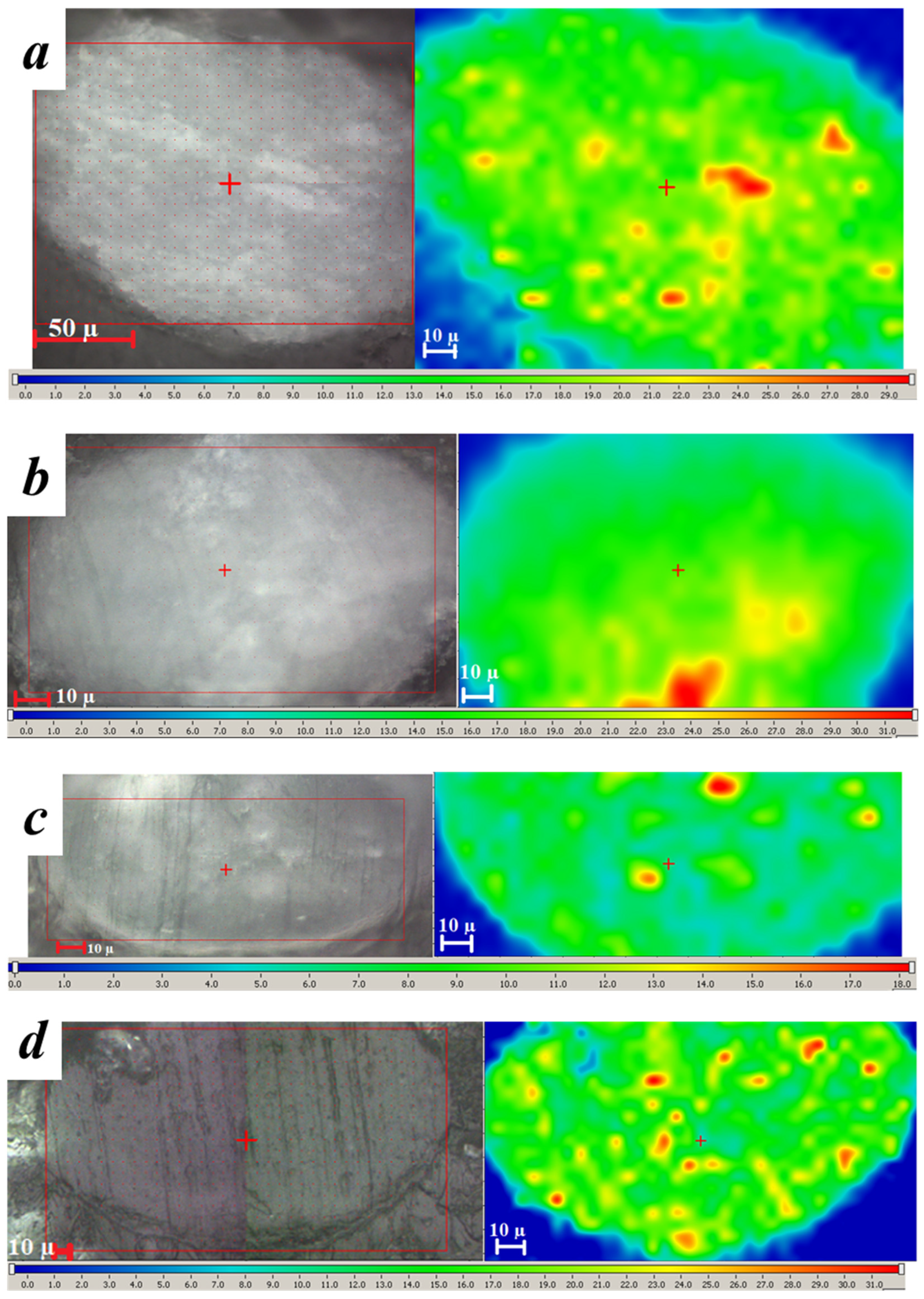
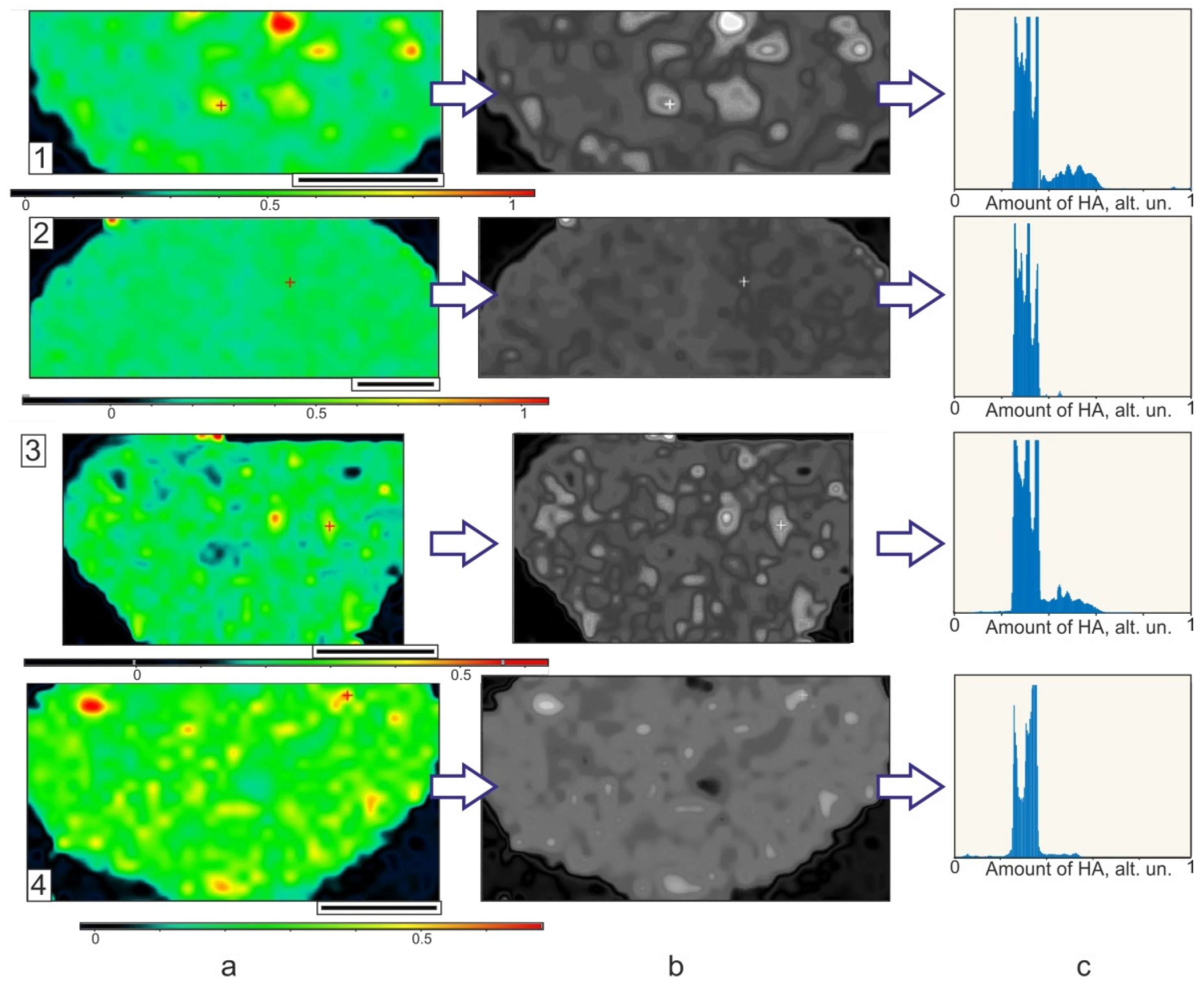
3. Results and Discussion
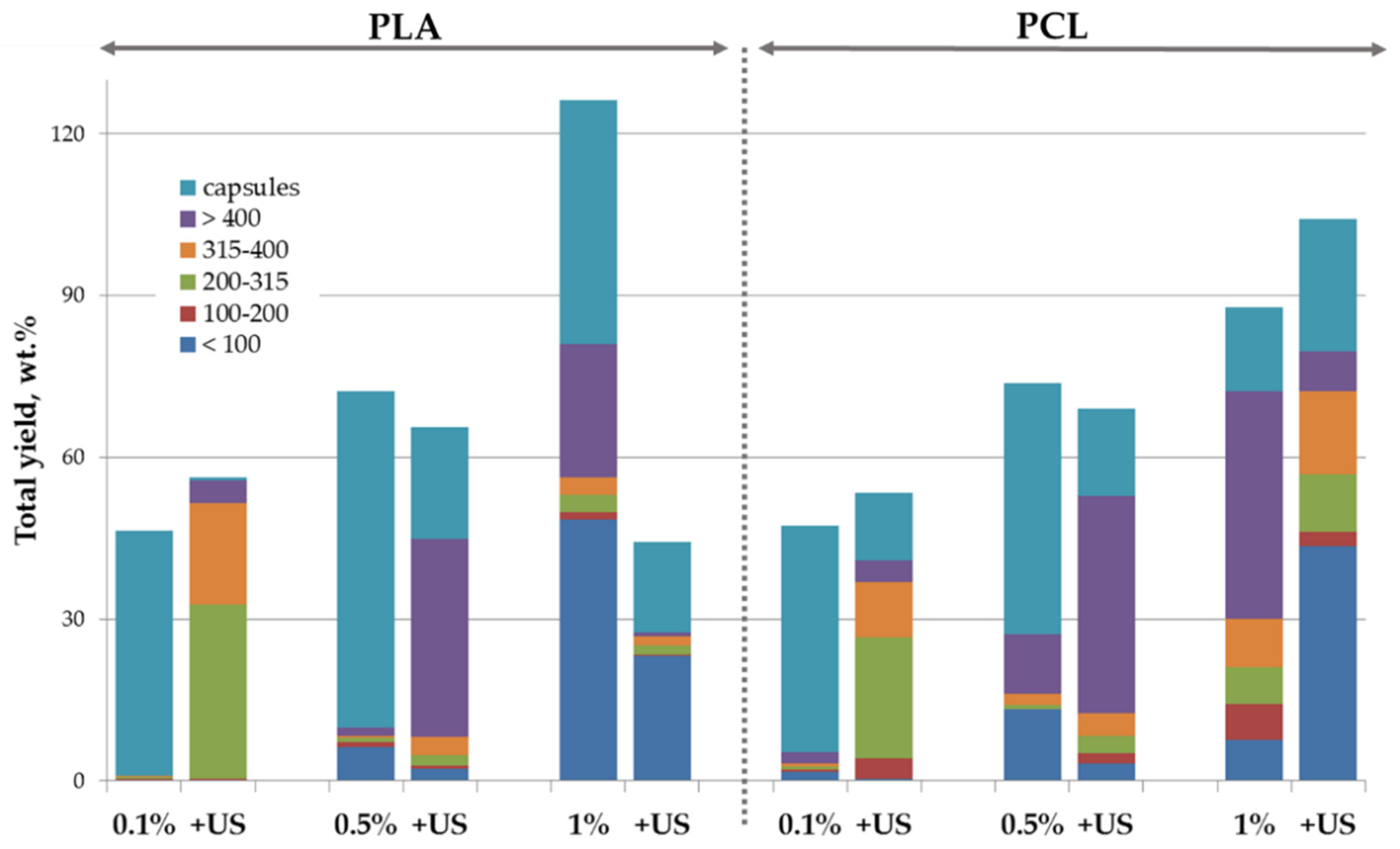
3.1. Core/Shell Microparticles Fabricated Using nHA as the Solid Emulsifier in the Aqueous Phase
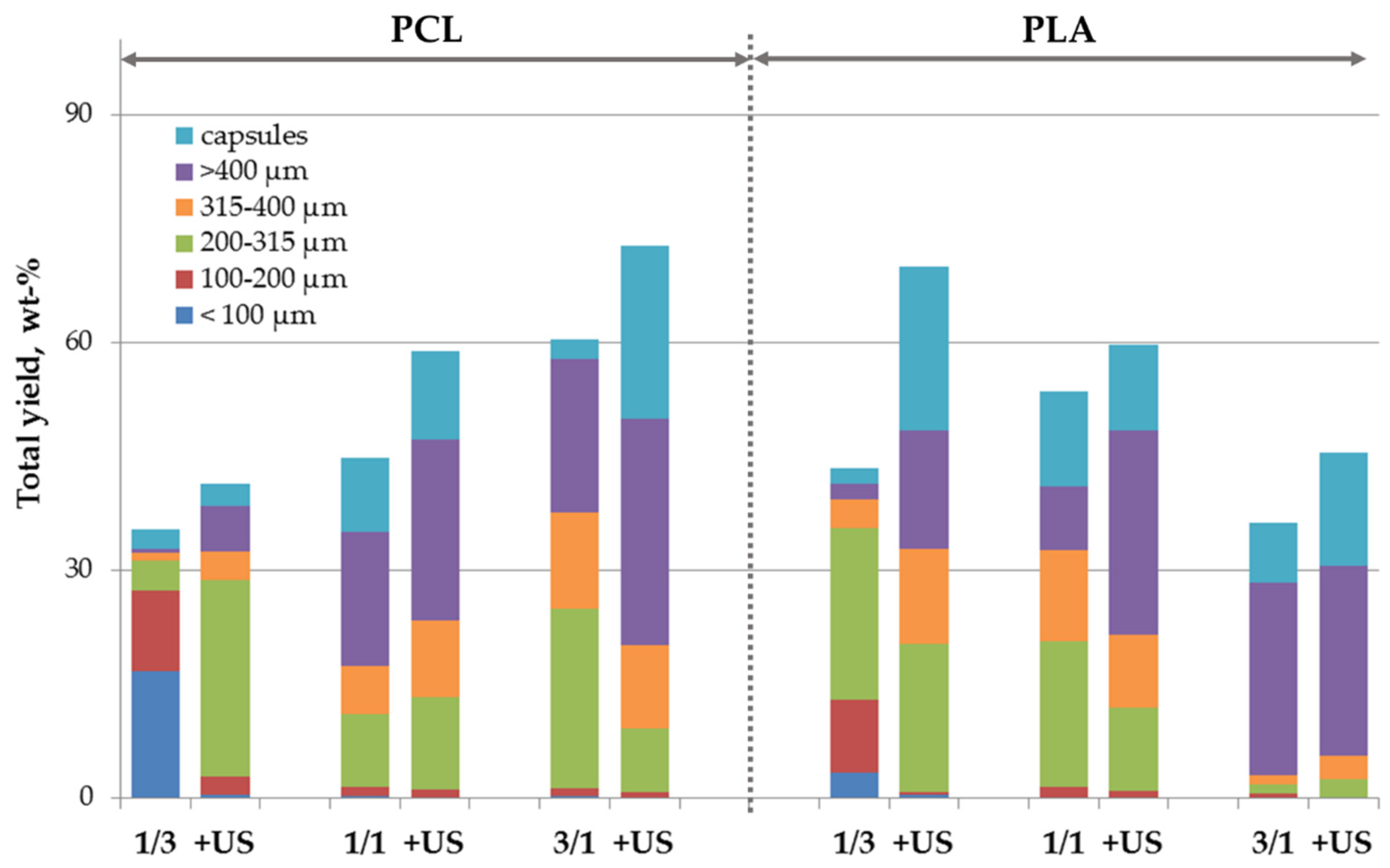
3.2. Polymeric Microparticles Filled with Hydroxyapatite Nanoparticles, i.e., the Addition of nHA within the Oil Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mota, H.P.; Quadrado, R.F.N.; Iglesias, B.A.; Fajardo, A.R. Enhanced Photocatalytic Degradation of Organic Pollutants Mediated by Zn(II)-Porphyrin/Poly(Acrylic Acid) Hybrid Microparticles. Appl. Catal. B Environ. 2020, 277, 119208. [Google Scholar] [CrossRef]
- Kudryavtseva, V.L.; Zhao, L.; Tverdokhlebov, S.I.; Sukhorukov, G.B. Fabrication of PLA/CaCO3 Hybrid Micro-Particles as Carriers for Water-Soluble Bioactive Molecules. Colloids Surf. B Biointerfaces 2017, 157, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bachhuka, A.; Christo, S.N.; Cavallaro, A.; Diener, K.R.; Mierczynska, A.; Smith, L.E.; Marian, R.; Manavis, J.; Hayball, J.D.; Vasilev, K. Hybrid Core/Shell Microparticles and Their Use for Understanding Biological Processes. J. Colloid Interface Sci. 2015, 457, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H. Functional Polymer Microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric Composites for Powder-Based Additive Manufacturing: Materials and Applications. Prog. Polym. Sci. 2019, 91, 141–168. [Google Scholar] [CrossRef]
