Mitigating Lithium Dissolution and Polysulfide Shuttle Effect Phenomena Using a Polymer Composite Layer Coating on the Anode in Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Synthesis
2.3. Electrochemical Measurements and Characterization
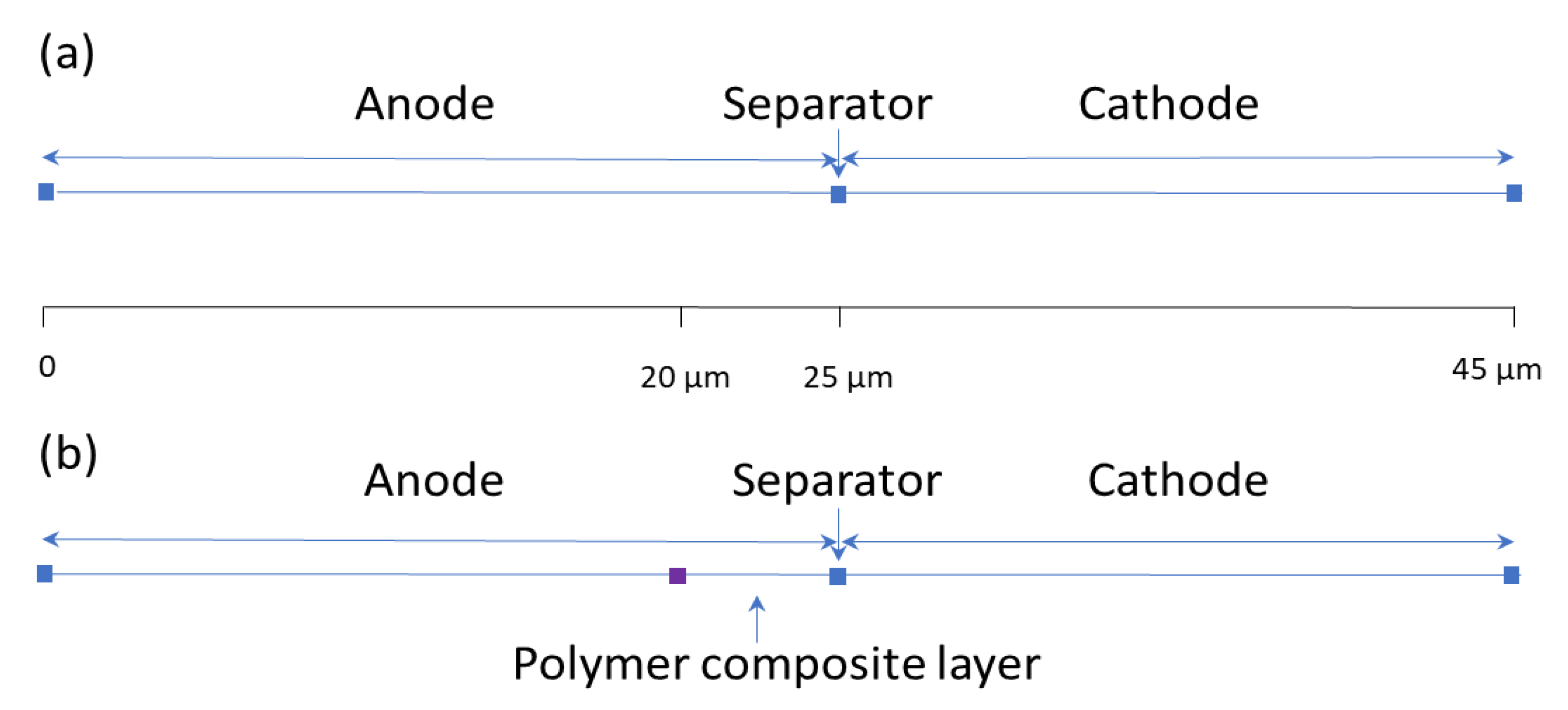
2.4. COMSOL Multiphysics Simulation
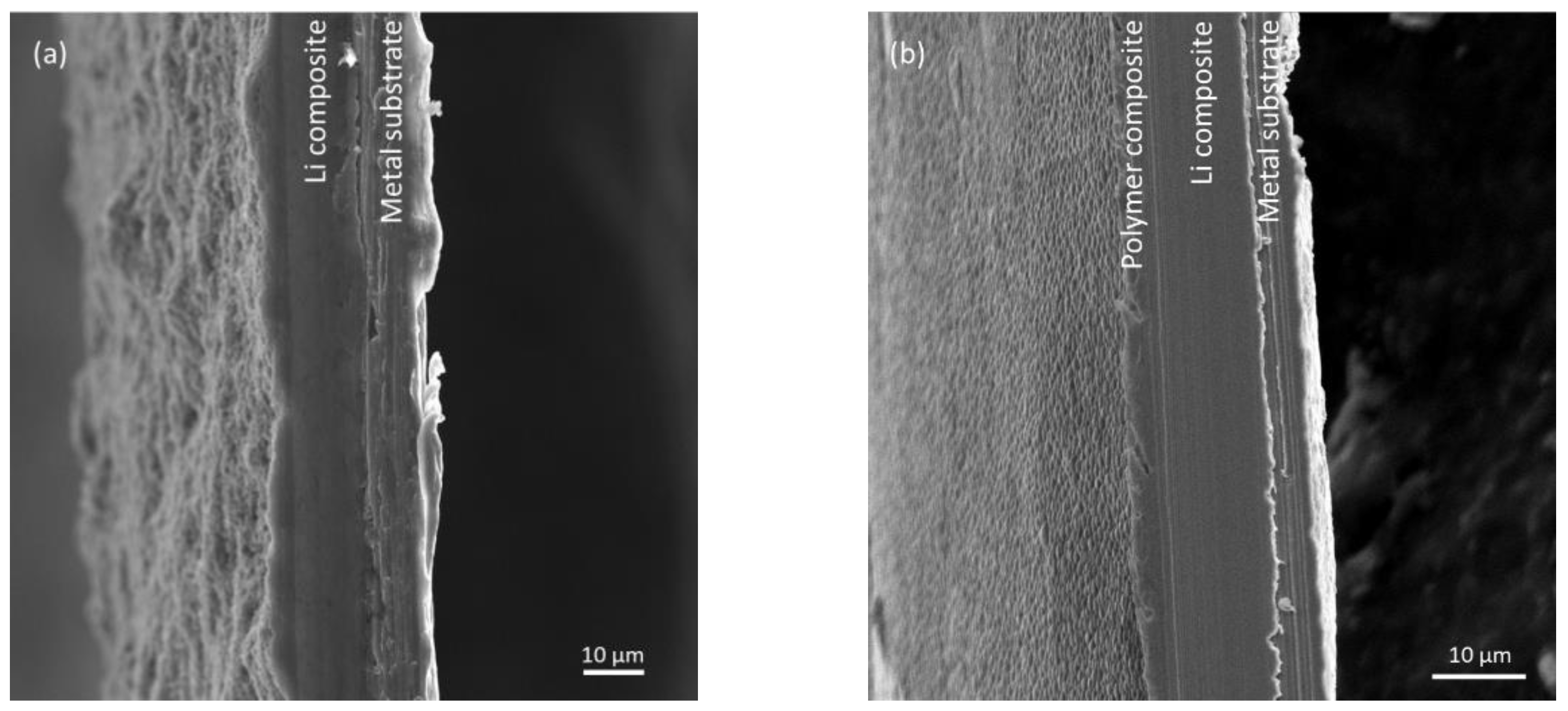
