Synergistic Membrane Disturbance Improves the Antibacterial Performance of Polymyxin B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
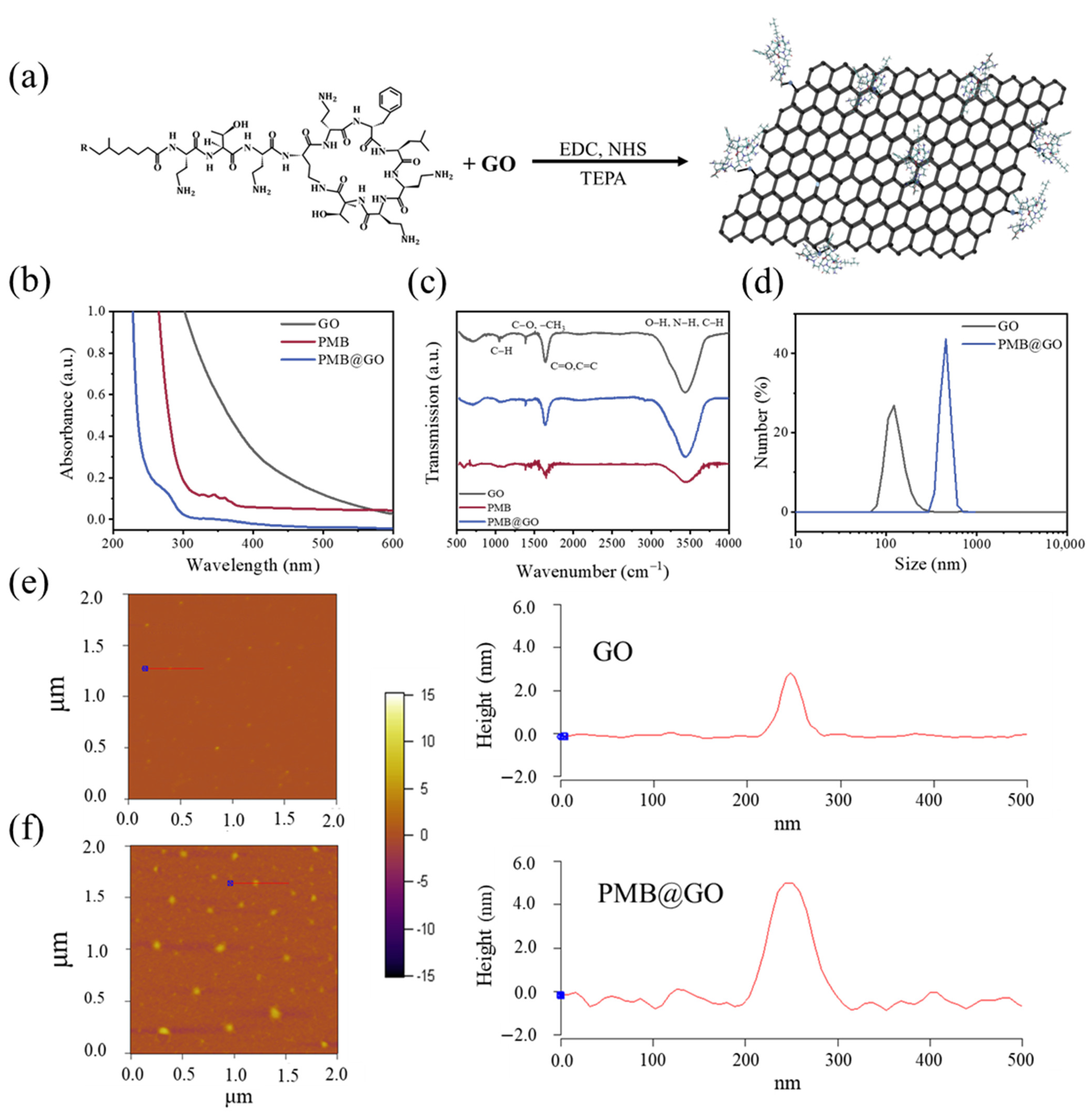
2.2. Synthesis and Characterizations
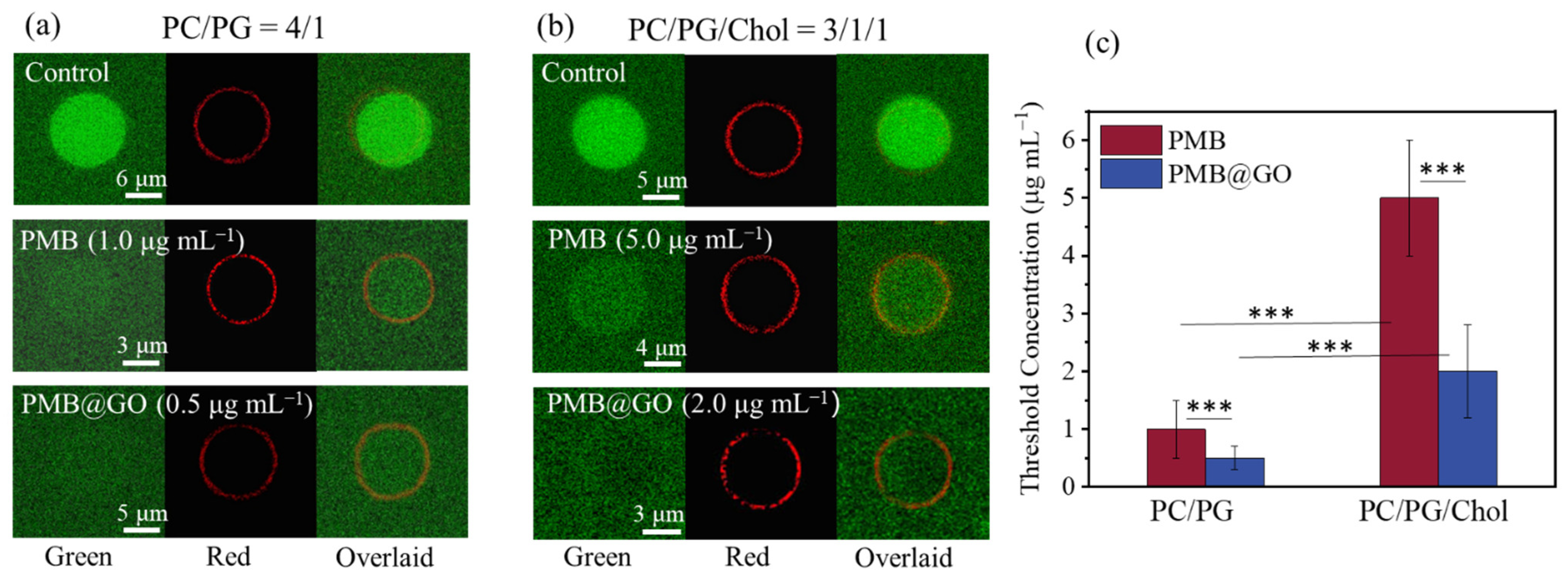
2.3. Membrane Activity Test
2.4. Antimicrobial Activity
2.5. Morphological Characterization of Drug-Treated Bacteria
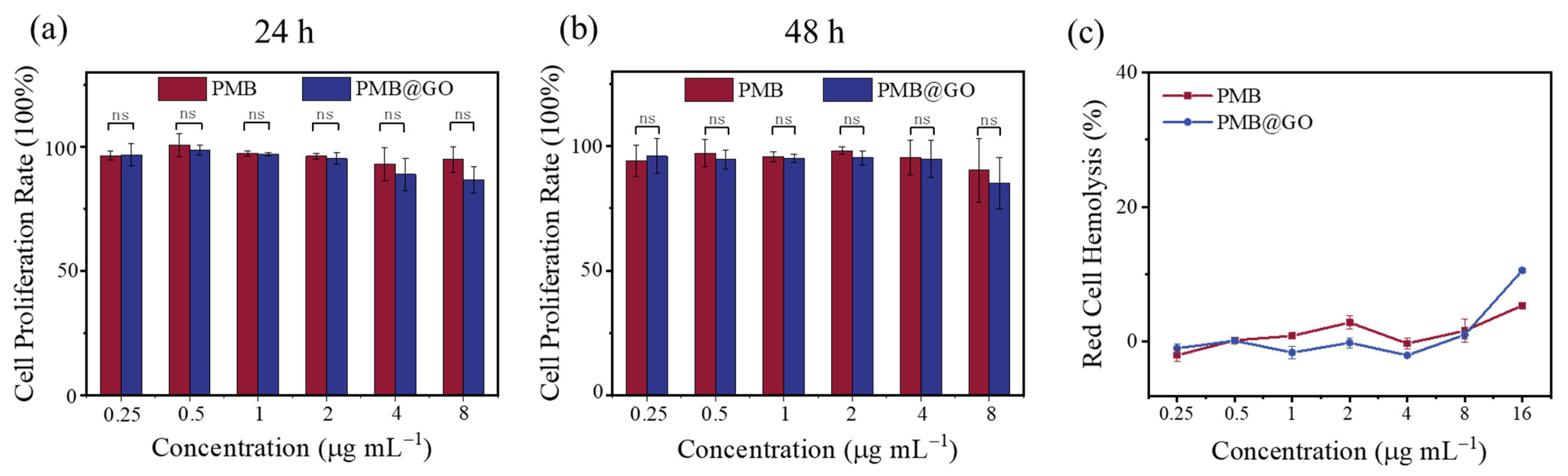
2.6. Cytotoxicity Assay
2.7. Hemolysis Assay
2.8. Simulation Models and Methods
3. Results and Discussion
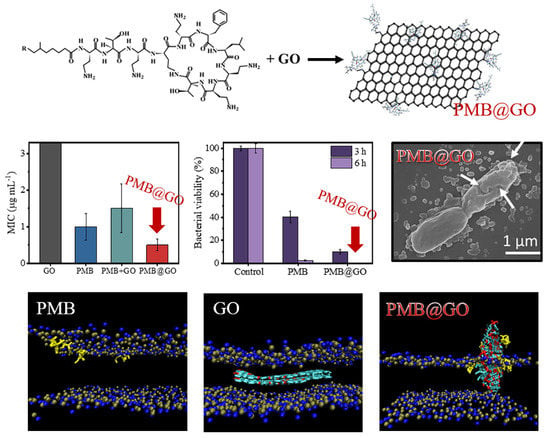
3.1. Characterization of PMB@GO
3.2. Growth Inhibition and Membrane Destabilization of PMB@GO over Bacteria
3.3. Hemolysis and Mammalian Cytotoxicity of PMB@GO Nanocomposite
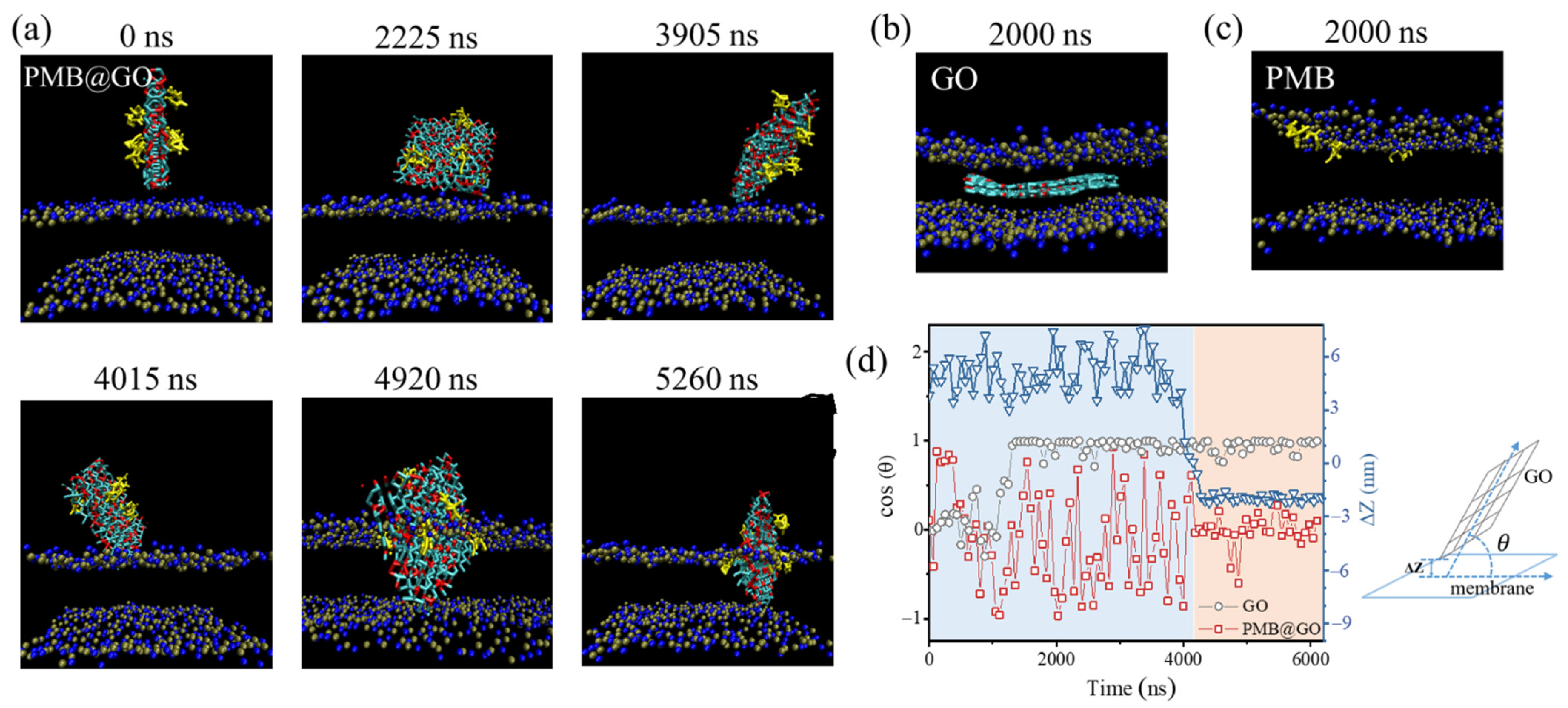
3.4. Analysis of the Physical Membrane-Disturbance Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.A.; Sill, M.; Laing, N.M.; Johnson, J.L.; Hoban, D.J.; Zhanel, G.G.; Grp, N. Molecular epidemiology of urinary tract isolates of vancomycin-resistant Enterococcus faecium from North America. Int. J. Antimicrob. Agents 2006, 27, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [Green Version]
- Berglund, N.A.; Piggot, T.J.; Jefferies, D.; Sessions, R.B.; Bond, P.J.; Khalid, S. Interaction of the antimicrobial peptide Polymyxin B1 with both membranes of E. coli: A molecular dynamics study. PLoS Comput. Biol. 2015, 11, e1004180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Lu, J.; Han, M.L.; Jiang, X.K.; Azad, M.A.K.; Patil, N.A.; Lin, Y.W.; Zhao, J.X.; Hu, Y.; Yu, H.H.; et al. Polymyxins bind to the cell surface of unculturable acinetobacter baumannii and cause unique dependent resistance. Adv. Sci 2020, 7, 2000704. [Google Scholar] [CrossRef]
- Vaara, M. New polymyxin derivatives that display improved efficacy in animal infection models as compared to polymyxin B and colistin. Med. Res. Rev. 2018, 38, 1661–1673. [Google Scholar] [CrossRef]