- Schoubben, A.; Blasi, P.; Giovagnoli, S.; Perioli, L.; Rossi, C.; Ricci, M. Novel Composite Microparticles for Protein Stabilization and Delivery. Eur. J. Pharm. Sci. 2009, 36, 226–234. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Mano, J.F. Polymer-Based Microparticles in Tissue Engineering and Regenerative Medicine. Biotechnol. Prog. 2011, 27, 897–912. [Google Scholar] [CrossRef]
- Dwivedi, P.; Han, S.; Mangrio, F.; Fan, R.; Dwivedi, M.; Zhu, Z.; Huang, F.; Wu, Q.; Khatik, R.; Cohn, D.E.; et al. Engineered Multifunctional Biodegradable Hybrid Microparticles for Paclitaxel Delivery in Cancer Therapy. Mater. Sci. Eng. C 2019, 102, 113–123. [Google Scholar] [CrossRef]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA Nanofibrous Nanocomposite Scaffolds for Bone Tissue Engineering. Acta Biomater. 2008, 5, 305–315. [Google Scholar] [CrossRef]
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Reis, R.L. Materials in Particulate Form for Tissue Engineering. 2. Applications in Bone. J. Tissue Eng. Regen. Med. 2007, 1, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jiang, J.; Sun, L.; Gan, Z. Hydrolysis and Biomineralization of Porous PLA Microspheres and Their Influence on Cell Growth. Colloids Surf. B Biointerfaces 2011, 85, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, R.; Xie, C.; Liang, Q.; Xiao, X. Biomimetic Mineralization of Nanocrystalline Hydroxyapatites on Aminated Modified Polylactic Acid Microspheres to Develop a Novel Drug Delivery System for Alendronate. Mater. Sci. Eng. C 2020, 110, 110655. [Google Scholar] [CrossRef]
