Fire Retardancy and Dielectric Strength of Cyclotriphosphazene Compounds with Schiff Base and Ester Linking Units Attached to the Electron-Withdrawing Side Arm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis and Testing Instrument
2.2.1. Fourier Transform Infra-Red (FTIR)
2.2.2. Nuclear Magnetic Resonance (NMR)
2.2.3. CHN Elemental Analysis
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Limiting Oxygen Index (LOI)
2.2.6. Scanning Electron Microscope (SEM)
2.2.7. Alternating Current Breakdown Voltage
2.3. Preparation of Samples
2.4. Synthesis Methods
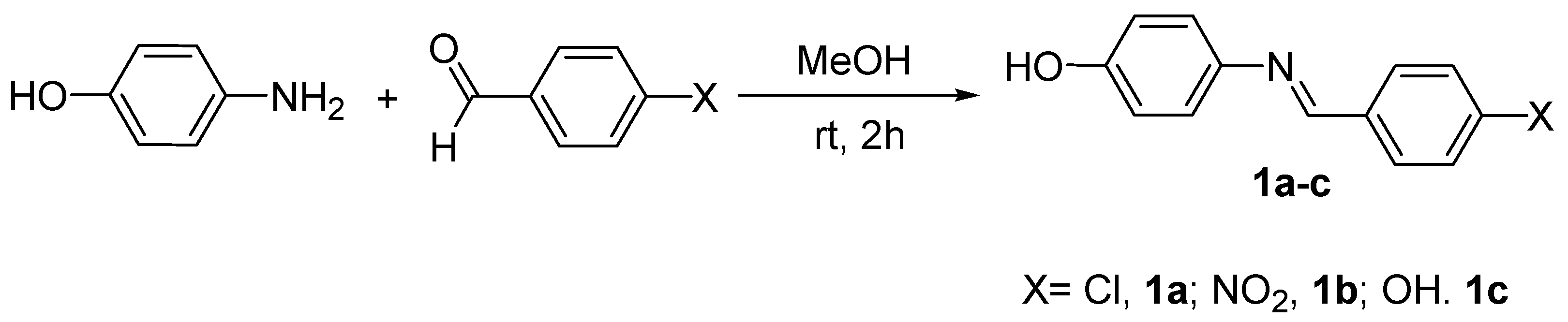
2.4.1. Synthesis of 4-[(4-substitutedbenzylidene)amino]phenol, 1a–c
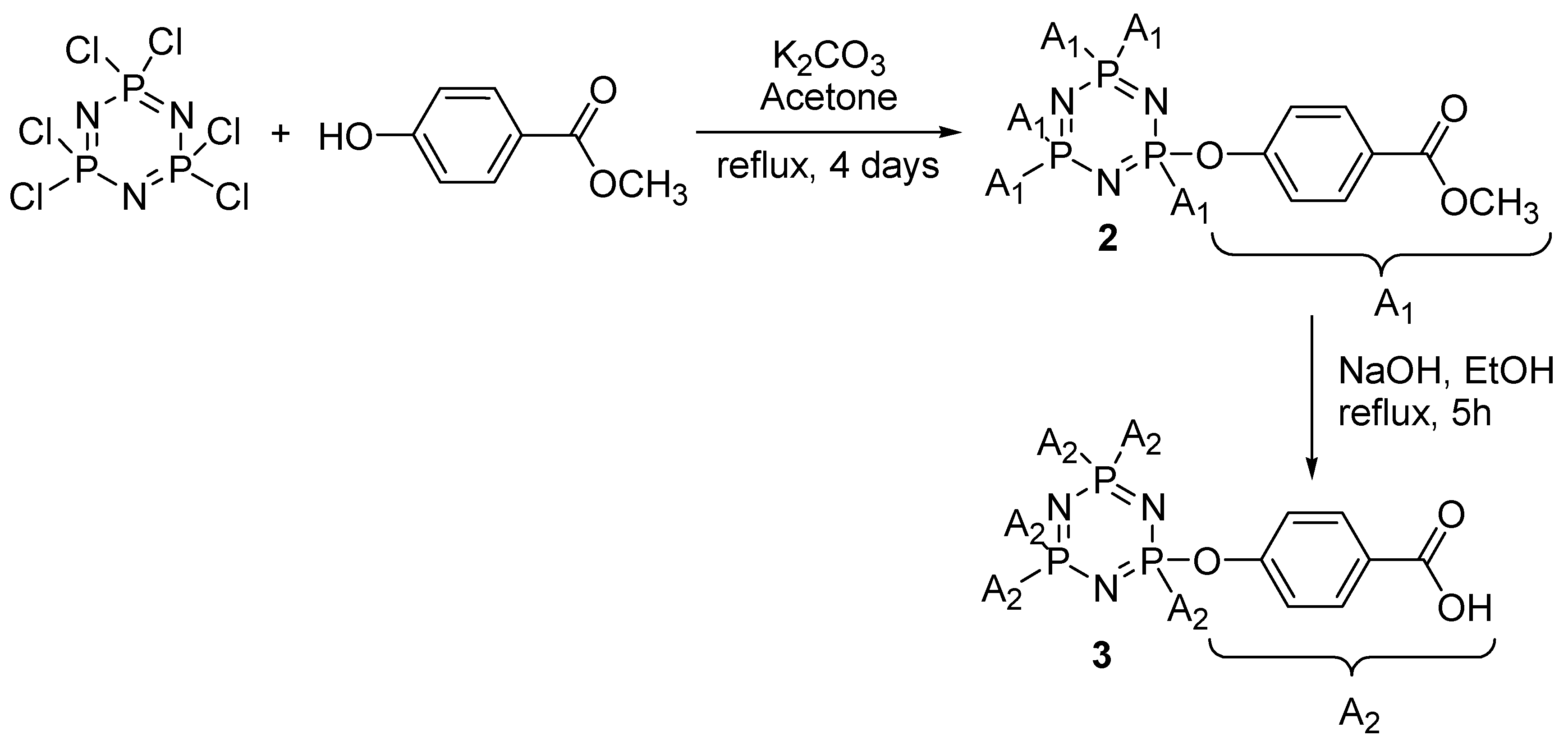
2.4.2. Synthesis of Hexakis(4-Benzoate-Phenoxy)Cyclotriphosphazene, 2