- Khondkerg, A.; Dhaliwal, A.K.; Saem, S.; Mahmood, A.; Fradin, C.; Moran-Mirabal, J.; Rheinstadte, M.C. Membrane charge and lipid packing determine polymyxin-induced membrane damage. Commun. Biol 2019, 2, 11. [Google Scholar]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-Activity relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Marshall, R.L.; Matheson, S.; Savage, P.B. Synthesis of lipid A derivatives and their interactions with polymyxin B and polymyxin B nonapeptide. J. Am. Chem. Soc. 2003, 125, 2426–2435. [Google Scholar] [CrossRef]
- Sun, Y.L.; Deng, Z.X.; Jiang, X.K.; Yuan, B.; Yang, K. Interactions between polymyxin B and various bacterial membrane mimics: A molecular dynamics study. Colloid Surf. B-Biointerfaces 2022, 211, 8. [Google Scholar] [CrossRef]
- Clausell, A.; Garcia-Subirats, M.; Pujol, M.; Busquets, M.A.; Rabanal, F.; Cajal, Y. Gram-negative outer and inner membrane models: Insertion of cyclic cationic lipopeptides. J. Phys. Chem. B 2007, 111, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.D.; Jiang, X.K.; Dou, Y.J.; Zhang, Z.H.; Li, J.; Yuan, B.; Yang, K. Biophysical impact of lipid A modification caused by bobile colistin resistance gene on bacterial outer membranes. J. Phys. Chem. Lett. 2021, 12, 11629–11635. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, S.; Hadjadj, L.; Rolain, J.M.; Olaitan, A.O. Molecular mechanisms of polymyxin resistance: Knowns and unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, X.; Gou, L.; Li, J.; Ma, Y.; Liu, J.; Yang, K.; Yuan, B. Graphene oxide as antibacterial sensitizer: Mechanically disturbed cell membrane for enhanced poration efficiency of melittin. Carbon 2019, 149, 248–256. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, Y.K.; Lu, X.M.; Chen, Z.L.; Liu, J.J.; Zhang, M.L.; Yang, K.; Yuan, B. Membrane perturbation of fullerene and graphene oxide distinguished by pore-forming peptide melittin. Carbon 2021, 180, 67–76. [Google Scholar] [CrossRef]
