Nanohybrid of Co3O4 Nanoparticles and Polyphosphazene-Decorated Ultra-Thin Boron Nitride Nanosheets for Simultaneous Enhancement in Fire Safety and Smoke Suppression of Thermoplastic Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
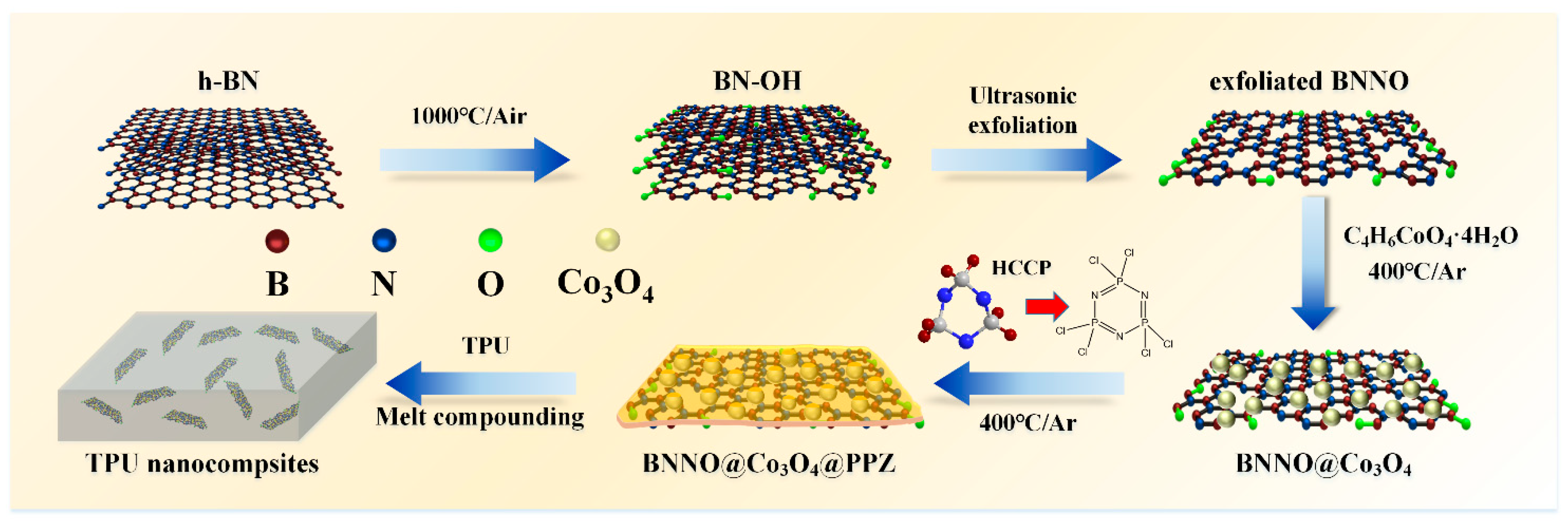
2.2. Preparation of BNNO@Co3O4@PPZ
2.3. Preparation of TPU Nanocomposites
2.4. Characterization
3. Results
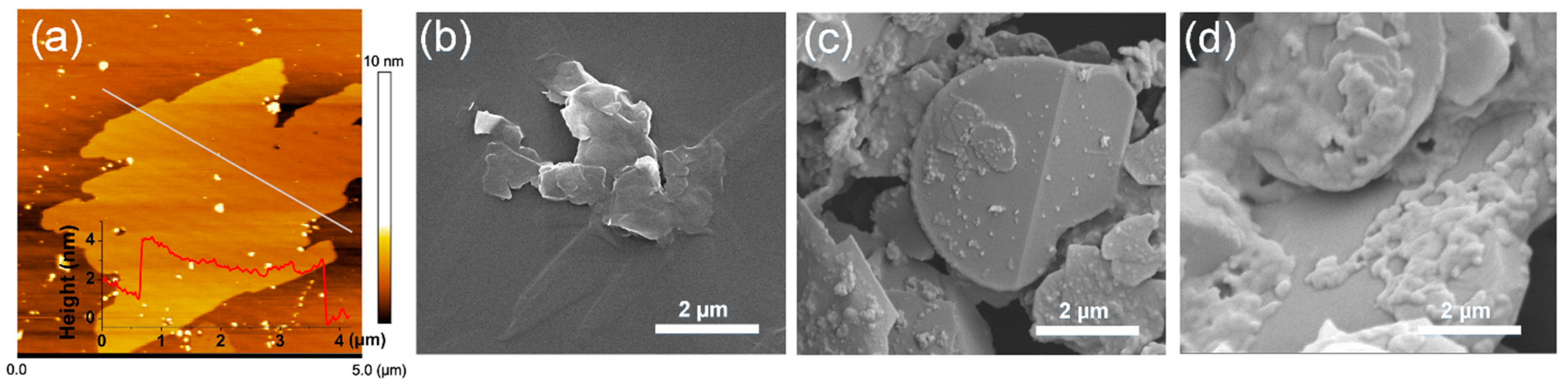
3.1. Characterization of BNNO@Co3O4@PPZ Hybrids
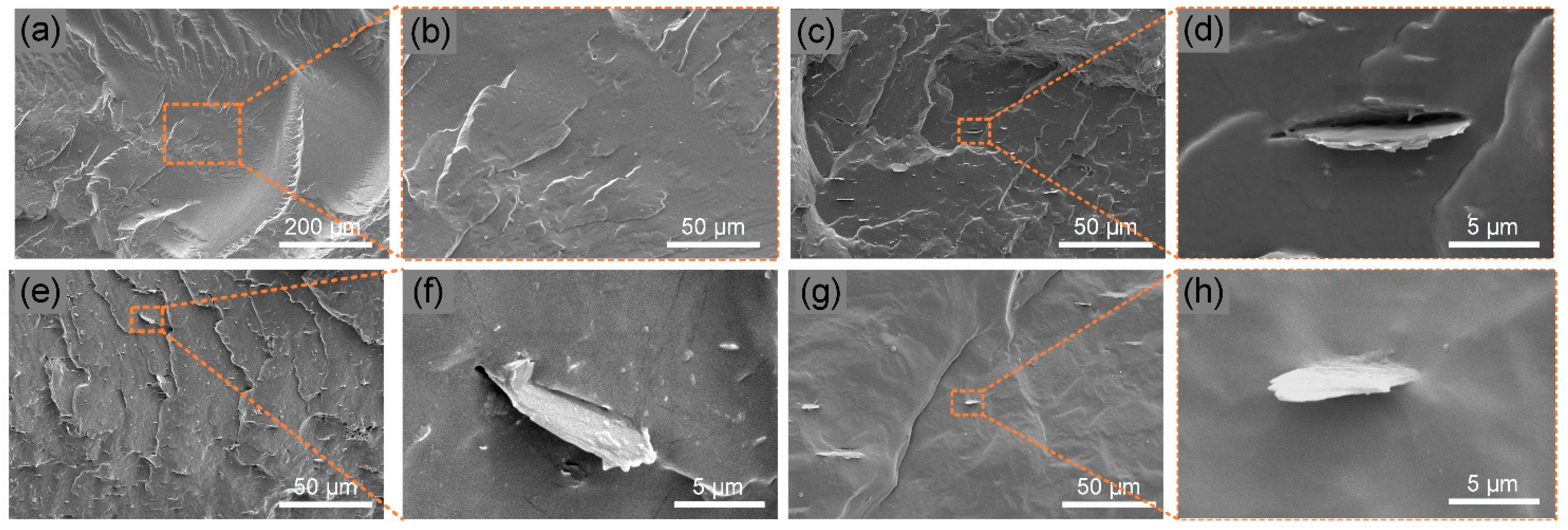
3.2. Fracture Surface Morphology of TPU Nanocomposites
3.3. Thermal Properties of TPU Nanocomposites
3.4. Fire Safety of TPU Nanocomposites
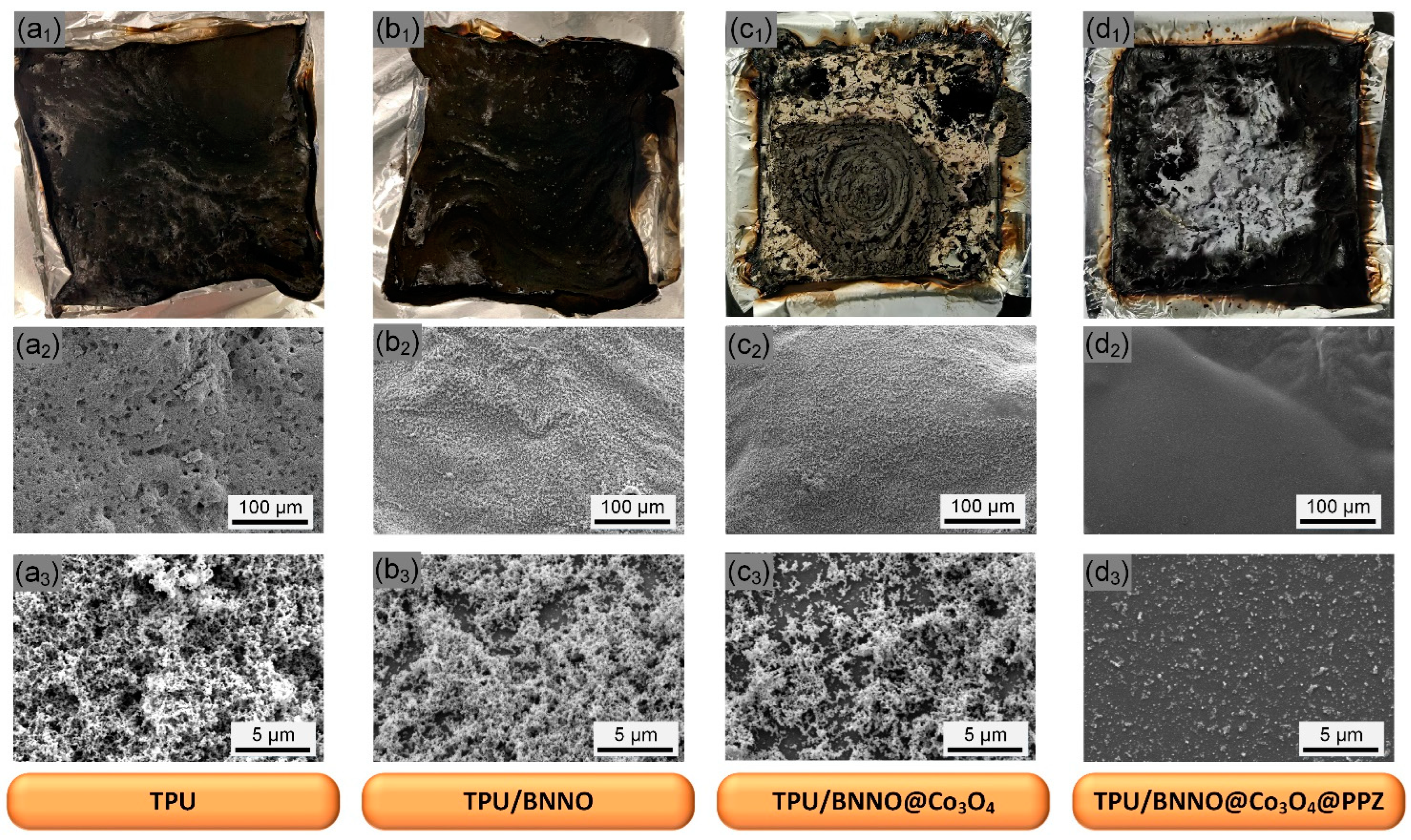
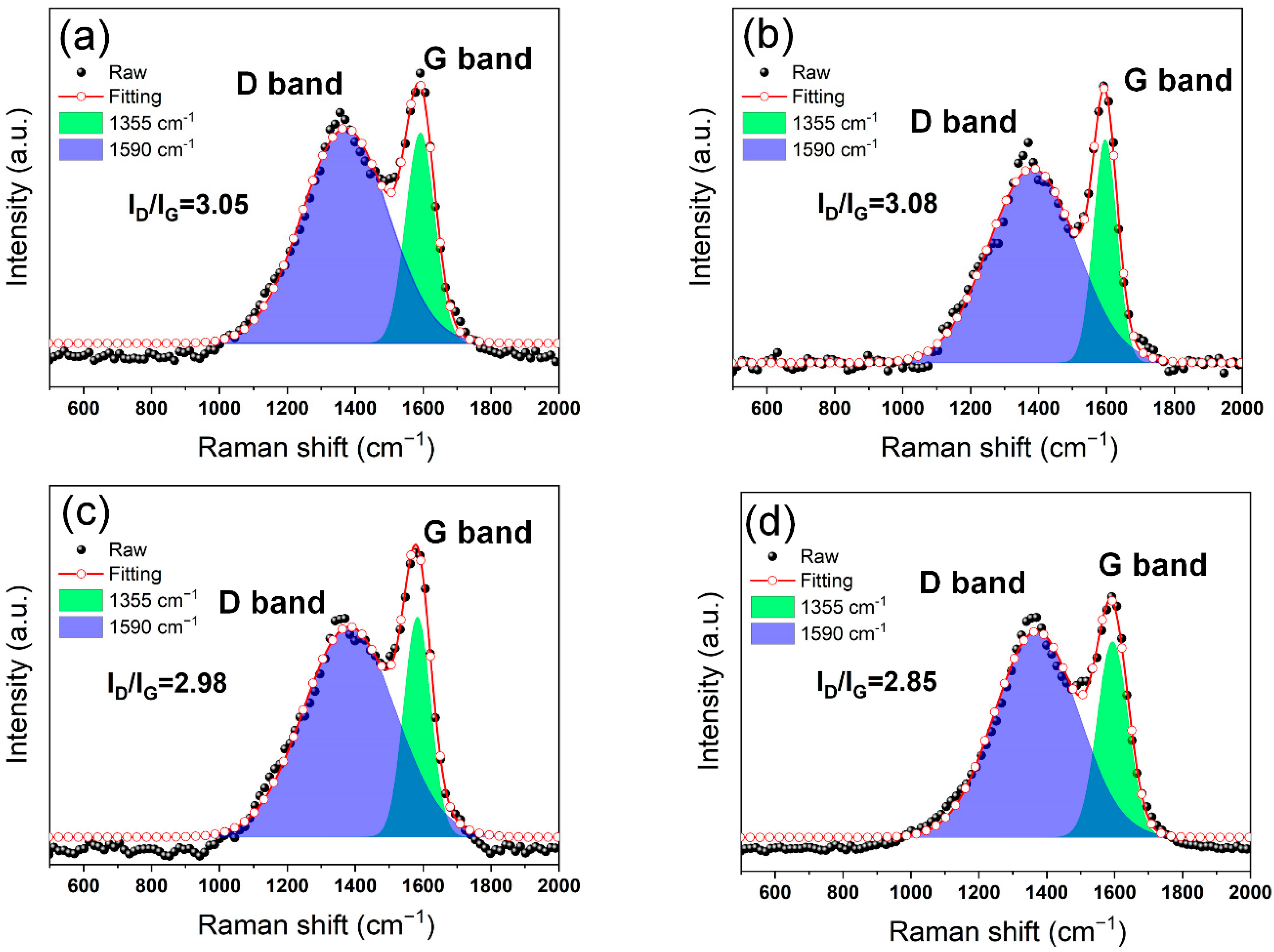
3.5. Analysis of Char Residues after Combustion
3.6. Vapor-Phase Analysis
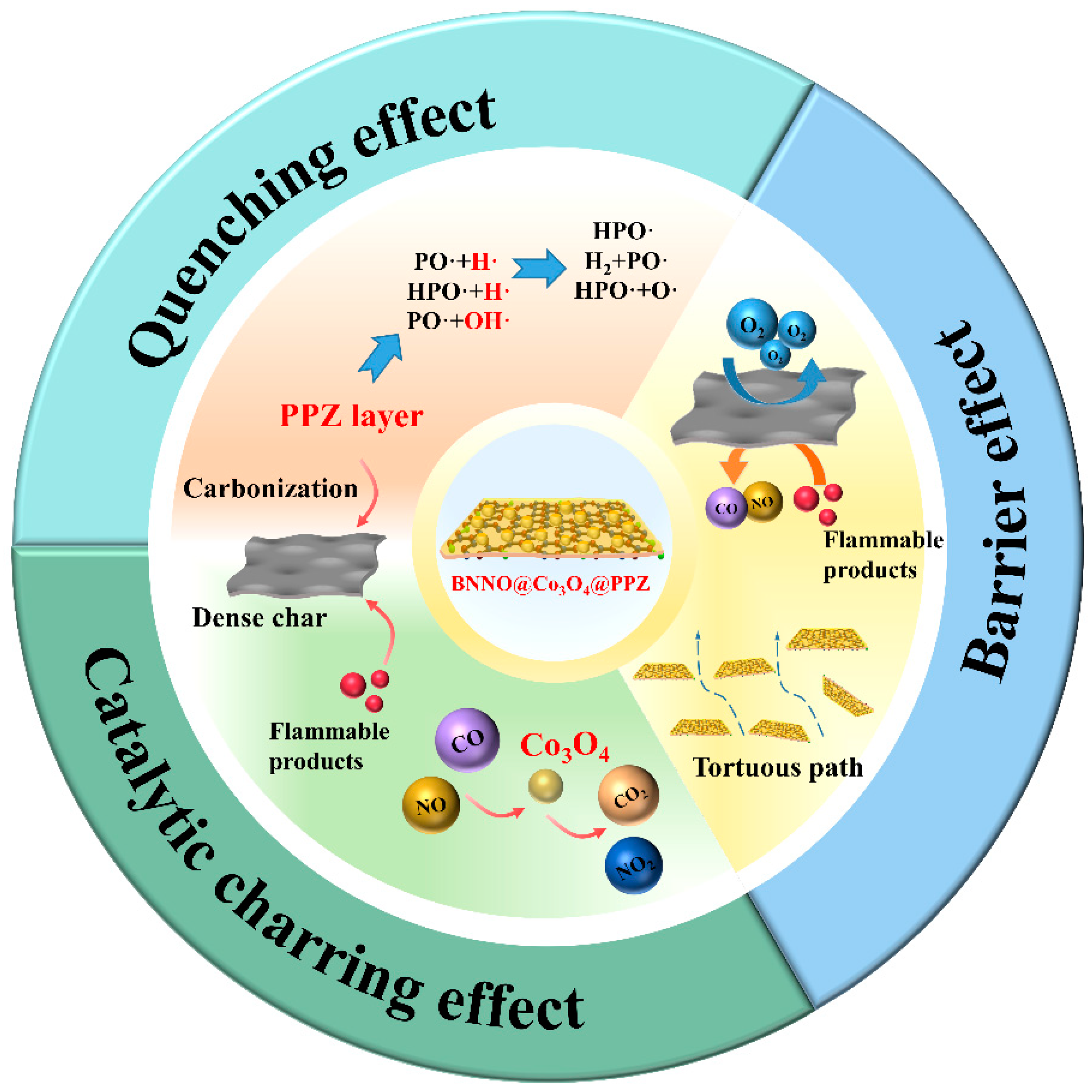
3.7. Flame Retardant Mechanism of TPU Nanocomposites
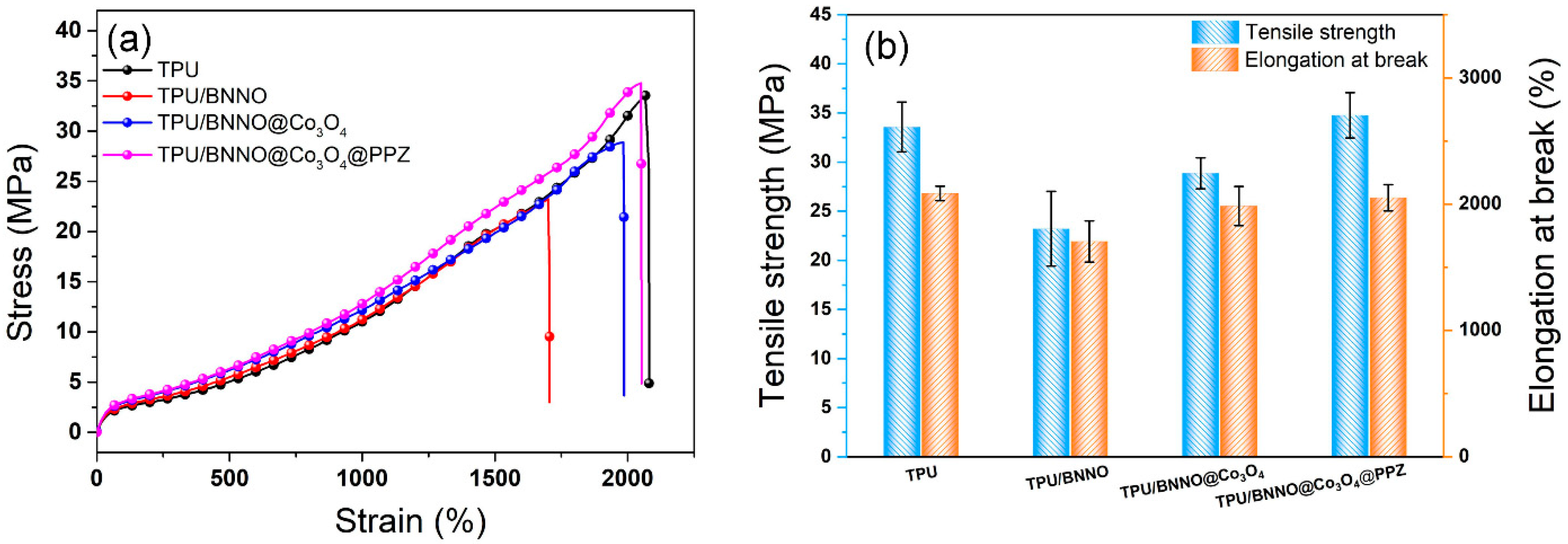
3.8. Mechanical Properties of TPU Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Jeon, H.; Shin, S.; Park, S.; Park, J. Superior toughness and fast self-healing at room temperature engineered by transparent elastomers. Adv. Mater. 2017, 30, 1705145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Q.; Wang, X.; Schubert, D.W.; Liu, X. Flexible, conductive, and anisotropic thermoplastic polyurethane/polydopamine/MXene foam for piezoresistive sensors and motion monitoring. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106838. [Google Scholar] [CrossRef]
- Chen, T.; Xie, Y.; Wang, Z.; Lou, J.; Liu, D.; Xu, R.; Cui, Z.; Li, S.; Panahi-Sarmad, M.; Xiao, X. Recent advances of flexible strain sensors based on conductive fillers and thermoplastic polyurethane matrixes. ACS Appl. Polym. Mater. 2021, 3, 5317–5338. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, W.; Li, Q.; Khan, M.R.; Hu, G.H.; Liu, Y.; Wu, W.; Huang, C.X.; Li, R.K.Y. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef] [PubMed]
- Jamsaz, A.; Goharshadi, E.K.; Barras, A.; Ifires, M.; Szunerits, S.; Boukherroub, R. Magnetically driven superhydrophobic/superoleophilic graphene-based polyurethane sponge for highly efficient oil/water separation and demulsification. Sep. Purif. Technol. 2021, 274, 118931. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Gong, X.; Sun, Q.; Cao, X.; Su, Y.; Yu, B.; Li, R.K.Y.; Vellaisamy, R.A.L. Surface decoration of Halloysite nanotubes with POSS for fire-safe thermoplastic polyurethane nanocomposites. J. Mater. Sci. Technol. 2021, 101, 107–117. [Google Scholar] [CrossRef]