- Ruphuy, G.; Saralegi, A.; Lopes, J.C.; Dias, M.M.; Barreiro, M.F. Spray Drying as a Viable Process to Produce Nano-Hydroxyapatite/Chitosan (n-HAp/CS) Hybrid Microparticles Mimicking Bone Composition. Adv. Powder Technol. 2016, 27, 575–583. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Wu, Q.; Yang, L.; Cao, S. Hollow Hydroxyapatite/Polyelectrolyte Hybrid Microparticles with Controllable Size, Wall Thickness and Drug Delivery Properties. J. Mater. Chem. B 2015, 3, 8162–8169. [Google Scholar] [CrossRef]
- Aslankoohi, N.; Mequanint, K. Intrinsically Fluorescent Bioactive Glass-Poly(Ester Amide) Hybrid Microparticles for Dual Drug Delivery and Bone Repair. Mater. Sci. Eng. C 2021, 128, 112288. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle Formation and Its Mechanism in Single and Double Emulsion Solvent Evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of Microspheres by the Solvent Evaporation Technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Lee, S.H.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective Laser Sintering of Porous Tissue Engineering Scaffolds from Poly(l-Lactide)/Carbonated Hydroxyapatite Nanocomposite Microspheres. J. Mater. Sci. Mater. Med. 2008, 19, 2535–2540. [Google Scholar] [CrossRef]
- Della Porta, G.; Campardelli, R.; Cricchio, V.; Oliva, F.; Maffulli, N.; Reverchon, E. Injectable PLGA/Hydroxyapatite/Chitosan Microcapsules Produced by Supercritical Emulsion Extraction Technology: An In Vitro Study on Teriparatide/Gentamicin Controlled Release. J. Pharm. Sci. 2016, 105, 2164–2172. [Google Scholar] [CrossRef]
