Molecular Organization in Exponentially Growing Multilayer Thin Films Assembled with Polyelectrolytes and Clay
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
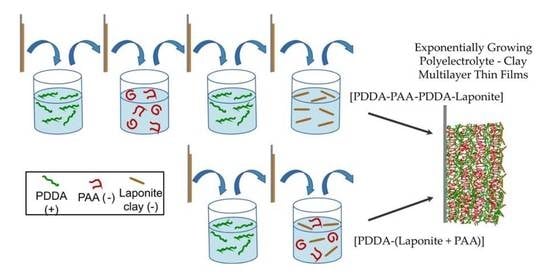
3.1. LbL Assembly Using Polyelectrolyte and Clay Alternately as Anionic Deposition Solution: Polycation-Polyanion-Polycation-Clay
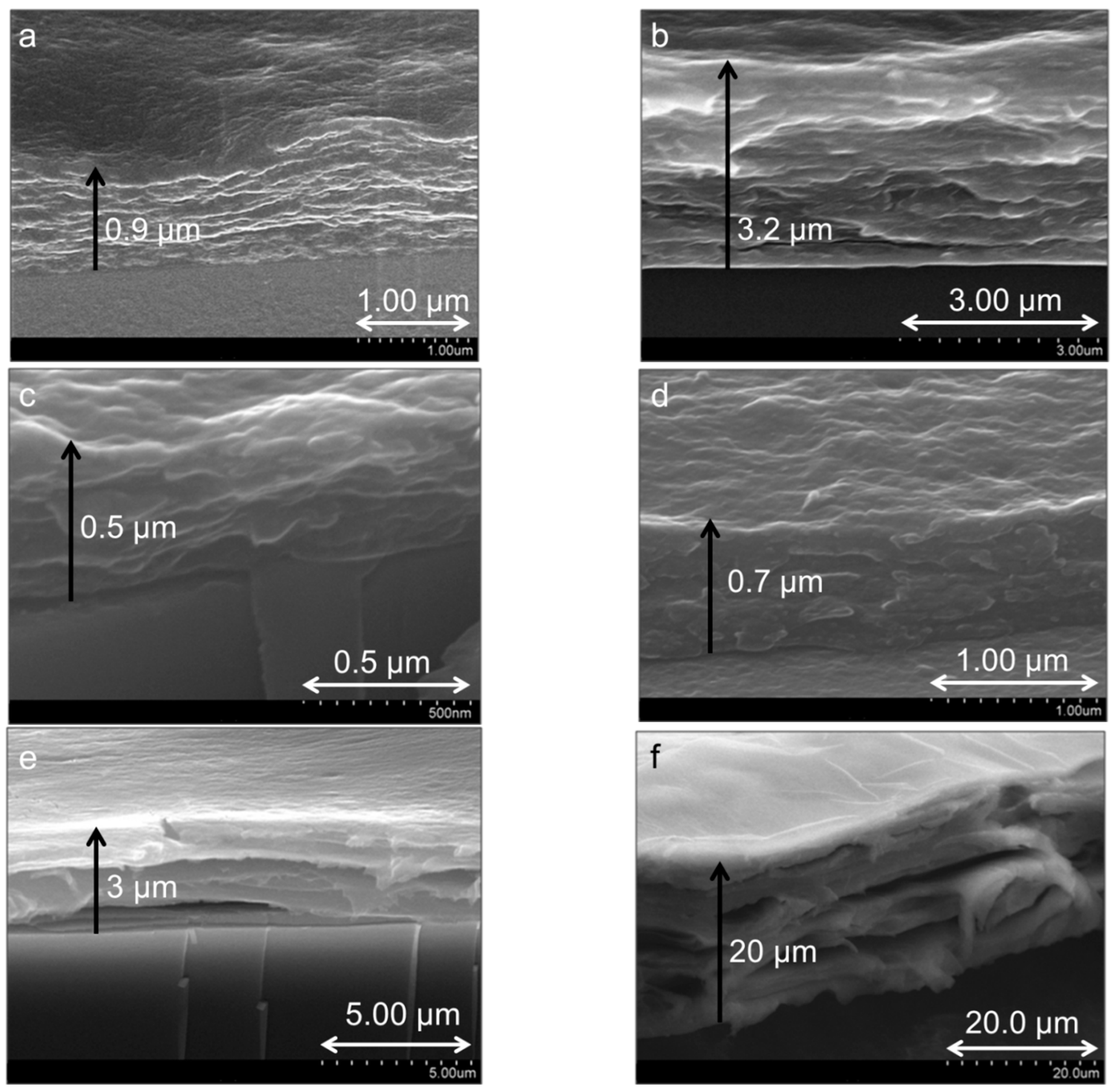
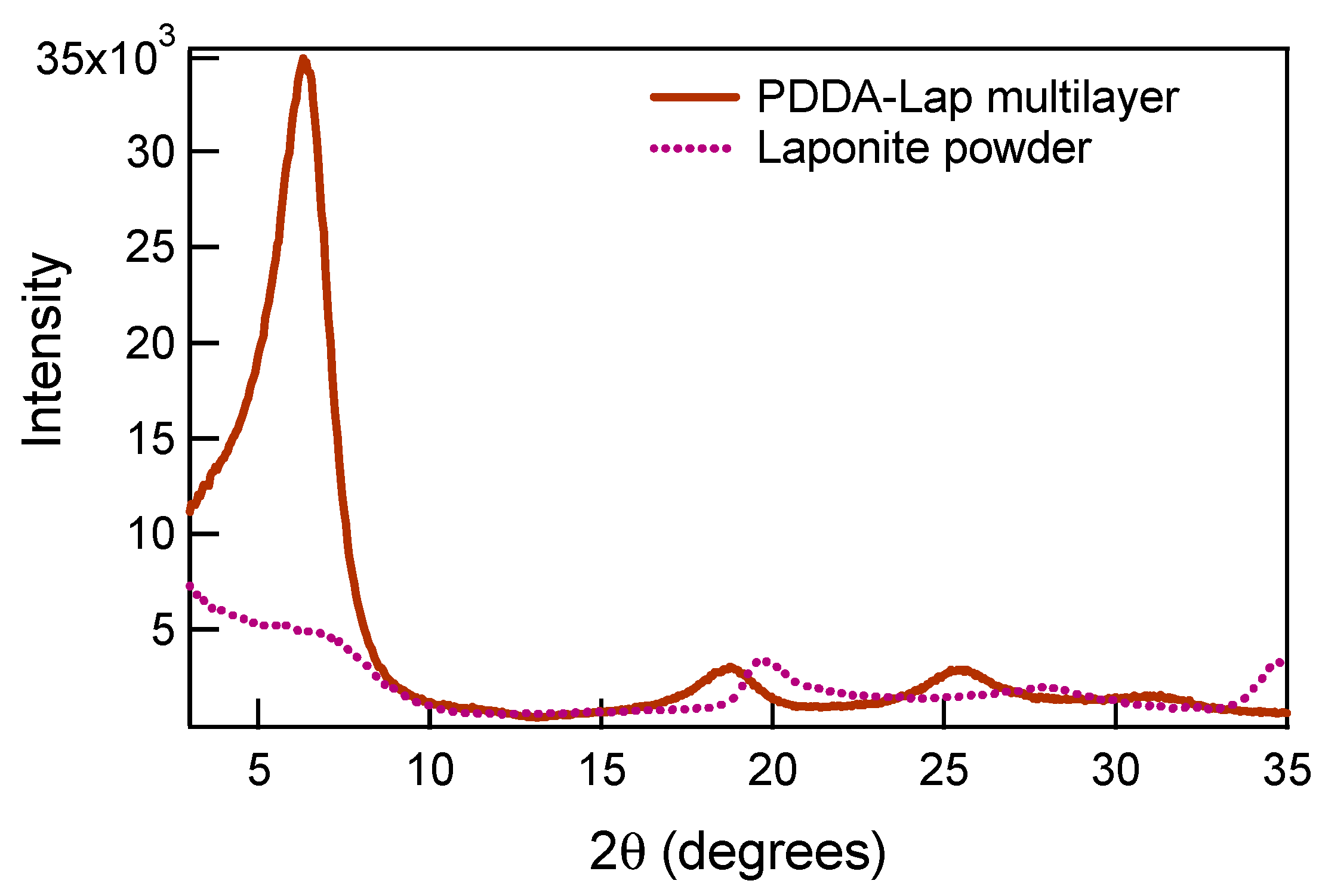
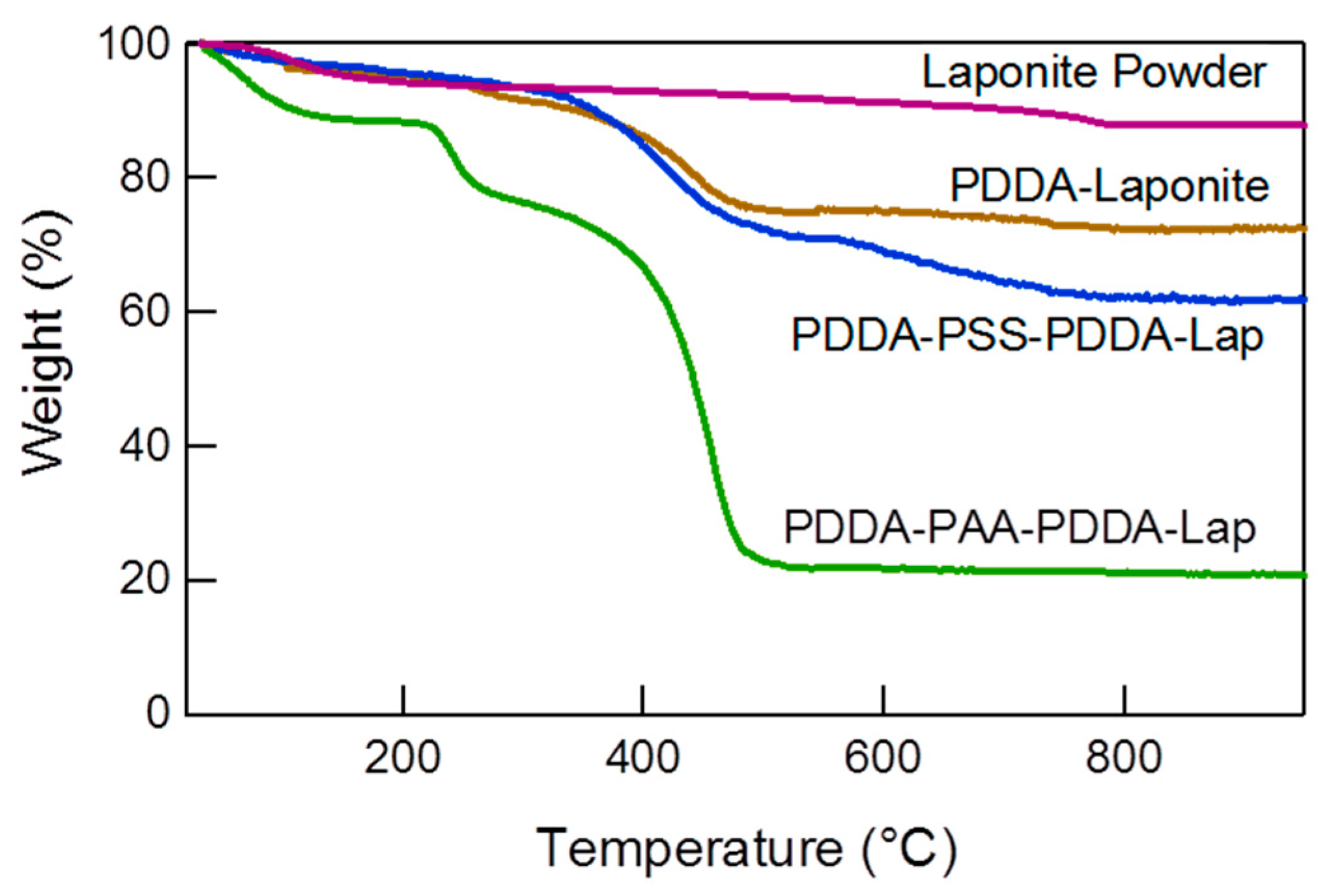
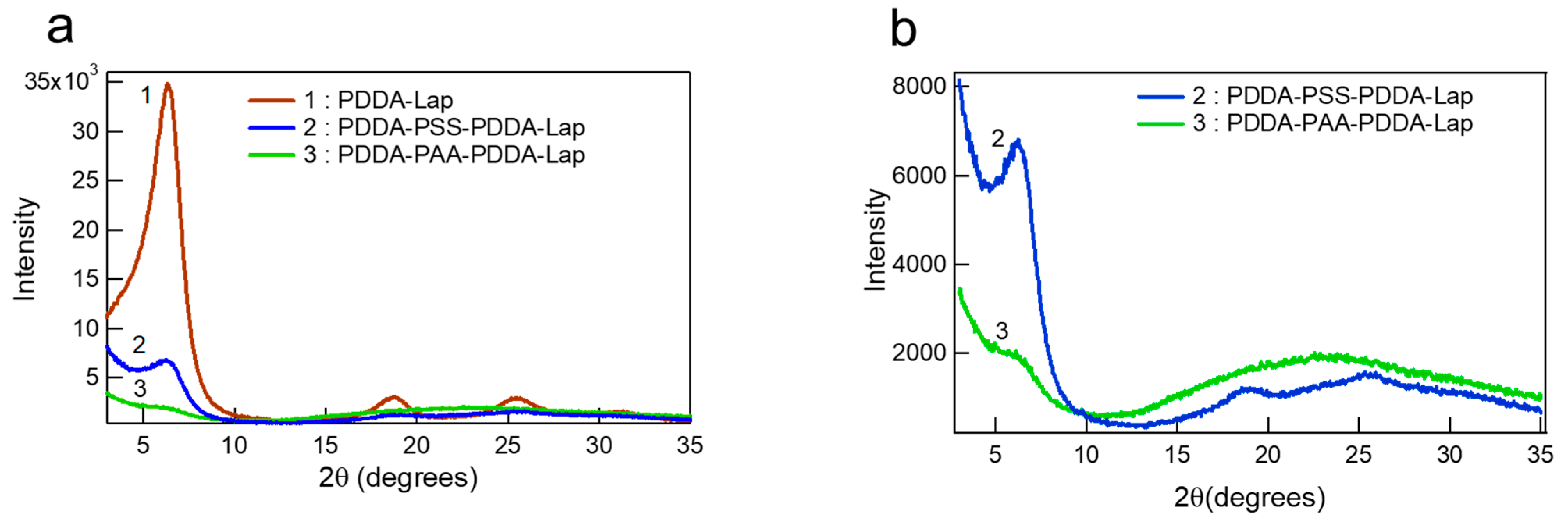
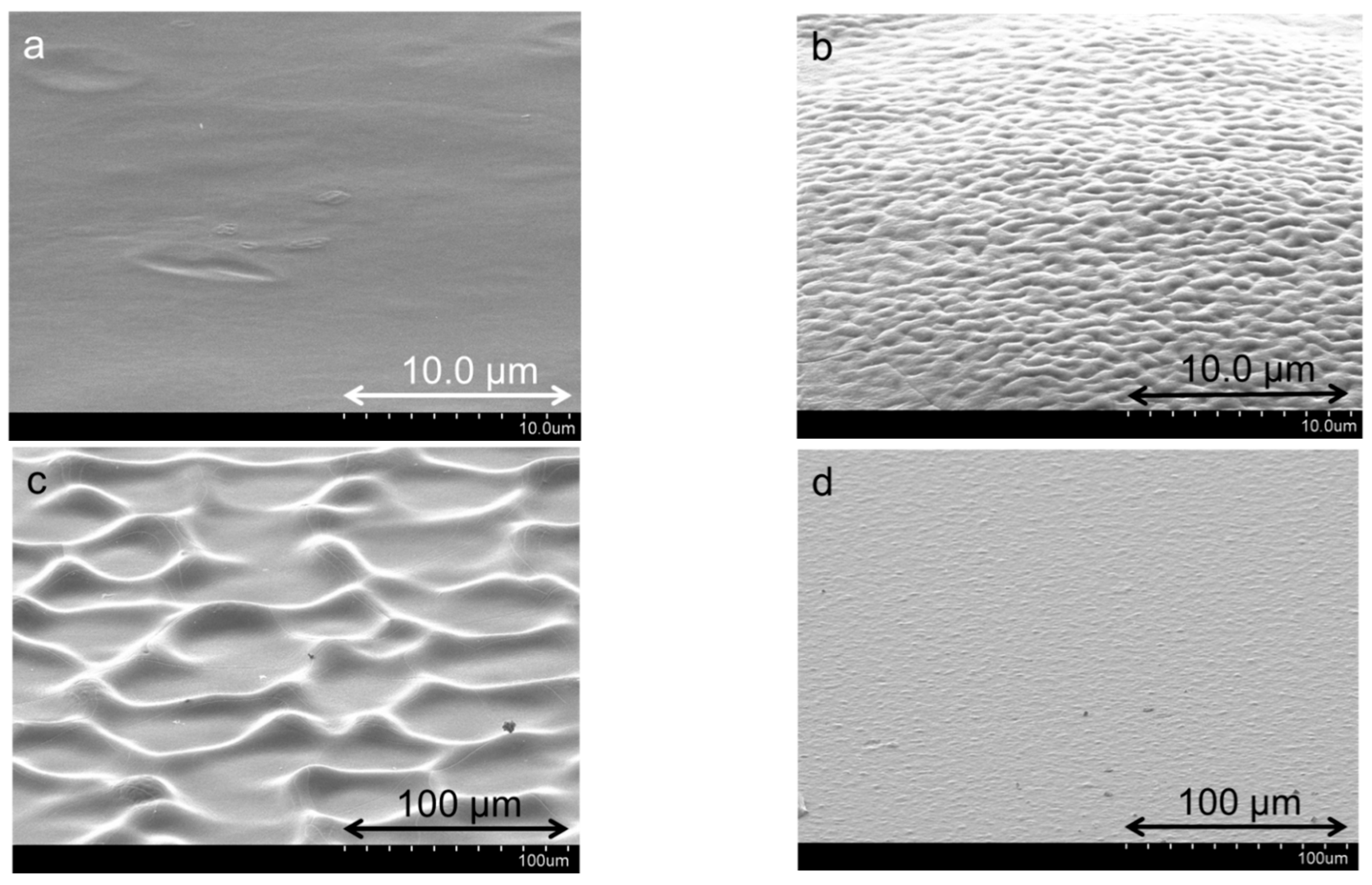
3.1.1. Structural Comparison between Polycation-Clay and Polycation-Polyanion-Polycation-Clay Systems
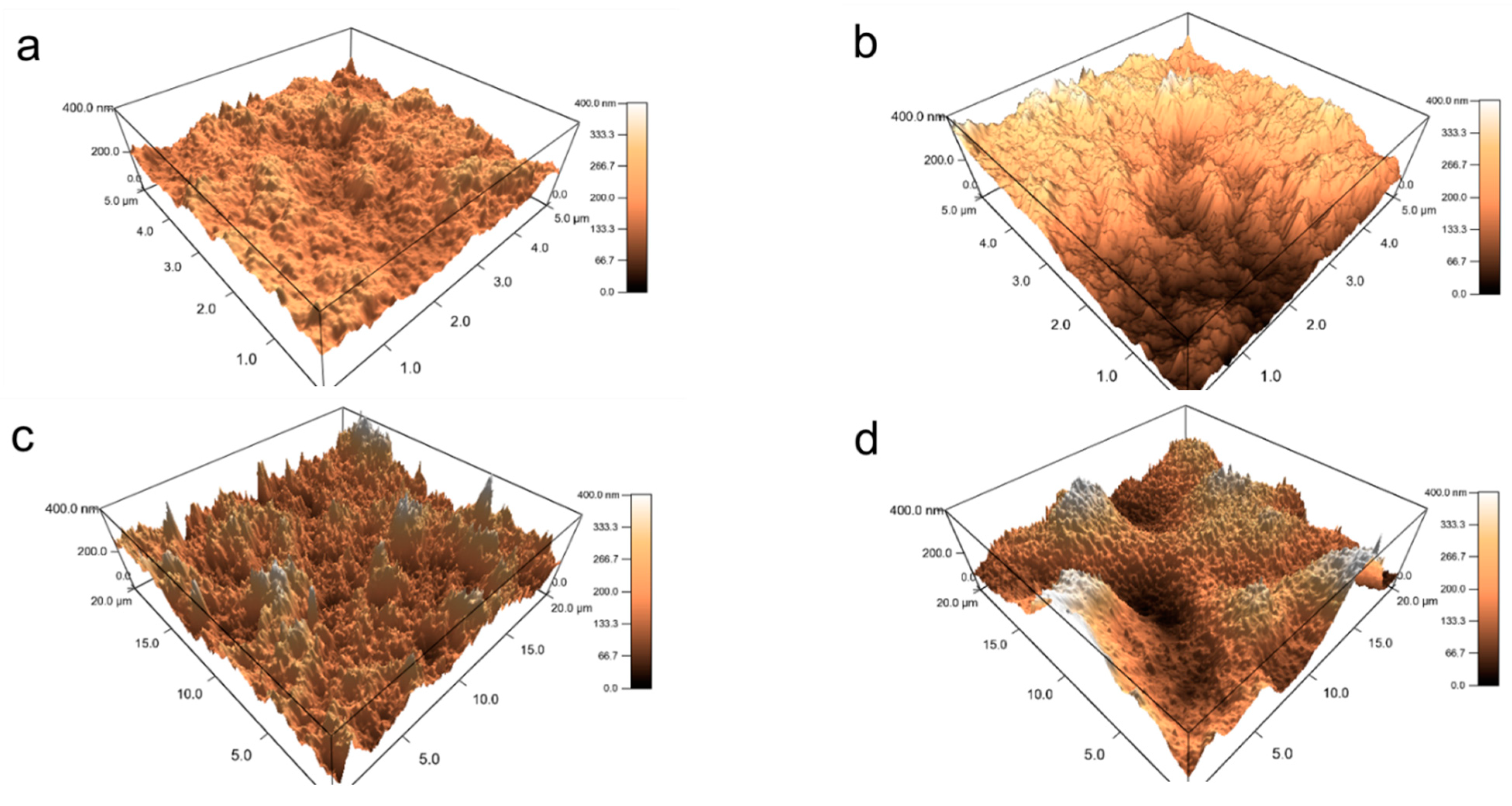
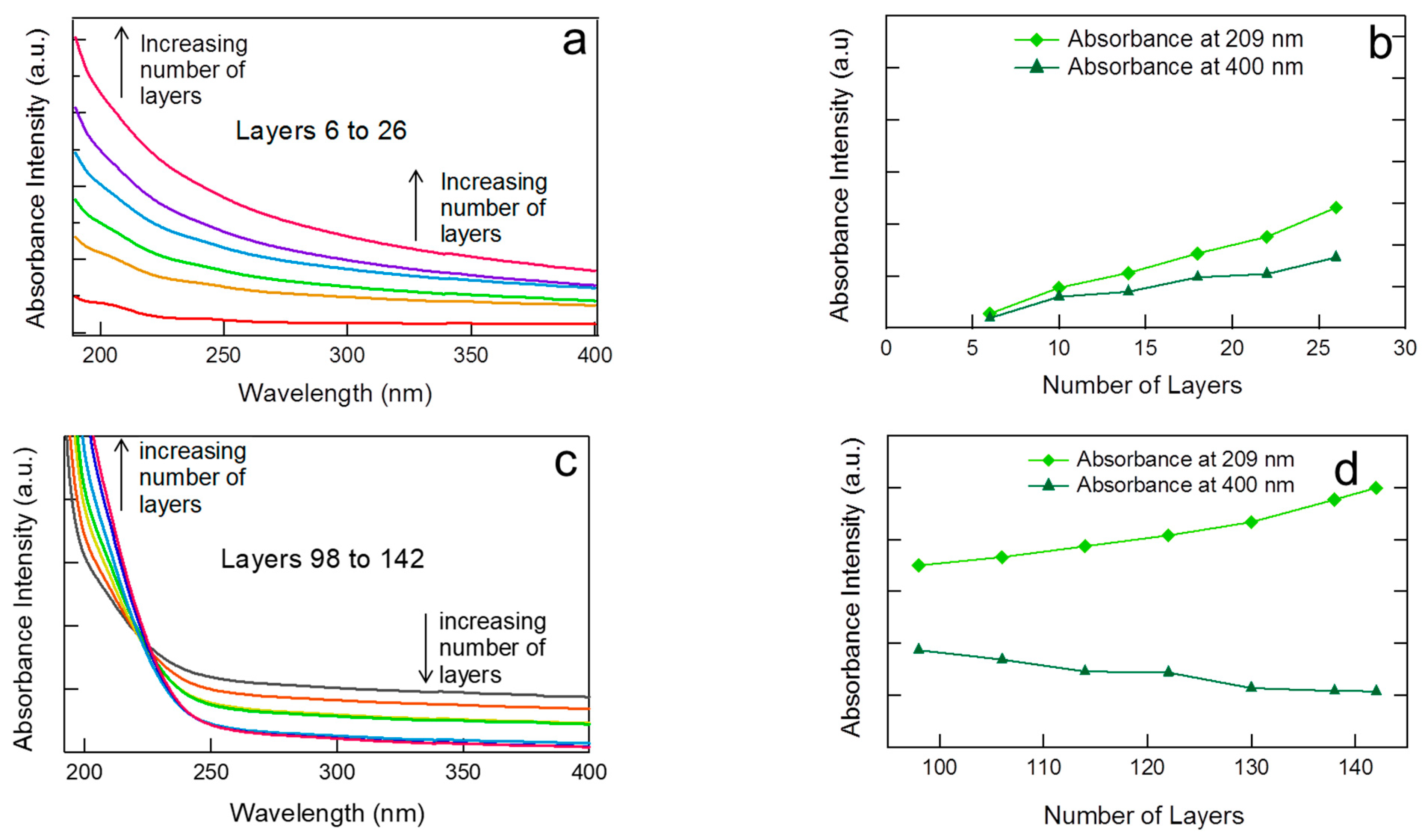
3.1.2. Internal Reorganization and Multilayer Growth Mechanism in (PDDA-PAA-PDDA-Laponite)n
3.2. LbL Assembly Using Polyelectrolyte and Clay in Combination as a ‘Single’ Deposition Solution: (Polycation-(Polyanion + Clay))n
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Das, B.P.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Humood, M.; Chowdhury, S.; Song, Y.; Tzeng, P.; Grunlan, J.C.; Polycarpou, A.A. Nanomechanical Behavior of High Gas Barrier Multilayer Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 11128–11138. [Google Scholar] [CrossRef]