- Jiao, C.; Li, M.; Chen, X.; Li, S. Flame retardancy and thermal decomposition behavior of TPU/chitosan composites. Polym. Adv. Technol. 2019, 31, 178–188. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Li, H.; Sun, J.; Gu, X.; Zhang, S. Constructing a novel synergistic flame retardant by hybridization of zeolitic imidazolate framework-67 and graphene oxide for thermoplastic polyurethane. Polym. Adv. Technol. 2022, 33, 2374–2385. [Google Scholar] [CrossRef]
- Jamsaz, A.; Goharshadi, E.K. Flame retardant, superhydrophobic, and superoleophilic reduced graphene oxide/orthoaminophenol polyurethane sponge for efficient oil/water separation. J. Mol. Liq. 2020, 307, 112979. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.-Q.; Yuen, A.C.Y.; Zhang, Z.-C.; Shen, L.-L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, J.; Yu, B.; Meng, Y.; Cao, X.; Zhang, Q.; Wu, W.; Shi, D.; Jiang, T.; Li, R.K. Facile preparation of phosphorus containing hyperbranched polysiloxane grafted graphene oxide hybrid toward simultaneously enhanced flame retardancy and smoke suppression of thermoplastic polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106614. [Google Scholar] [CrossRef]
- Cai, W.; Zhan, J.; Feng, X.; Yuan, B.; Liu, J.; Hu, W.; Hu, Y. Facile Construction of Flame-Retardant-Wrapped Molybdenum Disulfide Nanosheets for Properties Enhancement of Thermoplastic Polyurethane. Ind. Eng. Chem. Res. 2017, 56, 7229–7238. [Google Scholar] [CrossRef]
- Huang, S.-C.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Gao, Y.-Y.; Wang, Y.-Z. Phosphorus-containing organic-inorganic hybrid nanoparticles for the smoke suppression and flame retardancy of thermoplastic polyurethane. Polym. Degrad. Stab. 2020, 178, 109179. [Google Scholar] [CrossRef]
- Qian, Y.; Su, W.; Li, L.; Fu, H.; Li, J.; Zhang, Y. Synthesis of 3D hollow layered double hydroxide-molybdenum disulfide hybrid materials and their application in flame retardant thermoplastic polyurethane. Polymers 2022, 14, 1506. [Google Scholar] [CrossRef]
- Huang, S.-C.; Deng, C.; Chen, H.; Li, Y.-M.; Zhao, Z.-Y.; Wang, S.-X.; Wang, Y.-Z. Novel ultrathin layered double hydroxide nanosheets with in situ formed oxidized phosphorus as anions for simultaneous fire resistance and mechanical enhancement of thermoplastic polyurethane. ACS Appl. Polym. Mater. 2019, 1, 1979–1990. [Google Scholar] [CrossRef]