- Wijerathne, H.M.C.S.; Yan, D.; Zeng, B.; Xie, Y.; Hu, H.; Wickramaratne, M.N.; Han, Y. Effect of Nano-Hydroxyapatite on Protein Adsorption and Cell Adhesion of Poly (Lactic Acid)/Nano-Hydroxyapatite Composite Microspheres. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Krokos, A.; Gazinska, M.; Kryszak, B.; Dzienny, P.; Stepak, B.; Olejarczyk, M.; Gruber, P.; Kwiatkowski, R.; Bondyra, A.; Antonczak, A. Comparison of Thermal, Structural and Morphological Properties of Poly (L-Lactide) and Poly (L-Lactide)/Hydroxyapatite Microspheres for Laser Sintering Processes. Polimery 2020, 65, 605–612. [Google Scholar] [CrossRef]
- Nagata, F.; Miyajima, T.; Yokogawa, Y. Surfactant-Free Preparation of Poly (Lactic Acid)/Hydroxyapatite Microspheres. Chem. Lett. 2003, 32, 784–785. [Google Scholar] [CrossRef]
- Nagata, F.; Miyajima, T.; Yokogawa, Y. A Method to Fabricate Hydroxyapatite/Poly (Lactic Acid) Microspheres Intended for Biomedical Application. J. Eur. Ceram. Soc. 2006, 26, 533–535. [Google Scholar] [CrossRef]
- Nagata, F.; Miyajima, T.; Kato, K. Preparation of Phylloquinone-Loaded Poly (Lactic Acid)/Hydroxyapatite Core–Shell Particles and Their Drug Release Behavior. Adv. Powder Technol. 2016, 27, 903–907. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Nishimura, T.; Sugimoto, T.; Maeda, H.; Hamasaki, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite-Coated Poly (ε-Caprolactone) Microspheres Fabricated via a Pickering Emulsion Route: Effect of Fabrication Parameters on Diameter and Chemical Composition. Compos. Interfaces 2013, 20, 45–56. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Nishimura, T.; Maeda, H.; Sugimoto, T.; Hamasaki, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite-Armored Poly (ε-Caprolactone) Microspheres and Hydroxyapatite Microcapsules Fabricated via a Pickering Emulsion Route. J. Colloid Interface Sci. 2012, 374, 1–8. [Google Scholar] [CrossRef]