- Kim, S.; Byeon, C.C.; Kim, S.Y. Electrochemical Response of Clay/Polyelectrolyte Composite Barrier Coatings. Coatings 2020, 10, 1173. [Google Scholar] [CrossRef]
- Suarez-Martinez, P.C.; Robinson, J.; An, H.; Nahas, R.C.; Cinoman, D.; Lutkenhaus, J.L. Spray-On Polymer-Clay Multilayers as a Superior Anticorrosion Metal Pretreatment. Macromol. Mater. Eng. 2017, 302, 1600552. [Google Scholar] [CrossRef]
- Koerner, C.M.; Hopkinson, D.P.; Ziomek-Moroz, M.E.; Rodriguez, A.; Xiang, F. Environmentally Friendly Tannic Acid Multilayer Coating for Reducing Corrosion of Carbon Steel. Ind. Eng. Chem. Res. 2021, 60, 243–250. [Google Scholar] [CrossRef]
- Rajesh, S.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Nanofiber multilayer membranes with tailored nanochannels prepared by molecular layer-by-layer assembly for high throughput separation. J. Mater. Chem. A 2017, 5, 4616–4628. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Ordered assembly of hybrid room-temperature phosphorescence thin films showing polarized emission and the sensing of VOCs. Chem. Commun. 