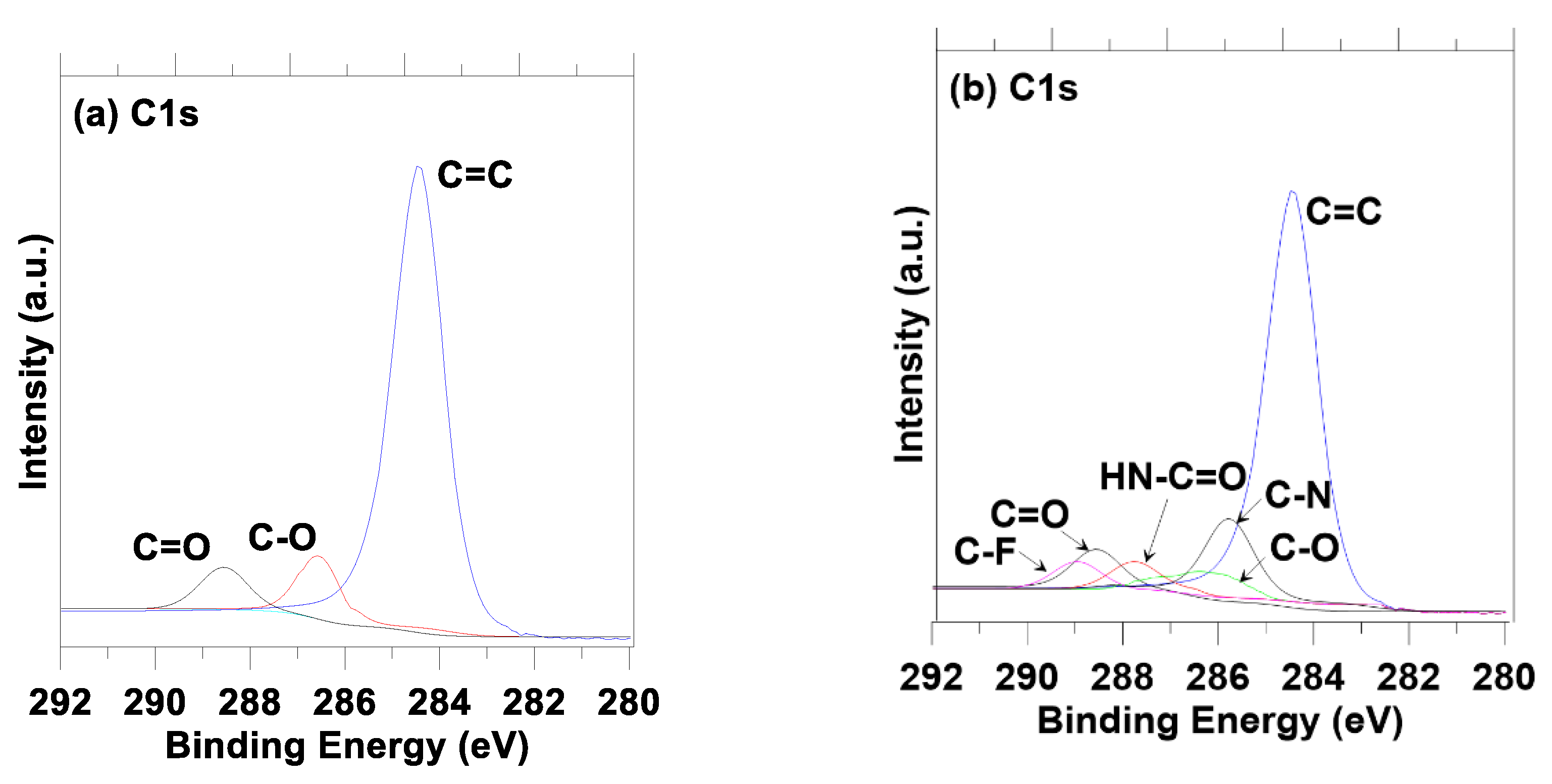
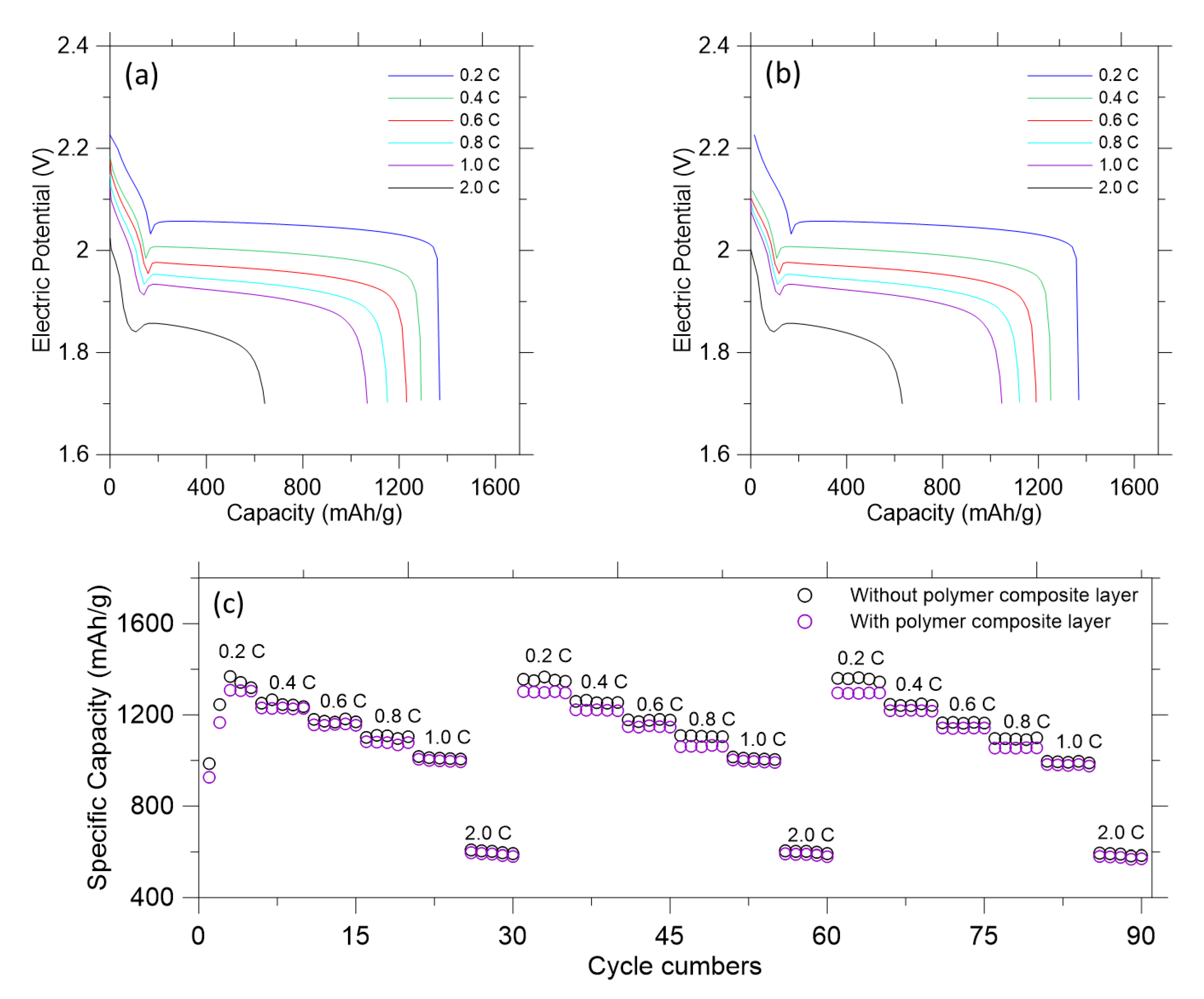
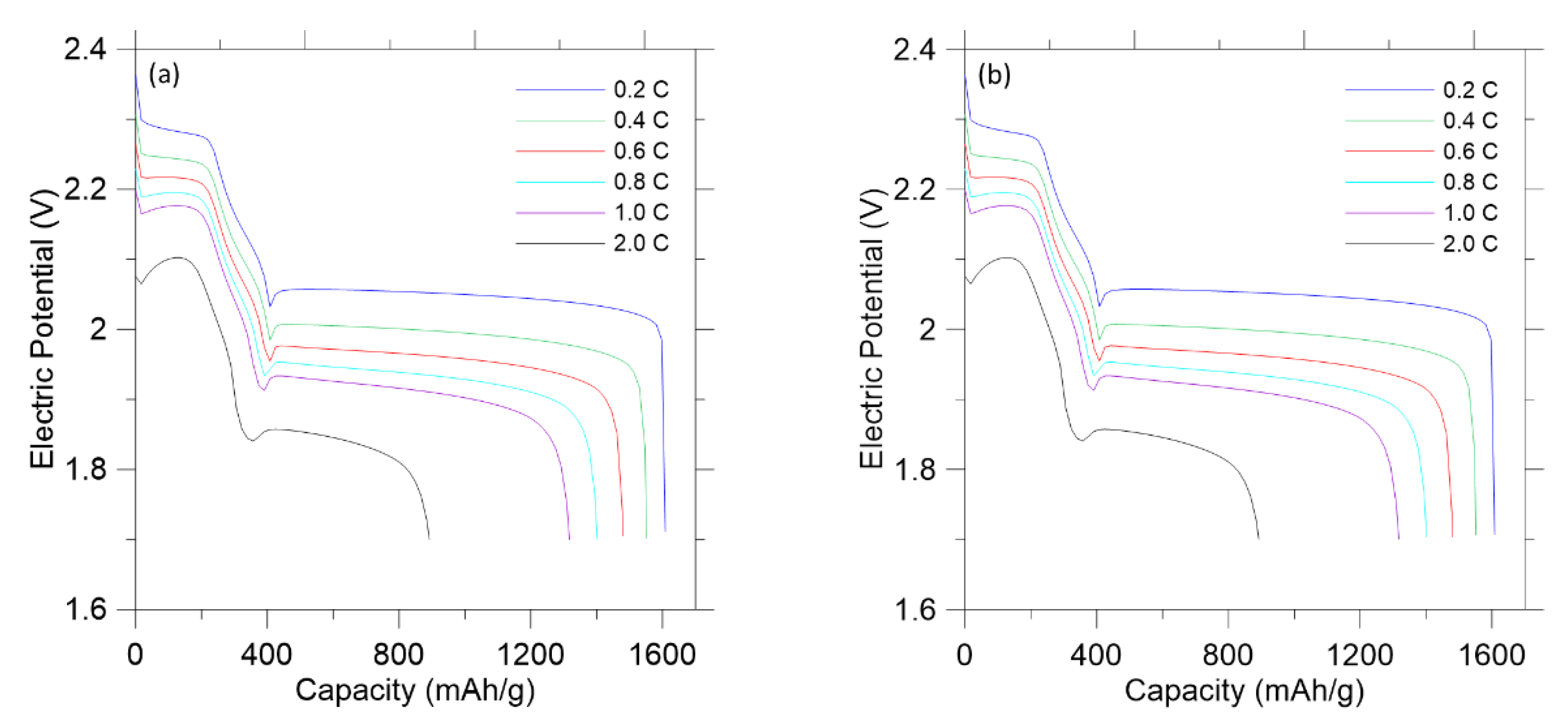
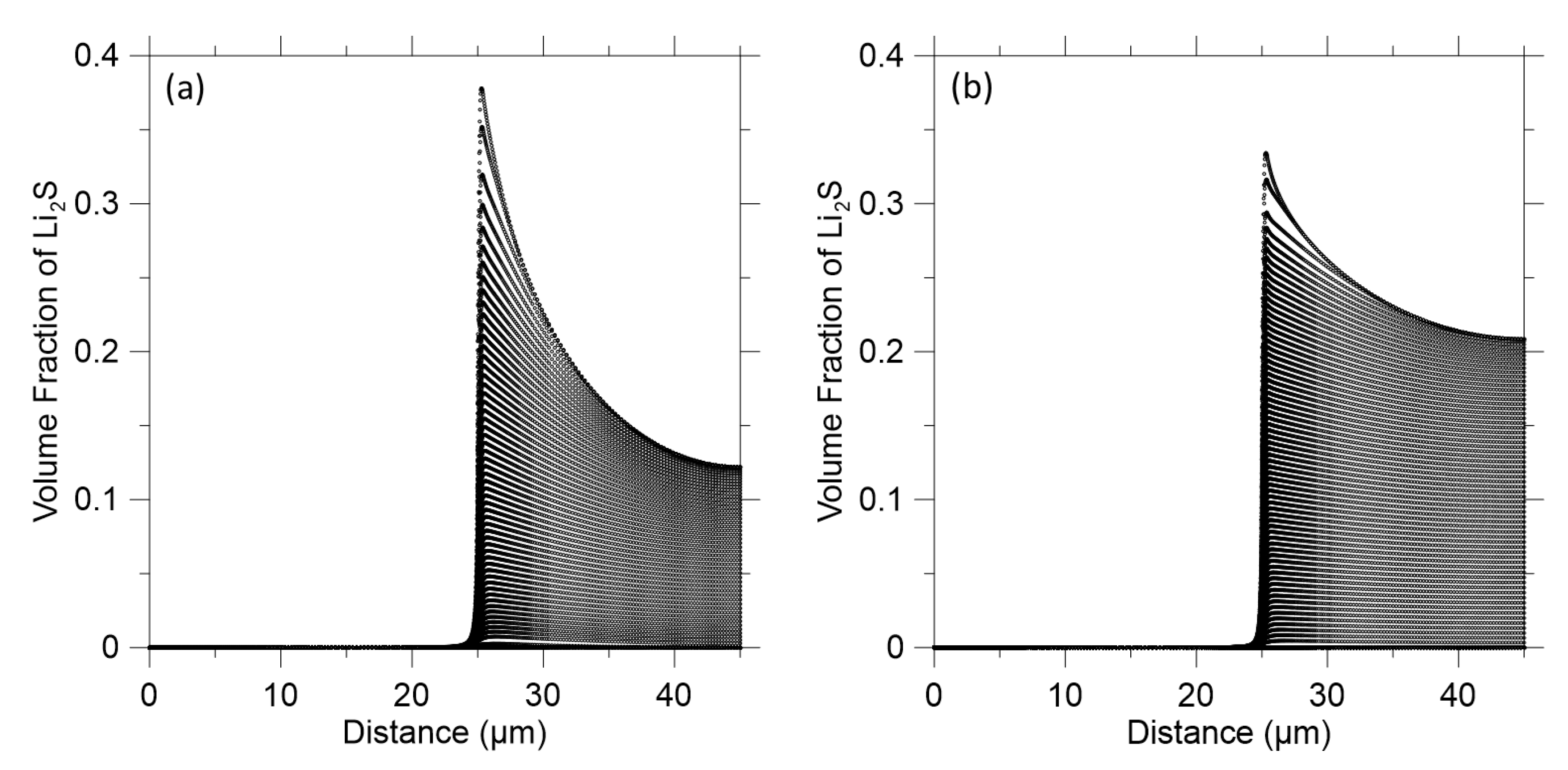
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Fu, K.K.; Chen, C.; Luo, W.; Gao, T.; Xu, S.; Dai, J.; Pastel, G.; Wang, Y.; Liu, B.; et al. Enabling High-Areal-Capacity Lithium–Sulfur Batteries: Designing Anisotropic and Low-Tortuosity Porous Architectures. Available online: https://pubs.acs.org/doi/pdf/10.1021/acsnano.7b01172 (accessed on 21 August 2022).