- Mokoena, T.E.; Magagula, S.I.; Mochane, M.J.; Mokhena, T.C. Mechanical properties, thermal conductivity, and modeling of boron nitride-based polymer composites: A review. Express Polym. Lett. 2021, 15, 1148–1173. [Google Scholar] [CrossRef]
- Zheng, Z.; Cox, M.; Li, B. Surface modification of hexagonal boron nitride nanomaterials: A review. J. Mater. Sci. 2018, 53, 66–99. [Google Scholar] [CrossRef]
- Fischer, A.J.; Zhong, Y.; Zhang, L.; Wu, W.; Drummer, D. Heat propagation in thermally conductive polymers of PA6 and hexagonal boron nitride. Fire Mater. 2019, 43, 928–935. [Google Scholar] [CrossRef]
- Yin, L.; Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Gui, Z.; Qian, L. Flame-retardant activity of ternary integrated modified boron nitride nanosheets to epoxy resin. J. Colloid Interface Sci. 2021, 608, 853–863. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.; Lan, T.; Xue, X.; Liu, X. Polybenzoxazine/boron nitride foam: A promising low-k, flame-retardant and robust material. J. Mater. Sci. 2021, 56, 18749–18761. [Google Scholar] [CrossRef]
- Cai, W.; Hong, N.; Feng, X.; Zeng, W.; Shi, Y.; Zhang, Y.; Wang, B.; Hu, Y. A facile strategy to simultaneously exfoliate and functionalize boron nitride nanosheets via Lewis acid-base interaction. Chem. Eng. J. 2017, 330, 309–321. [Google Scholar] [CrossRef]
- Cai, W.; Mu, X.; Pan, Y.; Guo, W.; Wang, J.; Yuan, B.; Feng, X.; Tai, Q.; Hu, Y. Facile fabrication of organically modified boron nitride nanosheets and its effect on the thermal stability, flame retardant, and mechanical properties of thermoplastic polyurethane. Polym. Adv. Technol. 2018, 29, 2545–2552. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.; Liu, L.; Zhou, X.; Chu, F.; Zhan, J.; Hu, Y.; Kan, Y.; Wang, X. An operable platform towards functionalization of chemically inert boron nitride nanosheets for flame retardancy and toxic gas suppression of thermoplastic polyurethane. Compos. Part B Eng. 2019, 178, 107462. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Zhang, Y.; Cai, W.; Yao, C.; Hu, Y.; Hu, W. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef]
- Yin, S.H.; Ren, X.L.; Lian, P.C.; Zhu, Y.Z.; Mei, Y. Synergistic Effects of Black Phosphorus/Boron Nitride Nanosheets on Enhancing the Flame-Retardant Properties of Waterborne Polyurethane and Its Flame-Retardant Mechanism. Polymers 2020, 12, 1487. [Google Scholar] [CrossRef]