2017, 53, 5408–5411. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Yu, J.; Lee, I. Role of clays in fouling-resistant clay-embedded polyelectrolyte multilayer membranes for wastewater effluent treatment. Sep. Sci. Technol. 2017, 52, 2108–2119. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Huh, S.-H.; Ullah, Z.; Pan, Y.-T.; Churchill, D.G.; Koo, B.H. LBL generated fire retardant nanocomposites on cotton fabric using cationized starch-clay-nanoparticles matrix. Carbohydr. Polym. 2021, 274, 118626. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Gill, R.; Arshad, M.; Siddiqi, H.M.; Qureshi, S.S. Layer-by-layer fabrication of nacre inspired epoxy/MMT multilayered composites. J. Appl. Polym. Sci. 2018, 135, 46079. [Google Scholar] [CrossRef]
- Batasheva, S.; Fakhrullin, R. Sequence Does Not Matter: The Biomedical Applications of DNA-Based Coatings and Cores. Int. J. Mol. Sci. 2021, 22, 12884. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, R.; Feng, S.; Wang, J. Influence of laponite on the drug loading and release performance of LbL polyurethane/poly(acrylic acid) multilayers. J. Appl. Polym. Sci. 2019, 136, 47348. [Google Scholar] [CrossRef]
- Kleinfeld, E.R.; Ferguson, G.S. Stepwise formation of multilayered nanostructural films from macromolecular precursors. Science 1994, 265, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Rubner, M. Materials science: Synthetic sea shell. Nature 2003, 423, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Kotov, N.A.; Magonov, S.; Ozturk, B. Nanostructured artificial nacre. Nat. Mater. 2003, 2, 413–418. [Google Scholar] [CrossRef]
- Ou, R.Q.; Zhang, J.G.; Deng, Y.L.; Ragauskas, A.J. Polymer clay self-assembly complexes on paper. J. Appl. Polym. Sci. 2007, 105, 1987–1992. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef]
- Kotov, N.A.; Magonov, S.; Tropsha, E. Layer-by-layer self-assembly of alumosilicate-polyelectrolyte composites: Mechanism of deposition, crack resistance, and perspectives for novel membrane materials. Chem. Mater. 1998, 10, 886–895. [Google Scholar] [CrossRef]