- Lu, X.M.; Liu, J.J.; Gou, L.; Li, J.L.; Yuan, B.; Yang, K.; Ma, Y.Q. Designing melittin-graphene hybrid complexes for enhanced antibacterial activity. Adv. Healthc. Mater. 2019, 8, 10. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.J.; Lin, Y.Q.; Wang, L.L.; Ma, R.Y.; Zhang, Y.L.; Feng, X.L.; Wu, J.R.; Chen, L.L.; Shao, L.Q. GO-based antibacterial composites: Application and design strategies. Adv. Drug Deliv. Rev. 2021, 178, 31. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Araujo, P.M. Antimicrobial polymer-based assemblies: A review. Int. J. Mol. Sci. 2021, 22, 27. [Google Scholar] [CrossRef]
- Zhang, P.S.; Ouyang, Q.H.; Zhai, T.S.; Sun, J.; Wu, J.; Qin, F.; Zhang, N.; Yue, S.S.; Yang, X.C.; Zhang, H.Y.; et al. An inflammation-targeted nanoparticle with bacteria forced release of polymyxin B for pneumonia therapy. Nanoscale 2022, 14. [Google Scholar] [CrossRef]
- Sandreschi, S.; Piras, A.M.; Batoni, G.; Chiellini, F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine 2016, 11, 1729–1744. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, J.J.; Deng, Z.X.; Wei, L.; Li, W.W.; Dou, Y.J.; Chen, Z.L.; Zhang, C.; Xia, Y.; Wang, J.; et al. A molecular architectural design that promises potent antimicrobial activity against multidrug-resistant pathogens. NPG Asia Mater. 2021, 13, 10. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, W.W.; Chen, Y.; Li, C.M.; Jiang, H.; Wang, X.M. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef]
- Pranantyo, D.; Raju, C.; Si, Z.Y.; Xu, X.F.; Pethe, K.; Kang, E.T.; Chan-Park, M.B. Nontoxic antimicrobial cationic peptide nanoconstructs with bacteria-displaceable polymeric counteranions. Nano Lett. 2021, 21, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, A. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Lozano, N.; Leon, V.; Scaini, D.; Musto, M.; Rago, I.; Severino, F.P.U.; Fabbro, A.; Casalis, L.; Vazquez, E.; et al. Graphene oxide nanosheets reshape synaptic function in cultured brain networks. Acs Nano 2016, 10, 4459–4471. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.S.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.R.; Huang, Q.; Fan, C.H.; Fang, H.P.; et al. Destructive extraction of phospholipids from escherichia coli membranes by graphene nanosheets. Nature Nanotechnology 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Zou, F.M.; Zhou, H.J.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.X.; Zhang, S.M.; Wu, S.J.; Xie, Z.P.; Ling, G.Y.; Kuang, Y.Q.; Yang, Y.L.; Yu, H.N.; Wang, Y.P. Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single-crystals - a new molecular-dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Pristovsek, P.; Kidric, J. Solution structure of polymyxins B and E and effect of binding to lipopolysaccharide: An MMR and molecular modeling study. J. Med. Chem. 1999, 42, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wan, M.W.; Zhang, S.; Gao, L.H.; Fang, W.H. Polymyxin B loosens lipopolysaccharide bilayer but stiffens phospholipid bilayer. Biophys. J. 2020, 118, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wei, Y.J.; Shi, X.H.; Gao, H.J. Cellular entry of graphene nanosheets: The role of thickness, oxidation and surface adsorption. RSC Adv. 2013, 3, 15776–15782. [Google Scholar] [CrossRef] [Green Version]
- Srimal, S.; Surolia, N.; Balasubramanian, S.; Surolia, A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem. J. 1996, 315, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.N.; Ferre, R.; Castanho, M. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef]
- Liu, T.; Gao, L.Q.; Zhao, J.; Cao, Y.; Tang, Y.G.; Miao, P. A polymyxin B-silver nanoparticle colloidal system and the application of lipopolysaccharide analysis. Analyst 2018, 143, 1053–1058. [Google Scholar] [CrossRef]