2.4.3. Synthesis of Hexakis(4-Carboxy-Phenoxy)Cyclotriphosphazene, 3
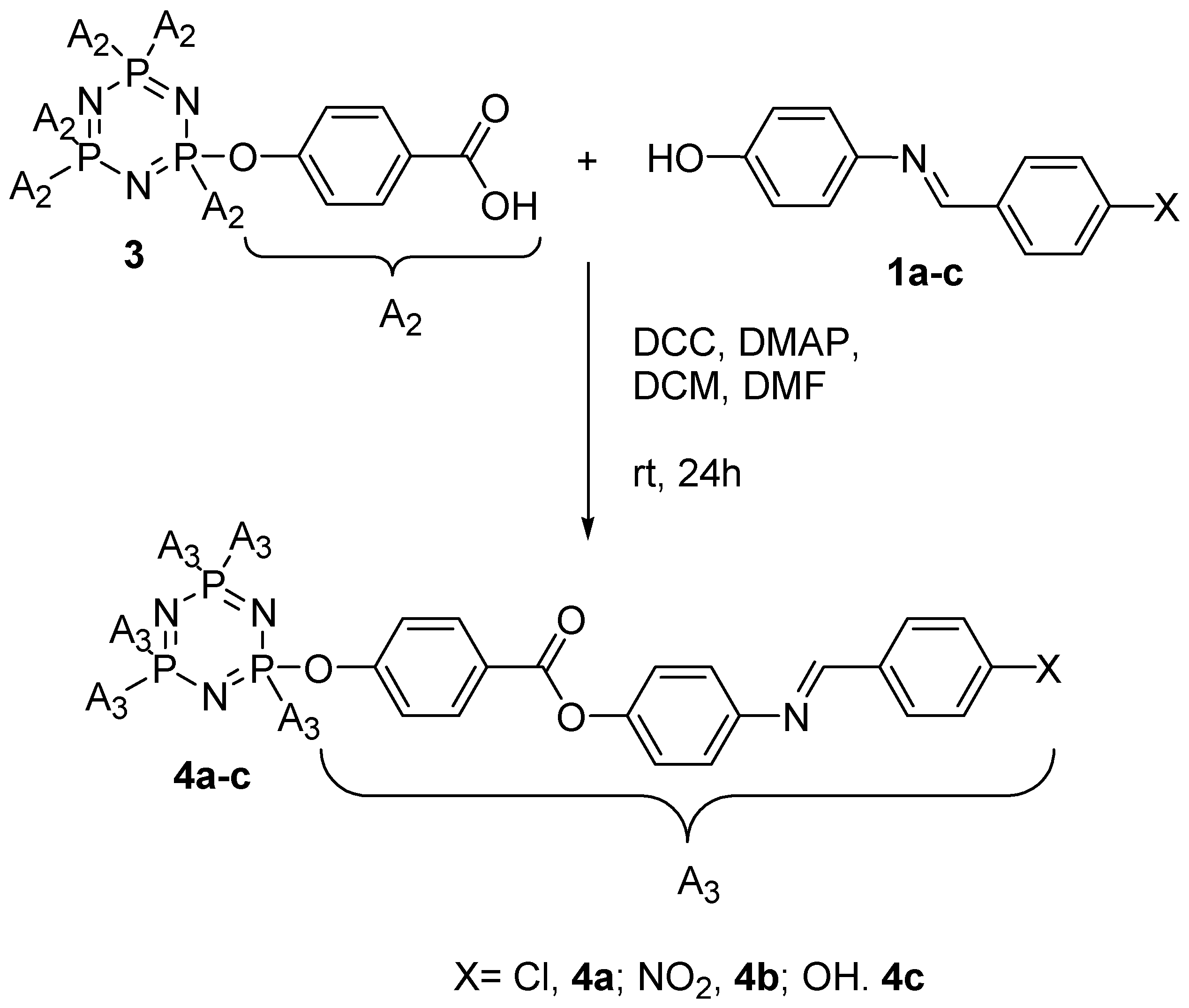
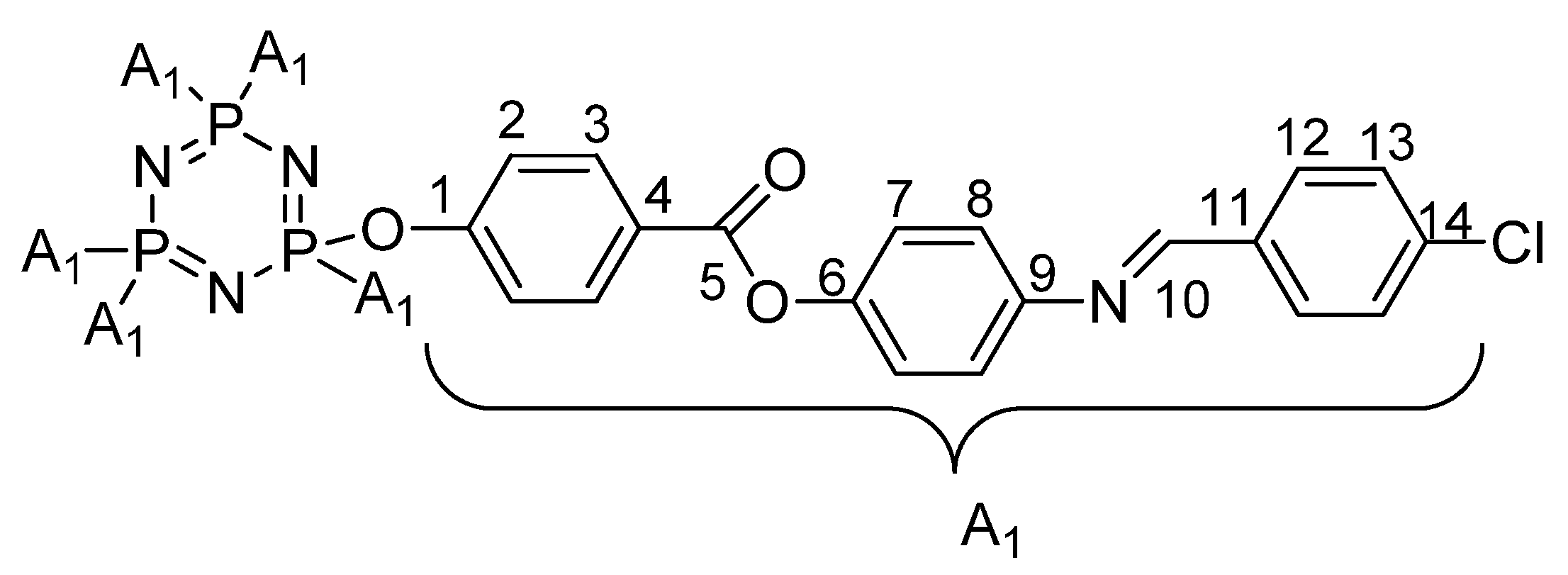
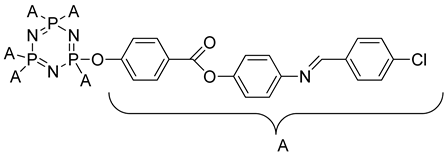
2.4.4. Synthesis of Hexakis[4-{((E)-4-(substituted)benzylidene)amino}phenyl]benzoate triazaphosphazene, 4a–c