- Jang, W.S.; Rawson, I.; Grunlan, J.C. Layer-by-layer assembly of thin film oxygen barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Qin, S.; Song, Y.; Floto, M.E.; Grunlan, J.C. Combined High Stretchability and Gas Barrier in Hydrogen-Bonded Multilayer Nanobrick Wall Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 7903–7907. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G.; Greenwell, H.C.; Boulet, P.; Coveney, P.V.; Bowden, A.A.; Whiting, A. A critical appraisal of polymer-clay nanocomposites. Chem. Soc. Rev. 2008, 37, 568–594. [Google Scholar] [CrossRef] [PubMed]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavalle, P.; Picart, C.; Mutterer, J.; Gergely, C.; Reiss, H.; Voegel, J.C.; Senger, B.; Schaaf, P. Modeling the buildup of polyelectrolyte multilayer films having exponential growth. J. Phys. Chem. B 2004, 108, 635–648. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation-polyanion pairing vs. polyelectrolyte-ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Michel, M.; Lee, J.; Verploegen, E.; Kam, N.W.S.; Ball, V.; Qi, Y.; Hart, A.J.; Hammond, P.T.; Kotov, N.A. Exponential growth of LbL films with incorporated inorganic sheets. Nano Lett. 2008, 8, 1762–1770. [Google Scholar] [CrossRef]