- Chernenok, T.V.; Kilyashova, L.A.; Israilova, N.N.; Popyrina, T.N.; Demina, T.S. Biodegradable Microparticles for Bone Tissue Regeneration Based on Polylactide and Hydroxyapatite Nanoparticles. Nanobiotechnol. Rep. 2021, 16, 505–509. [Google Scholar] [CrossRef]
- Tham, C.Y.; Chow, W.S. Poly (Lactic Acid) Microparticles with Controllable Morphology by Hydroxyapatite Stabilized Pickering Emulsions: Effect of PH, Salt, and Amphiphilic Agents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 275–285. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Demina, T.; Kilyashova, L.; Popyrina, T.; Svidchenko, E.; Bhuniya, S.; Akopova, T.; Grandfils, C. Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication. Polymers 2021, 13, 3045. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M. Encapsulation and Release of Biomolecules from Ca–P/PHBV Nanocomposite Microspheres and Three-Dimensional Scaffolds Fabricated by Selective Laser Sintering. Polym. Degrad. Stab. 2010, 95, 1655–1664. [Google Scholar] [CrossRef]
- Yan, D.; Zeng, B.; Han, Y.; Dai, H.; Liu, J.; Sun, Y.; Li, F. Preparation and Laser Powder Bed Fusion of Composite Microspheres Consisting of Poly (Lactic Acid) and Nano-Hydroxyapatite. Addit. Manuf. 2020, 34, 101305. [Google Scholar] [CrossRef]
- Demina, T.S.; Sevrin, C.; Kapchiekue, C.; Akopova, T.A.; Grandfils, C. Chitosan-g-polyester Microspheres: Effect of Length and Composition of Grafted Chains. Macromol. Mater. Eng. 2019, 304, 1900203. [Google Scholar] [CrossRef]