- Shen, W.; Li, K.; Lv, Y.; Xu, T.; Wei, D.; Liu, Z. Highly-Safe and Ultra-Stable All-Flexible Gel Polymer Lithium Ion Batteries Aiming for Scalable Applications. Adv. Energy Mater. 2020, 10, 1904281. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Q.; Zhen, C.; Niu, Y.; Han, Y.; Zeng, G.; Chen, D.; Feng, C.; Chen, N.; Lv, W.; et al. Recent Progress in Flame-Retardant Separators for Safe Lithium-Ion Batteries. Energy Storage Mater. 2021, 37, 628–647. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Jiang, D.; Yang, L.; Zhang, J.; Yao, M.; Ji, X.; Wang, G.; Zhang, S. Enhanced transport and favorable distribution of Li-ion in a poly (ionic liquid) based electrolyte facilitated by Li1. 3Al0. 3Ti1. 7 (PO4) 3 nanoparticles for highly-safe lithium metal batteries. Electrochim. Acta 2021, 368, 137581. [Google Scholar] [CrossRef]
- Zou, Y.; Cao, Z.; Zhang, J.; Wahyudi, W.; Wu, Y.; Liu, G.; Li, Q.; Cheng, H.; Zhang, D.; Park, G.-T.; et al. Interfacial Model Deciphering High-Voltage Electrolytes for High Energy Density, High Safety, and Fast-Charging Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2102964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Yue, F.-S.; Li, S.-C.; Zhang, Y.; Tian, Z.-R.; Xu, Q.; Xin, S.; Guo, Y.-G. Advances of Polymer Binders for Silicon-Based Anodes in High Energy Density Lithium-Ion Batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Ge, S.; Leng, Y.; Liu, T.; Longchamps, R.S.; Yang, X.-G.; Gao, Y.; Wang, D.; Wang, D.; Wang, C.-Y. A New Approach to Both High Safety and High Performance of Lithium-Ion Batteries. Sci. Adv. 2020, 6, 7633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.-Y.; Kang, H.; Park, M.J. High-Capacity, Sustainable Lithium–Sulfur Batteries Based on Multifunctional Polymer Binders. ACS Appl. Energy Mater. 2021, 4, 2696–2706. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How Far Away Are Lithium-Sulfur Batteries From Commercialization? Front. Energy Res. 2019, 7, 123. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Chien, Y.-C.; Caballero, Á.; Morales, J.; Brandell, D. Recent Advances in Lithium-Sulfur Batteries Using Biomass-Derived Carbons as Sulfur Host. Renew. Sustain. Energy Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Danuta, H.; Juliusz, U. Electric Dry Cells and Storage Batteries. US3043896A, 10 July 1962. [Google Scholar]
- Peled, E.; Sternberg, Y.; Gorenshtein, A.; Lavi, Y. Lithium-Sulfur Battery: Evaluation of Dioxolane-Based Electrolytes. J. Electrochem. Soc. 1989, 136, 1621. [Google Scholar] [CrossRef]
- Ye, H.; Lee, J.Y. Solid Additives for Improving the Performance of Sulfur Cathodes in Lithium–Sulfur Batteries—Adsorbents, Mediators, and Catalysts. Small Methods 2020, 4, 1900864. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bakenova, Z.; Bakenov, Z. Carbon/Sulfur Composite Cathodes for Flexible Lithium/Sulfur Batteries: Status and Prospects. Front. Energy Res. 2015, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Kolosnitsyn, V.S.; Karaseva, E.V. Lithium-Sulfur Batteries: Problems and Solutions. Ehlektrokhimiya 2008, 44, 548–552. [Google Scholar] [CrossRef]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the Surface Chemical Aspects of Very High Energy Density, Rechargeable Li–Sulfur Batteries. J. Electrochem. Soc. 2009, 156, A694. [Google Scholar] [CrossRef]
- Chen, L.; Lv, A.; Guo, F.; Wang, M.; Jiao, S. Solid–Liquid Coexisting LiNO3 Electrolyte for Extremely Stable Lithium Metal Anodes on a Bare Cu Foil. ACS Sustain. Chem. Eng. 2020, 8, 706–713. [Google Scholar] [CrossRef]