- Laachachi, A.; Ball, V.; Apaydin, K.; Toniazzo, V.; Ruch, D. Diffusion of Polyphosphates into (Poly(allylamine)-montmorillonite) Multilayer Films: Flame Retardant-Intumescent Films with Improved Oxygen Barrier. Langmuir 2011, 27, 13879–13887. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine-montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Debreczeny, M.; Ball, V.; Boulmedais, F.; Szalontai, B.; Voegel, J.C.; Schaaf, P. Multilayers built from two component polyanions and single component polycation solutions: A way to engineer films with desired secondary structure. J. Phys. Chem. B 2003, 107, 12734–12739. [Google Scholar] [CrossRef]
- Campbell, V.E.; Chiarelli, P.A.; Kaur, S.; Johal, M.S. Coadsorption of a polyanion and an azobenzene dye in self-assembled and spin-assembled polyelectrolyte multilayers. Chem. Mater. 2005, 17, 186–190. [Google Scholar] [CrossRef]
- Johal, M.S.; Ozer, B.H.; Casson, J.L.; John, A.S.; Robinson, J.M.; Wang, H.L. Coadsorption of sodium dodecyl sulfate and a polyanion onto poly(ethylenimine) in multilayered thin films. Langmuir 2004, 20, 2792–2796. [Google Scholar] [CrossRef] [PubMed]
- Laponite Technical Brochure; Southern Clay Products, Inc.: Austin, TX, USA.