- Zhi, Y.-R.; Yu, B.; Yuen, A.C.Y.; Liang, J.; Wang, L.-Q.; Yang, W.; Lu, H.-D.; Yeoh, G. Surface Manipulation of Thermal-Exfoliated Hexagonal Boron Nitride with Polyaniline for Improving Thermal Stability and Fire Safety Performance of Polymeric Materials. ACS Omega 2018, 3, 14942–14952. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, H.; Guo, J.; Sun, J.; Li, H.; Zhang, S.; Gu, X. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Compos. Part B Eng. 2020, 182, 107498. [Google Scholar] [CrossRef]
- Zhou, K.; Gong, K.; Gao, F.; Yin, L. Facile strategy to synthesize MXene@LDH nanohybrids for boosting the flame retardancy and smoke suppression properties of epoxy. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106912. [Google Scholar] [CrossRef]
- Gong, K.; Yin, L.; Zhou, K.; Qian, X.; Shi, C.; Gui, Z.; Yu, B.; Qian, L. Construction of interface-engineered two-dimensional nanohybrids towards superb fire resistance of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106707. [Google Scholar] [CrossRef]
- Kalali, E.N.; Guo, W.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Effect of metal-based nanoparticles decorated graphene hybrids on flammability of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105694. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, X.; Li, Q.; Li, H.; Chao, Y.; Li, M.; Mahurin, S.M.; Li, H.; Zhu, H.; Dai, S. Controlled Gas Exfoliation of Boron Nitride into Few-Layered Nanosheets. Angew. Chem. Int. Ed. 2016, 55, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, R.; Liu, J.; Zou, X.; Qiu, L.; Kang, F.; Liu, B.; Cheng, H.-M. Simultaneous Production and Functionalization of Boron Nitride Nanosheets by Sugar-Assisted Mechanochemical Exfoliation. Adv. Mater. 2019, 31, e1804810. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Lei, S.; Liu, Z. Flame-retardant activity of modified boron nitride nanosheets to cotton. Text. Res. J. 2019, 90, 512–522. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Wang, D.; Mu, X.; Cai, W.; Song, L.; Ma, C.; Hu, Y. Constructing phosphorus, nitrogen, silicon-co-contained boron nitride nanosheets to reinforce flame retardant properties of unsaturated polyester resin. Compos. Part A Appl. Sci. Manuf. 2018, 109, 546–554. [Google Scholar] [CrossRef]