- Pan, H.; Wei, X.; Henderson, W.A.; Shao, Y.; Chen, J.; Bhattacharya, P.; Xiao, J.; Liu, J. On the Way Toward Understanding Solution Chemistry of Lithium Polysulfides for High Energy Li–S Redox Flow Batteries. Adv. Energy Mater. 2015, 5, 1500113. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhao, M.; Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Huang, J.-Q. Toward Practical High-Energy-Density Lithium–Sulfur Pouch Cells: A Review. Adv. Mater. 2022, 34, 2201555. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Yan, C.; Huang, J.-Q.; Li, P.; Zhu, L.; Zhao, L.; Zhang, Y.; Zhu, W.; Yang, S.-T.; Zhang, Q. The Gap between Long Lifespan Li-S Coin and Pouch Cells: The Importance of Lithium Metal Anode Protection. Energy Storage Mater. 2017, 6, 18–25. [Google Scholar] [CrossRef]
- Schönherr, K.; Schumm, B.; Hippauf, F.; Lissy, R.; Althues, H.; Leyens, C.; Kaskel, S. Liquid Lithium Metal Processing into Ultrathin Metal Anodes for Solid State Batteries. Chem. Eng. J. Adv. 2022, 9, 100218. [Google Scholar] [CrossRef]
- Dong, L.; Liu, J.; Chen, D.; Han, Y.; Liang, Y.; Yang, M.; Yang, C.; He, W. Suppression of Polysulfide Dissolution and Shuttling with Glutamate Electrolyte for Lithium Sulfur Batteries. ACS Nano 2019, 13, 14172–14181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, X.; Liu, W.; Sun, X. Research Progress of the Solid State Lithium-Sulfur Batteries. Front. Energy Res. 2019, 7, 112. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium–Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, K.; Li, L.; Huang, H.; Liang, C.; Gan, Y.; He, X.; Zhang, W.; Zhang, J. High-Performance All-Solid-State Lithium–Sulfur Batteries Enabled by Slurry-Coated Li6PS5Cl/S/C Composite Electrodes. Front. Energy Res. 2021, 8, 606494. [Google Scholar] [CrossRef]
- Ohno, S.; Zeier, W.G. Toward Practical Solid-State Lithium–Sulfur Batteries: Challenges and Perspectives. Acc. Mater. Res. 2021, 2, 869–880. [Google Scholar] [CrossRef]
- Zech, C.; Hönicke, P.; Kayser, Y.; Risse, S.; Grätz, O.; Stamm, M.; Beckhoff, B. Polysulfide Driven Degradation in Lithium–Sulfur Batteries during Cycling—Quantitative and High Time-Resolution Operando X-ray Absorption Study for Dissolved Polysulfides Probed at Both Electrode Sides. J. Mater. Chem. A 2021, 9, 10231–10239. [Google Scholar] [CrossRef]
- Kumta, P.; Hepp, A.; Datta, M.; Velikokhatnyi, O. Lithium-Sulfur Batteries: Advances in High-Energy Density Batteries, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-819676-2. [Google Scholar]
- Kim, S.; Lim, W.-G.; Cho, A.; Jeong, J.; Jo, C.; Kang, D.; Han, S.M.; Han, J.W.; Lee, J. Simultaneous Suppression of Shuttle Effect and Lithium Dendrite Growth by Lightweight Bifunctional Separator for Li–S Batteries. ACS Appl. Energy Mater. 2020, 3, 2643–2652. [Google Scholar] [CrossRef]
- Suzanowicz, A.M.; Mei, C.W.; Mandal, B.K. Approaches to Combat the Polysulfide Shuttle Phenomenon in Li–S Battery Technology. Batteries 2022, 8, 45. [Google Scholar] [CrossRef]
- Yu, S.-H.; Huang, X.; Brock, J.D.; Abruña, H.D. Regulating Key Variables and Visualizing Lithium Dendrite Growth: An Operando X-Ray Study. J. Am. Chem. Soc. 2019, 141, 8441–8449. [Google Scholar] [CrossRef]
- He, Y.; Chang, Z.; Wu, S.; Zhou, H. Effective Strategies for Long-Cycle Life Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 6155–6182. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon–Sulphur Cathode for Lithium–Sulphur Batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Liu, Y.; Zhao, X.; Ananth, N.; Zheng, B.; Peng, L.; Huang, T.; Gao, W.; Gao, C. High-Quality Graphene Microflower Design for High-Performance Li–S and Al-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700051. [Google Scholar] [CrossRef]