- Podsiadlo, P.; Paternel, S.; Rouillard, J.M.; Zhang, Z.F.; Lee, J.; Lee, J.W.; Gulari, L.; Kotov, N.A. Layer-by-layer assembly of nacre-like nanostructured composites with antimicrobial properties. Langmuir 2005, 21, 11915–11921. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Liu, Z.Q.; Paterson, D.; Messersmith, P.B.; Kotov, N.A. Fusion of seashell nacre and marine bioadhesive analogs: High-strength nanocompoisite by layer-by-layer assembly of clay and L-3,4-dihydroxyphenylaianine polymer. Adv. Mater. 2007, 19, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Azinfar, A.; Neuber, S.; Vancova, M.; Sterba, J.; Stranak, V.; Helm, C.A. Self-Patterning Polyelectrolyte Multilayer Films: Influence of Deposition Steps and Drying in a Vacuum. Langmuir 2021, 37, 10490–10498. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, S.; Balzer, B.N.; Hugel, T.; Laschewsky, A.; von Klitzing, R. Effect of Ionic Strength and Layer Number on Swelling of Polyelectrolyte Multilayers in Water Vapour. Soft Mater. 2013, 11, 157–164. [Google Scholar] [CrossRef]
- Dodoo, S.; Steitz, R.; Laschewsky, A.; von Klitzing, R. Effect of ionic strength and type of ions on the structure of water swollen polyelectrolyte multilayers. Phys. Chem. Chem. Phys. 2011, 13, 10318–10325. [Google Scholar] [CrossRef] [Green Version]
- Nestler, P.; Passvogel, M.; Helm, C.A. Influence of Polymer Molecular Weight on the Parabolic and Linear Growth Regime of PDADMAC/PSS Multilayers. Macromolecules 2013, 46, 5622–5629. [Google Scholar] [CrossRef]
- Li, Y.C.; Schulz, J.; Grunlan, J.C. Polyelectrolyte/nanosilicate thin-film assemblies: Influence of pH on growth, mechanical behavior, and flammability. ACS Appl. Mater. Interfaces 2009, 1, 2338–2347. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Tang, Z.Y.; Shim, B.S.; Kotov, N.A. Counterintuitive effect of molecular strength and role of molecular rigidity on mechanical properties of layer-by-layer assembled nanocomposites. Nano Lett. 2007, 7, 1224–1231. [Google Scholar] [CrossRef]
- Kim, D.W.; Kumar, J.; Blumstein, A. Ordered assembly of conjugated ionic polyacetylenes within clay nanoplatelets: Layer-by-layer assembly and intercalative polymerization. Appl. Clay Sci. 2005, 30, 134–140. [Google Scholar] [CrossRef]
- Ladam, G.; Schaad, P.; Voegel, J.C.; Schaaf, P.; Decher, G.; Cuisinier, F. In situ determination of the structural properties of initially deposited polyelectrolyte multilayers. Langmuir 2000, 16, 1249–1255. [Google Scholar] [CrossRef]
- Vertlib, V.; Dietiker, M.; Plotze, M.; Yezek, L.; Spolenak, R.; Puzrin, A.M. Fast assembly of bio-inspired nanocomposite films. J. Mater. Res. 2008, 23, 1026–1035. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Michel, M.; Critchley, K.; Srivastava, S.; Qin, M.; Lee, J.W.; Verploegen, E.; Hart, A.J.; Qi, Y.; Kotov, N.A. Diffusional self-organization in exponential layer-by-layer films with micro- and nanoscale periodicity. Angew. Chem. Int. Ed. 2009, 48, 7073–7077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjipto, E.; Quinn, J.F.; Caruso, F. Layer-by-layer assembly of weak-strong copolymer polyelectrolytes: A route to morphological control of thin films. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4341–4351. [Google Scholar] [CrossRef]
- Ruths, J.; Essler, F.; Decher, G.; Riegler, H. Polyelectrolytes I: Polyanion/polycation multilayers at the air/monolayer/water interface as elements for quantitative polymer adsorption studies and preparation of hetero-superlattices on solid surfaces. Langmuir 2000, 16, 8871–8878. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Dubas, S.T. Mechanism of polyelectrolyte multilayer growth: Charge overcompensation and distribution. Macromolecules 2001, 34, 592–598. [Google Scholar] [CrossRef]