- Neuendorf, R.E.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Adhesion between Biodegradable Polymers and Hydroxyapatite: Relevance to Synthetic Bone-like Materials and Tissue Engineering Scaffolds. Acta Biomater. 2008, 4, 1288–1296. [Google Scholar] [CrossRef]
- Okada, M.; Maeda, H.; Fujii, S.; Nakamura, Y.; Furuzono, T. Formation of Pickering Emulsions Stabilized via Interaction between Nanoparticles Dispersed in Aqueous Phase and Polymer End Groups Dissolved in Oil Phase. Langmuir 2012, 28, 9405–9412. [Google Scholar] [CrossRef]
- Demina, T.S.; Drozdova, M.G.; Sevrin, C.; Compère, P.; Akopova, T.A.; Markvicheva, E.; Grandfils, C. Biodegradable Cell Microcarriers Based on Chitosan/Polyester Graft-Copolymers. Molecules 2020, 25, 1949. [Google Scholar] [CrossRef]
- Fu, H.; Rahaman, M.N.; Day, D.E.; Brown, R.F. Hollow Hydroxyapatite Microspheres as a Device for Controlled Delivery of Proteins. J. Mater. Sci. Mater. Med. 2011, 22, 579–591. [Google Scholar] [CrossRef]
- Fu, H.; Rahaman, M.N.; Brown, R.F.; Day, D.E. Evaluation of BSA Protein Release from Hollow Hydroxyapatite Microspheres into PEG Hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2245–2250. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Sawa, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite Nanoparticles as Particulate Emulsifier: Fabrication of Hydroxyapatite-Coated Biodegradable Microspheres. Langmuir 2009, 25, 9759–9766. [Google Scholar] [CrossRef]