- Yang, L.; Guo, J.; Zhang, L.; Li, C. Significant Improvement in the Flame Retardancy and Thermal Conductivity of the Epoxy Resin via Constructing a Branched Flame Retardant Based on SI-ATRP Initiated by Dopamine-Modified Boron Nitride. Ind. Eng. Chem. Res. 2022, 61, 8031–8042. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Mu, X.; Zhou, M.; Cai, W.; Song, L.; Xing, W.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. Part B Eng. 2020, 202, 108397. [Google Scholar] [CrossRef]
- Singh, K.P.; Mishra, A.; Kumar, N.; Tripathi, D.; Shami, T.C. Evaluation of thermal, morphological and flame-retardant properties of thermoplastic polyurethane/polyphosphazene blends. Polym. Bull. 2018, 75, 2415–2430. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, K.K.R.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef]
- Qiu, S.; Hu, Y.; Shi, Y.; Hou, Y.; Kan, Y.; Chu, F.; Sheng, H.; Yuen, K.K.R.; Xing, W. In situ growth of polyphosphazene particles on molybdenum disulfide nanosheets for flame retardant and friction application. Compos. Part A Appl. Sci. Manuf. 2018, 114, 407–417. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Wang, G.; Zhu, Y. Fabrication of Anisotropic Polyphosphazene/Bio-based Poly(urethane-acrylate) composite foams with High Thermal Insulation and Flame Retardancy. Polymer 2021, 231, 124108. [Google Scholar] [CrossRef]
- Hong, J.; Wu, T.; Wang, X.; Lu, Z.; Zhang, J.; Zeng, B.; Yuan, C.; Dai, L. Copper-catalyzed pyrolysis of halloysites@polyphosphazene for efficient carbonization and smoke suppression. Compos. Part B Eng. 2022, 230, 109547. [Google Scholar] [CrossRef]
- Qiu, S.; Xing, W.; Mu, X.; Feng, X.; Ma, C.; Yuen, K.K.R.; Hu, Y. A 3D Nanostructure Based on Transition-Metal Phosphide Decorated Heteroatom-Doped Mesoporous Nanospheres Interconnected with Graphene: Synthesis and Applications. ACS Appl. Mater. Interfaces 2016, 8, 32528–32540. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cui, J.; Zhang, H.; Yang, B.; Guo, J.; Mu, B.; Wang, Z.; Li, H.; Tian, L. A novel flame retardant consisting of functionalized Salen-Ni based polyphosphazene microspheres. High Perform. Polym. 2022, 34, 914–927. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Xing, W.; Gangireddy, C.S.R.; Gui, Z.; Hu, Y. Hierarchical Polyphosphazene@Molybdenum Disulfide Hybrid Structure for Enhancing the Flame Retardancy and Mechanical Property of Epoxy Resins. ACS Appl. Mater. Interfaces 2017, 9, 29147–29156. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Lu, H.; Song, L.; Hu, Y. The effect of metal oxide decorated graphene hybrids on the improved thermal stability and the reduced smoke toxicity in epoxy resins. Chem. Eng. J. 2014, 250, 214–221. [Google Scholar] [CrossRef]