- Dreher, Y.; Jahnke, K.; Schroter, M.; Gopfrich, K. Light-Triggered cargo loading and division of DNA-Containing giant unilamellar lipid vesicles. Nano Lett. 2021, 21, 5952–5957. [Google Scholar] [CrossRef]
- Sharmin, S.; Islam, M.Z.; Karal, M.A.; Shibly, S.U.; Dohra, H.; Yamazaki, M. Effects of lipid composition on the entry of cell-penetrating peptide oligoarginine into single vesicles. Biochemistry 2016, 55, 4154–4165. [Google Scholar] [CrossRef]
- Lyu, Y.; Xiang, N.; Zhu, X.; Narsimhan, G. Potential of mean force for insertion of antimicrobial peptide melittin into a pore in mixed DOPC/DOPG lipid bilayer by molecular dynamics simulation. J. Chem. Phys. 2017, 146, 12. [Google Scholar] [CrossRef]
- Khondker, A.; Alsop, R.J.; Dhaliwal, A.; Saem, S.; Moran-Mirabal, J.M.; Rheinstadter, M.C. Membrane cholesterol reduces Polymyxin B nephrotoxicity in renal membrane analogs. Biophys. J. 2017, 113, 2016–2028. [Google Scholar] [CrossRef] [Green Version]
- Bouquiaux, C.; Castet, F.; Champagne, B. Unravelling the effects of cholesterol on the second-order nonlinear optical responses of Di-8-ANEPPS dye embedded in phosphatidylcholine lipid bilayers. J. Phys. Chem. B 2021, 125, 10195–10212. [Google Scholar] [CrossRef]
- Alves, E.D.; Colherinhas, G.; Mendanha, S.A. Assessing the DOPC-cholesterol interactions and their influence on fullerene C-60 partitioning in lipid bilayers. J. Mol. Liq. 2020, 315, 11. [Google Scholar] [CrossRef]
- Chakraborty, S.; Doktorova, M.; Molugu, T.R.; Heberle, F.A.; Scott, H.L.; Dzikovski, B.; Nagao, M.; Stingaciu, L.R.; Standaert, R.F.; Barrera, F.N.; et al. How cholesterol stiffens unsaturated lipid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 21896–21905. [Google Scholar] [CrossRef]