- Demina, T.S.; Popyrina, T.N.; Minaeva, E.D.; Dulyasova, A.A.; Minaeva, S.A.; Tilkin, R.; Yusupov, V.I.; Grandfils, C.; Akopova, T.A.; Minaev, N.V.; et al. Polylactide Microparticles Stabilized by Chitosan Graft-Copolymer as Building Blocks for Scaffold Fabrication via Surface-Selective Laser Sintering. J. Mater. Res. 2022, 37, 933–942. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minaev, N.V.; Minaeva, S.A.; Sherstneva, A.A.; Chernenok, T.V.; Sedova, Y.K.; Minaeva, E.D.; Yusupov, V.I.; Akopova, T.A.; Timashev, P.S.; Demina, T.S. Controlled Structure of Polyester/Hydroxyapatite Microparticles Fabricated via Pickering Emulsion Approach. Polymers 2022, 14, 4309. https://doi.org/10.3390/polym14204309
Minaev NV, Minaeva SA, Sherstneva AA, Chernenok TV, Sedova YK, Minaeva ED, Yusupov VI, Akopova TA, Timashev PS, Demina TS. Controlled Structure of Polyester/Hydroxyapatite Microparticles Fabricated via Pickering Emulsion Approach. Polymers. 2022; 14(20):4309. https://doi.org/10.3390/polym14204309
Chicago/Turabian StyleMinaev, Nikita V., Svetlana A. Minaeva, Anastasia A. Sherstneva, Tatiana V. Chernenok, Yulia K. Sedova, Ekaterina D. Minaeva, Vladimir I. Yusupov, Tatiana A. Akopova, Peter S. Timashev, and Tatiana S. Demina. 2022. "Controlled Structure of Polyester/Hydroxyapatite Microparticles Fabricated via Pickering Emulsion Approach" Polymers 14, no. 20: 4309. https://doi.org/10.3390/polym14204309
APA StyleMinaev, N. V., Minaeva, S. A., Sherstneva, A. A., Chernenok, T. V., Sedova, Y. K., Minaeva, E. D., Yusupov, V. I., Akopova, T. A., Timashev, P. S., & Demina, T. S. (2022). Controlled Structure of Polyester/Hydroxyapatite Microparticles Fabricated via Pickering Emulsion Approach. Polymers, 14(20), 4309. https://doi.org/10.3390/polym14204309