- Guender, D.; Watanabe, K.; Taniguchi, T.; Witte, G. Van der Waals Bound Organic/2D Insulator Hybrid Structures: Epitaxial Growth of Acene Films on hBN(001) and the Influence of Surface Defects. ACS Appl. Mater. Interfaces 2020, 12, 38757–38767. [Google Scholar] [CrossRef]
- Mu, X.; Pan, Y.; Ma, C.; Zhan, J.; Song, L. Novel Co3O4/covalent organic frameworks nanohybrids for conferring enhanced flame retardancy, smoke and CO suppression and thermal stability to polypropylene. Mater. Chem. Phys. 2018, 215, 20–30. [Google Scholar] [CrossRef]
- Ponnamma, D.; Nair, S.S.; Parangusan, H.; Hassan, M.K.; Adham, S.; Karim, A.; Al-Maadeed, M.A.A. White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption. Polymers 2019, 12, 4. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Xie, Y.; Wang, H.; Bai, J. Synthesis of carbon-coated Co3O4 composite with dendrite-like morphology and its electrochemical performance for lithium-ion batteries. J. Nanoparticle Res. 2013, 15, 1740. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, Y.; Liu, H.; Huang, Z. Superior Sodium-Ion Storage Performance of Co3O4@Nitrogen-Doped Carbon: Derived from a Metal-Organic Framework. J. Mater. Chem. A 2016, 4, 5428–5435. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.-Z.; Zhao, W.-J.; Wu, W.; Zhang, D.-L.; He, G.-J.; Yang, Z.-T.; Cao, X.-W. Realizing enhanced dielectric and mechanical performance of polyvinylidene fluoride/SiC nanocomposites through a bio-inspired interface design. Adv. Compos. Hybrid Mater. 2022, 5, 263–277. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106113. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, Z.; Tanchak, R.N.; Wang, Q. A review on cone calorimeter for assessment of flame-retarded polymer composites. J. Therm. Anal. Calorim. 2022, 147, 10209–10234. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Ye, D.; Zhu, Y.; Cui, M.; Xi, J.; Liu, J.; Xing, W. Bio-based polyphenol tannic acid as universal linker between metal oxide nanoparticles and thermoplastic polyurethane to enhance flame retardancy and mechanical properties. Compos. Part B Eng. 2021, 224, 109206. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.-Y. Nano-architectured mesoporous silica decorated with ultrafine Co3O4 toward an efficient way to delaying ignition and improving fire retardancy of polystyrene. Mater. Des. 2017, 129, 69–81. [Google Scholar] [CrossRef]