- Konwer, S.; Gogoi, J.P.; Kalita, A.; Dolui, S.K. Synthesis of Expanded Graphite Filled Polyaniline Composites and Evaluation of Their Electrical and Electrochemical Properties. J. Mater. Sci. Mater. Electron. 2011, 22, 1154–1161. [Google Scholar] [CrossRef]
- Chulkin, P.; Łapkowski, M. An Insight into Ionic Conductivity of Polyaniline Thin Films. Materials 2020, 13, 2877. [Google Scholar] [CrossRef]
- Anderson, M.R.; Mattes, B.R.; Reiss, H.; Kaner, R.B. Conjugated Polymer Films for Gas Separations. Science 1991, 252, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- McVerry, B.; Anderson, M.; He, N.; Kweon, H.; Ji, C.; Xue, S.; Rao, E.; Lee, C.; Lin, C.-W.; Chen, D.; et al. Next-Generation Asymmetric Membranes Using Thin-Film Liftoff. Nano Lett. 2019, 19, 5036–5043. [Google Scholar] [CrossRef]
- Kweon, H.; Lin, C.-W.; Faruque Hasan, M.M.; Kaner, R.; Sant, G.N. Highly Permeable Polyaniline–Graphene Oxide Nanocomposite Membranes for CO2 Separations. ACS Appl. Polym. Mater. 2019, 1, 3233–3241. [Google Scholar] [CrossRef]
- Park, C.; Johnston, A.S.; Kweon, H. Physical Filtration Efficiency Analysis of a Polyaniline Hybrid Composite Filter with Graphite Oxide for Particulate Matter 2.5. J. Appl. Polym. Sci. 2020, 137, 49149. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Reddy, M.V.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials 2021, 11, 2476. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, S.L.; Wu, Z.-P.; Luan, D.; Lou, X.W. (David) A Highly Stable Lithium Metal Anode Enabled by Ag Nanoparticle–Embedded Nitrogen-Doped Carbon Macroporous Fibers. Sci. Adv. 2021, 7, eabg3626. [Google Scholar] [CrossRef]
- Luo, J.; Lee, R.-C.; Jin, J.-T.; Weng, Y.-T.; Fang, C.-C.; Wu, N.-L. A Dual-Functional Polymer Coating on a Lithium Anode for Suppressing Dendrite Growth and Polysulfide Shuttling in Li–S Batteries. Chem. Commun. 2017, 53, 963–966. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li–S Batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of Long Stability of Sulfur Cathode by Encapsulating Sulfur into Micropores of Carbon Spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553. [Google Scholar] [CrossRef]

| Year | Main Milestones | Challenges/Limitations | Approaches | References |
|---|---|---|---|---|
| 1960–2000s |
|
|
| [11,12] |
| 2002 |
|
|
| [13,14] |
| 2004–2010 |
|
|
| [15,16,17] |
| 2013–2017 |
|
|
| [18,19,20,21] |
| 2018–present |
|
|
| [22,23,24,25,26,27] |
| Future |
|
| [28] |
| Variable | Units | Definition |
|---|---|---|
| F | C/mol | Faraday’s constant |
| R | J/(mol K) | Universal gas constant |
| T | K | Temperature of the simulation |
| - | Volumetric fraction of the electrolyte phase | |
| S/m | Intrinsic electrolyte conductivity (tensor) | |
| m2/s | Intrinsic electrolyte diffusivity (scalar) | |
| - | Transference number for ions | |
| ) | - | Ratio between the effective and intrinsic electrolyte properties (diffusivity and conductivity) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweon, H.; Kim-Shoemaker, W. Mitigating Lithium Dissolution and Polysulfide Shuttle Effect Phenomena Using a Polymer Composite Layer Coating on the Anode in Lithium–Sulfur Batteries. Polymers 2022, 14, 4359. https://doi.org/10.3390/polym14204359
Kweon H, Kim-Shoemaker W. Mitigating Lithium Dissolution and Polysulfide Shuttle Effect Phenomena Using a Polymer Composite Layer Coating on the Anode in Lithium–Sulfur Batteries. Polymers. 2022; 14(20):4359. https://doi.org/10.3390/polym14204359
Chicago/Turabian StyleKweon, Hyukmin, and William Kim-Shoemaker. 2022. "Mitigating Lithium Dissolution and Polysulfide Shuttle Effect Phenomena Using a Polymer Composite Layer Coating on the Anode in Lithium–Sulfur Batteries" Polymers 14, no. 20: 4359. https://doi.org/10.3390/polym14204359