- Buron, C.C.; Filiâtre, C.; Membrey, F.; Bainier, C.; Charraut, D.; Foissy, A. Early steps in layer-by-layer construction of polyelectrolyte films: The transition from surface/polymer to polymer/polymer determining interactions. J. Colloid Interface Sci. 2007, 314, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Zhang, X.; Guan, Y.; Xu, J.; Zhang, X. From cloudy to transparent: Chain rearrangement in hydrogen-bonded layer-by-layer assembled films. Chemphyschem 2007, 8, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Shiratori, S.S.; Rubner, M.F. Controlling bilayer composition and surface wettability of sequentially adsorbed multilayers of weak polyelectrolytes. Macromolecules 1998, 31, 4309–4318. [Google Scholar] [CrossRef]
- Choi, J.; Rubner, M.F. Influence of the degree of ionization on weak polyelectrolyte multilayer assembly. Macromolecules 2005, 38, 116–124. [Google Scholar] [CrossRef]
- Buetergerds, D.; Kateloe, C.; Cramer, C.; Schoenhoff, M. Influence of the Degree of Ionization on the Growth Mechanism of Poly(Diallyldimethylammonium)/Poly(Acrylic Acid) Multilayers. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 425–434. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Sukhishvili, S.A. Ionization and pH stability of multilayers formed by self-assembly of weak polyelectrolytes. Langmuir 2003, 19, 1235–1243. [Google Scholar] [CrossRef]
- Elzbieciak, M.; Wodka, D.; Zapotoczny, S.; Nowak, P.; Warszynski, P. Characteristics of model polyelectrolyte multilayer films containing laponite clay nanoparticles. Langmuir 2010, 26, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hagen, D.A.; Song, Y.X.; Saucier, L.; Milhorn, A.; Stevens, B.; Grunlan, J.C. Balancing polyelectrolyte diffusion and clay deposition for high gas barrier. Green Mater. 2016, 4, 6. [Google Scholar] [CrossRef]
- Das, B.P. Assembly and Organization in Nanostructured Polymer Thin Films. Ph.D. Dissertation, University at Buffalo, The State University of New York, Buffalo, NY, USA, 2014. [Google Scholar]
- Van Duffel, B.; Schoonheydt, R.A.; Grim, C.P.M.; De Schryver, F.C. Multilayered clay films: Atomic force microscopy study and modeling. Langmuir 1999, 15, 7520–7529. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Hagen, D.A.; Stevens, B.; Regev, O.; Grunlan, J.C. Nanobrick wall multilayer thin films grown faster and stronger using electrophoretic deposition. Nanotechnology 2015, 26, 185703. [Google Scholar] [CrossRef] [PubMed]
- Schönhoff, M. Layered polyelectrolyte complexes: Physics of formation and molecular properties. J. Phys. Condens. Matter 2003, 15, R1781–R1808. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]




| Multilayers | PDDA-Laponite | PDDA-PSS-PDDA-Laponite | PDDA-PAA-PDDA-Laponite | |
|---|---|---|---|---|
| Properties | ||||
| Thickness | 80 layers | 0.9 µm | 0.5 µm | 3 µm |
| 140 layers | 1.3 µm | 0.7 µm | 10 µm | |
| 180 layers | - | 0.7 µm | 20 µm | |
| 360 layers | 3.2 µm | - | - | |
| Composition | Clay content | 80 wt.% | 50 wt.% | 22 wt.% |
| Water content | Negligible | Negligible | 12 wt.% | |
| Crystallographic properties | 2θ | 6.30° (Sharp peak) | 6.25° (broad and low intensity peak) | No peak, only shoulder |
| d Spacing | 1.40 nm | 1.41 nm | - | |
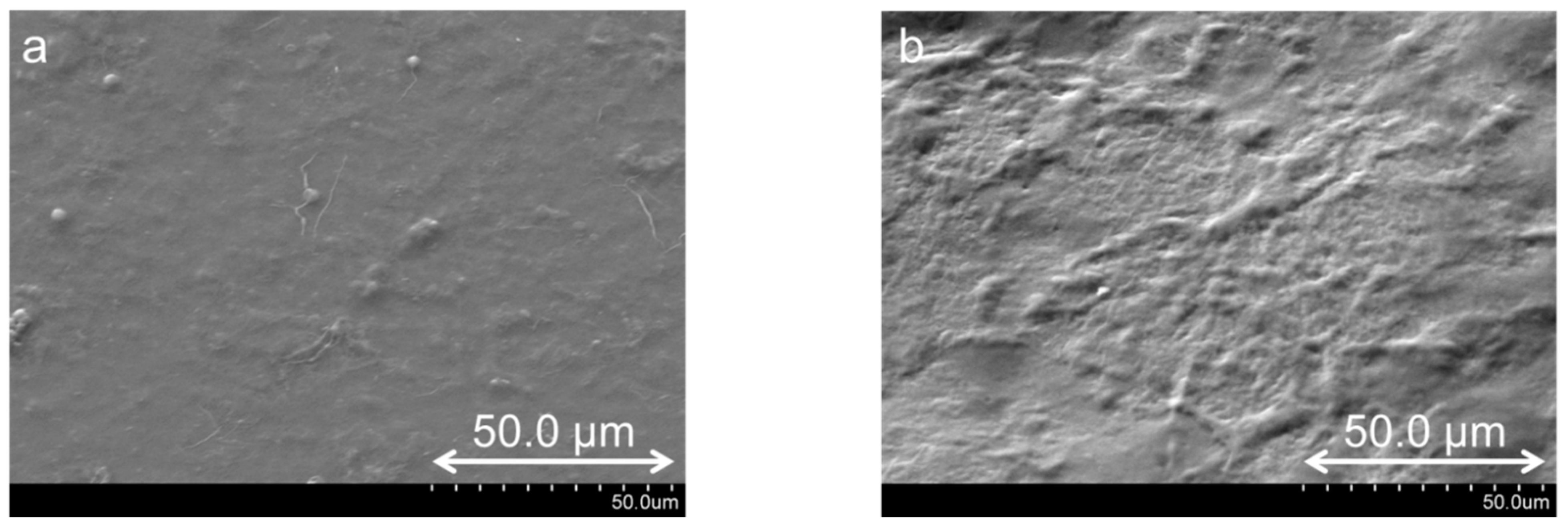
| Surface roughness | 40 layers | - | 70 nm | 145 nm |
| 160 layers | - | 115 nm | 560 nm |
| Multilayers | PDDA-[Laponite + PAA] | ||||
|---|---|---|---|---|---|
| Laponite: PAA (Weight Ratio of Aqueous Lap and PAA Solutions) | |||||
| Properties | 4:1 | 3:2 | 2:3 | 1:4 | |
| Thickness | 100 layers | ~3 µm | 10–15 µm | 11 µm | 13 µm |
| Composition | Clay content | 35 wt.% | 25 wt.% | 5 wt.% | Negligible |
| Water content | 17 wt.% | 21 wt.% | 13 wt.% | 14 wt.% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.P.; Tsianou, M. Molecular Organization in Exponentially Growing Multilayer Thin Films Assembled with Polyelectrolytes and Clay. Polymers 2022, 14, 4333. https://doi.org/10.3390/polym14204333
Das BP, Tsianou M. Molecular Organization in Exponentially Growing Multilayer Thin Films Assembled with Polyelectrolytes and Clay. Polymers. 2022; 14(20):4333. https://doi.org/10.3390/polym14204333
Chicago/Turabian StyleDas, Biswa P., and Marina Tsianou. 2022. "Molecular Organization in Exponentially Growing Multilayer Thin Films Assembled with Polyelectrolytes and Clay" Polymers 14, no. 20: 4333. https://doi.org/10.3390/polym14204333