- Bennett, W.F.D.; MacCallum, J.L.; Tieleman, D.P. Thermodynamic analysis of the effect of cholesterol on dipalmitoylphosphatidylcholine lipid membranes. J. Am. Chem. Soc. 2009, 131, 1972–1978. [Google Scholar] [CrossRef]
- Jiang, X.K.; Zhang, S.; Azad, M.A.K.; Roberts, K.D.; Wan, L.; Gong, B.; Yang, K.; Yuan, B.; Uddin, H.; Li, J.L.; et al. Structure-Interaction relationship of polymyxins with the membrane of human kidney proximal tubular cells. ACS Infact. Dis. 2020, 6, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Chen, X.K.; Yang, J.J.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Quaternized silicon nanoparticles with polarity-sensitive fluorescence for selectively imaging and killing gram-positive bacteria. Adv. Funct. Mater. 2016, 26, 5958–5970. [Google Scholar] [CrossRef]
- Wu, Y.H.; Long, Y.B.; Li, Q.L.; Han, S.Y.; Ma, J.B.; Yang, Y.W.; Gaot, H. Layer-by-Layer (LBL) self-assembled biohybrid nanomaterials for efficient antibacterial applications. ACS Appl. Mater. Interfaces 2015, 7, 17255–17263. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, C.; Lu, X.; Sun, S.; Yang, K.; Yuan, B. Synergistic Membrane Disturbance Improves the Antibacterial Performance of Polymyxin B. Polymers 2022, 14, 4316. https://doi.org/10.3390/polym14204316
Li W, Zhang C, Lu X, Sun S, Yang K, Yuan B. Synergistic Membrane Disturbance Improves the Antibacterial Performance of Polymyxin B. Polymers. 2022; 14(20):4316. https://doi.org/10.3390/polym14204316
Chicago/Turabian StyleLi, Wenwen, Che Zhang, Xuemei Lu, Shuqing Sun, Kai Yang, and Bing Yuan. 2022. "Synergistic Membrane Disturbance Improves the Antibacterial Performance of Polymyxin B" Polymers 14, no. 20: 4316. https://doi.org/10.3390/polym14204316
APA StyleLi, W., Zhang, C., Lu, X., Sun, S., Yang, K., & Yuan, B. (2022). Synergistic Membrane Disturbance Improves the Antibacterial Performance of Polymyxin B. Polymers, 14(20), 4316. https://doi.org/10.3390/polym14204316