- Liu, C.; Yao, A.; Chen, K.; Shi, Y.; Feng, Y.; Zhang, P.; Yang, F.; Liu, M.; Chen, Z. MXene based core-shell flame retardant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part B Eng. 2021, 226, 109363. [Google Scholar] [CrossRef]
- Li, H.; Ning, N.; Zhang, L.; Wang, Y.; Liang, W.; Tian, M.; Chan, T.W. Effect of content of organophosphorus on flame retardancy mode of thermoplastic polyurethane. Polymer 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, W.; Huang, J.; He, Y.; Liang, X.; Su, Y.; Wu, W.; Li, R.K. Interface engineering of graphene oxide containing phosphorus/nitrogen towards fire safety enhancement for thermoplastic polyurethane. Compos. Commun. 2021, 27, 100821. [Google Scholar] [CrossRef]
- Pan, H.; Ma, W.; Zhang, Z.; Liu, Y.; Lu, F.; Yu, B.; Zhang, X. Co-Effect Flame Retardation of Co3O4-Loaded Titania Nanotubes and α-Zirconium Phosphate in the Epoxy Matrix. ACS Omega 2020, 5, 28475–28482. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.-W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Wu, W.; He, H.; Liu, T.; Wei, R.; Cao, X.; Sun, Q.; Venkatesh, S.; Yuen, R.K.; Roy, V.A.; Li, R.K. Synergetic enhancement on flame retardancy by melamine phosphate modified lignin in rice husk ash filled P34HB biocomposites. Compos. Sci. Technol. 2018, 168, 246–254. [Google Scholar] [CrossRef]
- Qiu, S.; Liang, J.; Hou, Y.; Zhou, X.; Zhou, Y.; Wang, J.; Zou, B.; Xing, W.; Hu, Y. Hindered phenolic antioxidant passivation of black phosphorus affords air stability and free radical quenching. J. Colloid Interface Sci. 2022, 606, 1395–1409. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y.; Jiang, S.; Tang, G. The influence of cobalt oxide–graphene hybrids on thermal degradation, fire hazards and mechanical properties of thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2016, 88, 10–18. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Yang, L.; Wang, D.-Y. Few layered Co(OH)2 ultrathin nanosheet-based polyurethane nanocomposites with reduced fire hazard: From eco-friendly flame retardance to sustainable recycling. Green Chem. 2016, 18, 3066–3074. [Google Scholar] [CrossRef]





| Samples | Tempd5% (°C) | Tempmax (°C) | R800°C (wt%) |
|---|---|---|---|
| TPU | 314.0 | 413.6 | 5.08 |
| TPU/BNNO | 314.2 | 413.8 | 6.81 |
| TPU/BNNO@Co3O4 | 301.6 | 365.8 | 7.8 |
| TPU/BNNO@Co3O4@PPZ | 318.7 | 414.5 | 7.42 |
| Samples | TTI(s) | PHRR (kW/m2) | THR (MJ/m2) | PSPR (m2/s) | TSP (m2) | PCOPR (g/s) | PCO2PR (g/s) |
|---|---|---|---|---|---|---|---|
| Pure TPU | 72 | 900.8 | 63.5 | 0.1497 | 10.8 | 0.0142 | 0.758 |
| TPU/BNNO | 72 | 724.7 | 64.5 | 0.1462 | 9.8 | 0.0123 | 0.663 |
| TPU/BNNO@Co3O4 | 53 | 732.9 | 60.0 | 0.0685 | 4.9 | 0.0043 | 0.454 |
| TPU/BNNO@Co3O4@PPZ | 68 | 503.1 | 56.9 | 0.073 | 8.6 | 0.0039 | 0.308 |
| Sample | PHRR | PSPR | PCOPR | PCO2PR |
|---|---|---|---|---|
| BN@P-PEI [22] | −34.4% | −26.8% | - | - |
| h-BN@SiO2@PA [24] | −23.5% | −29.2% | −26.8% | −11.0% |
| h-BN-PPy-PA-Cu2+ [25] | −35.6% | −31.8% | - | - |
| BNO/PANI [27] | −32.6% | +9.1% | 0% | −32.2% |
| Co3O4-Tannic acid [56] | −18.9% | −33.3% | −11.4% | −10.5% |
| GO-DOPO [60] | −35.8% | −50% | −57.1% | −36.5% |
| Black phosphorus-HPL [64] | −49.9% | −45.8% | −37.5% | −32.5% |
| Co3O4/GNS [65] | −(<)10% | - | −18.2% | - |
| Co(OH)2 [66] | −38.7% | −33.3% | −81.0% | - |
| BNNO@Co3O4@PPZ (This work) | −44.1% | −51.2% | −72.5% | −59.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Wu, W.; Zhao, W.; Xing, Y.; Zhang, H.; Wang, C.; Chen, T.B.Y.; Yuen, A.C.Y.; Yu, B.; Cao, X.; et al. Nanohybrid of Co3O4 Nanoparticles and Polyphosphazene-Decorated Ultra-Thin Boron Nitride Nanosheets for Simultaneous Enhancement in Fire Safety and Smoke Suppression of Thermoplastic Polyurethane. Polymers 2022, 14, 4341. https://doi.org/10.3390/polym14204341
Tong Y, Wu W, Zhao W, Xing Y, Zhang H, Wang C, Chen TBY, Yuen ACY, Yu B, Cao X, et al. Nanohybrid of Co3O4 Nanoparticles and Polyphosphazene-Decorated Ultra-Thin Boron Nitride Nanosheets for Simultaneous Enhancement in Fire Safety and Smoke Suppression of Thermoplastic Polyurethane. Polymers. 2022; 14(20):4341. https://doi.org/10.3390/polym14204341
Chicago/Turabian StyleTong, Yizhang, Wei Wu, Wanjing Zhao, Yurui Xing, Hongti Zhang, Cheng Wang, Timothy B. Y. Chen, Anthony C. Y. Yuen, Bin Yu, Xianwu Cao, and et al. 2022. "Nanohybrid of Co3O4 Nanoparticles and Polyphosphazene-Decorated Ultra-Thin Boron Nitride Nanosheets for Simultaneous Enhancement in Fire Safety and Smoke Suppression of Thermoplastic Polyurethane" Polymers 14, no. 20: 4341. https://doi.org/10.3390/polym14204341