3. Results and Discussion
3.1. CHN Elemental Analysis
3.2. FTIR Spectral Discussion
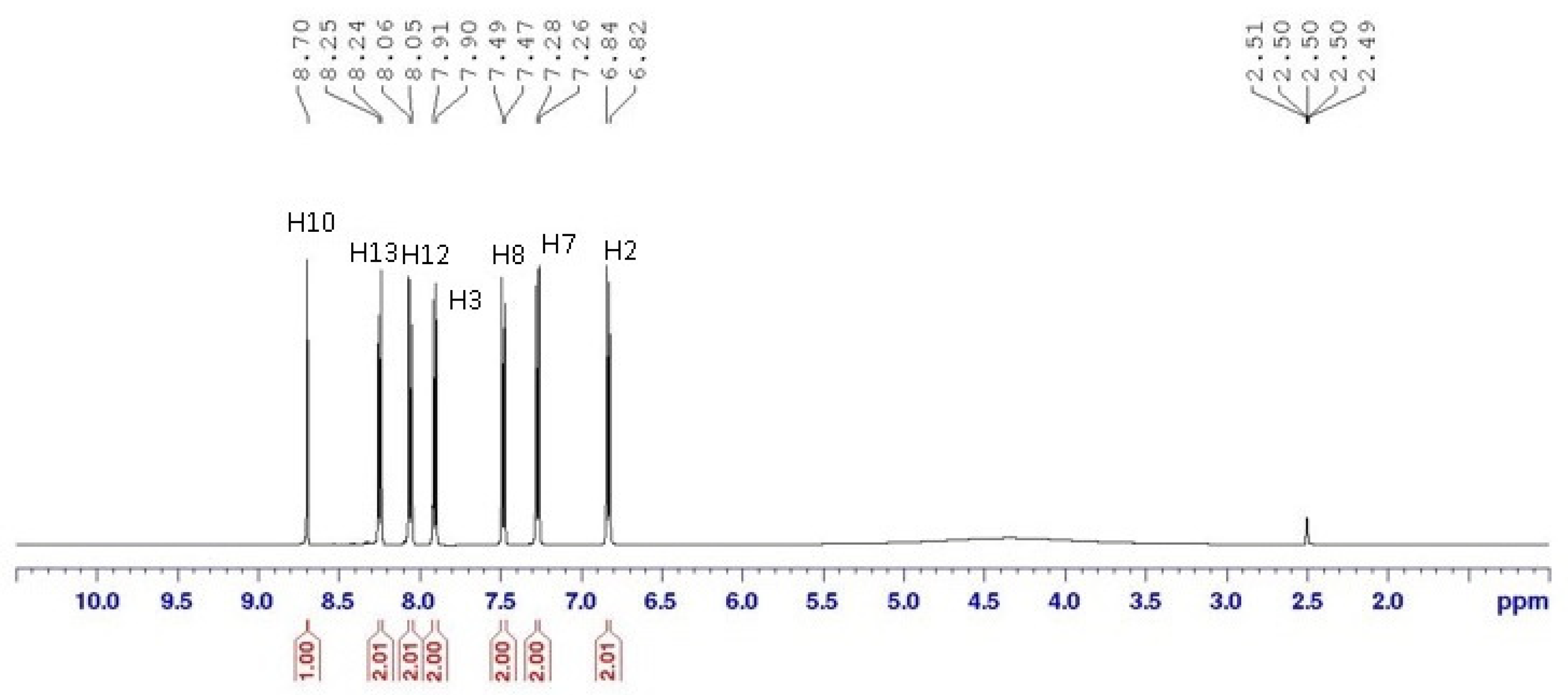
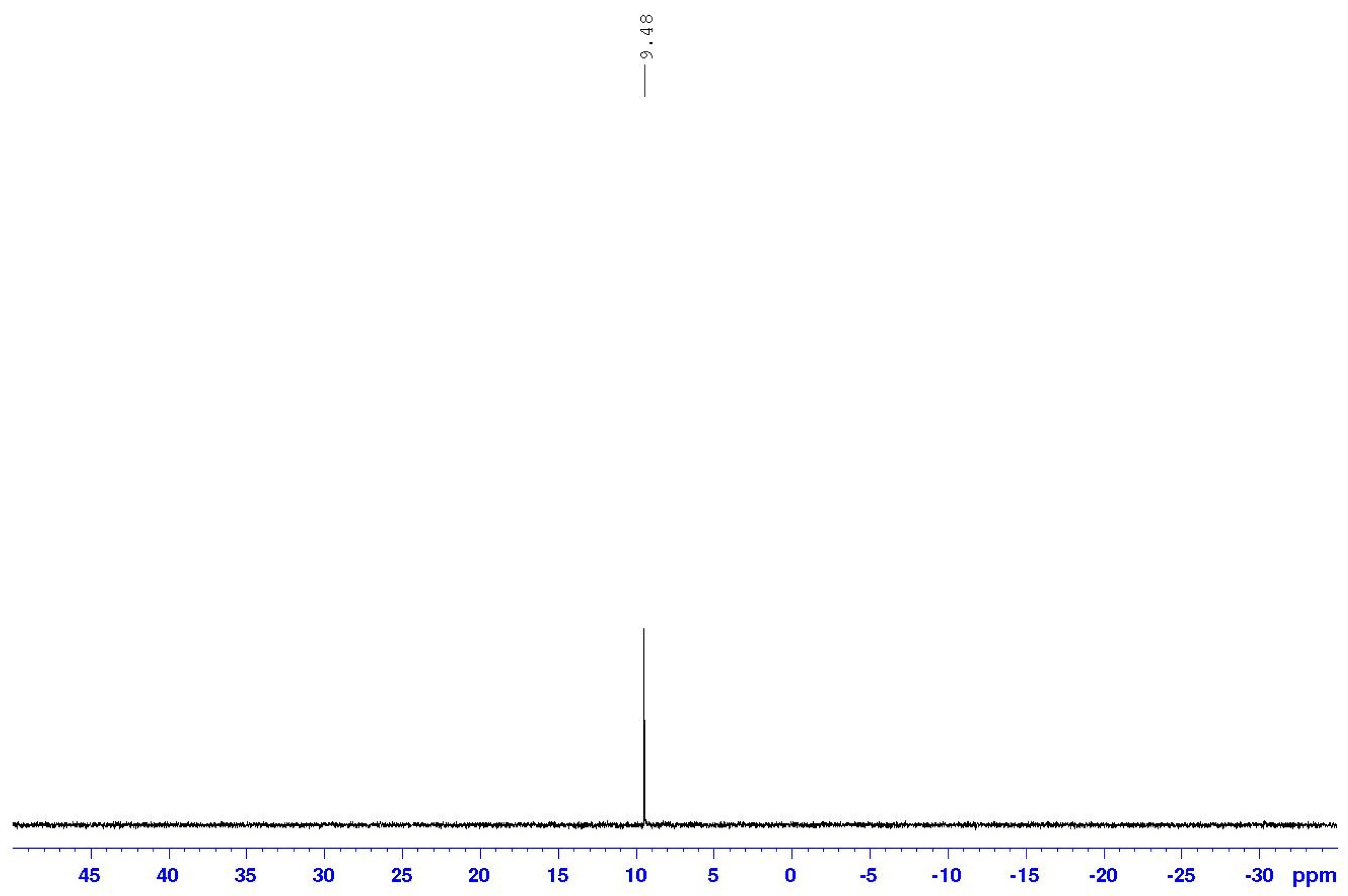
3.3. NMR Spectral Discussion
3.4. Thermogravimetric Analysis (TGA)
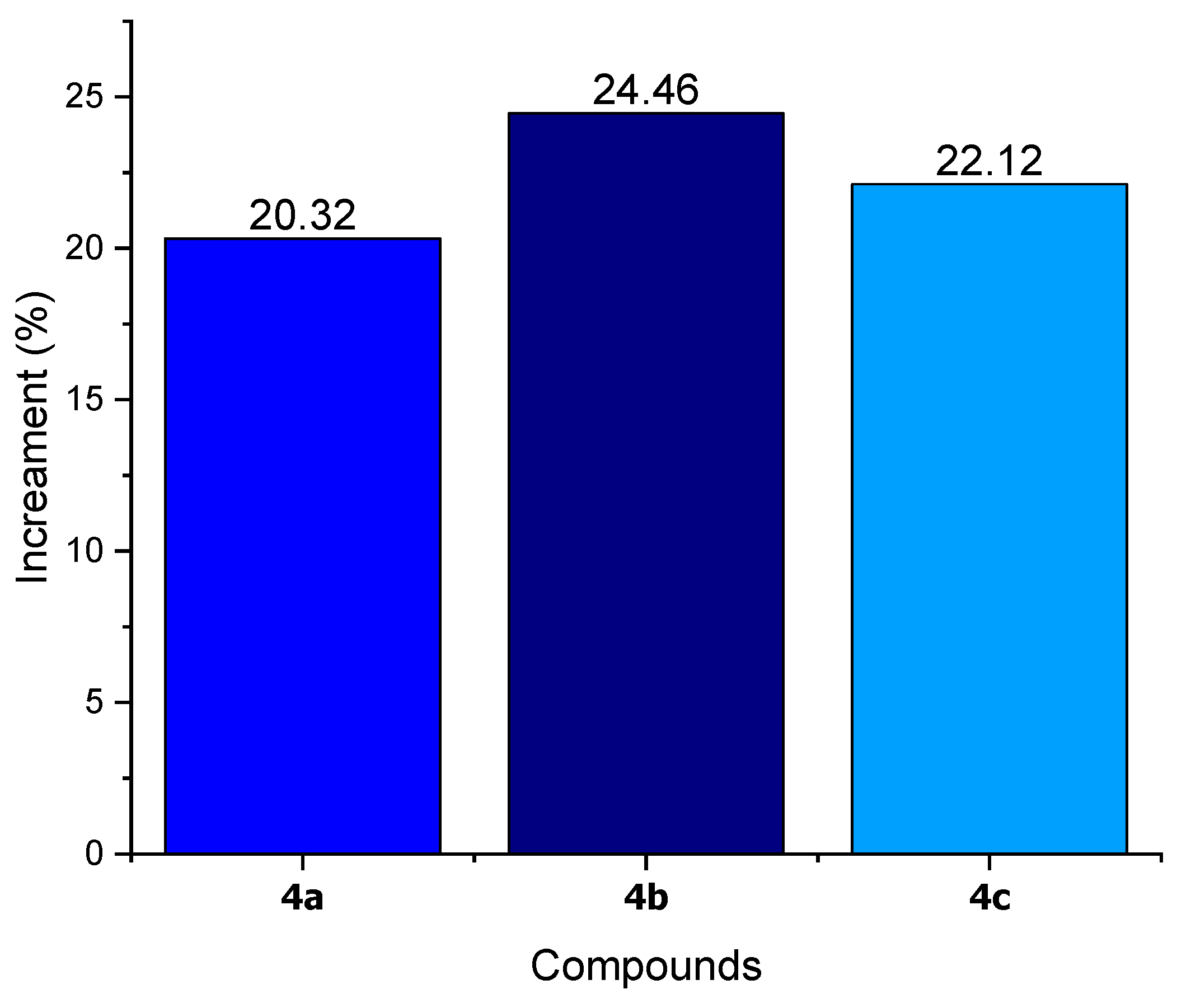
3.5. Fire Retardant Testing
3.6. Residue Analysis
3.7. Dielectric Properties Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Billah, S.M. Dielectric Polymers. In Functional Polymers. Polymers and Polymeric Composites: A Reference Series; Jafar Mazumder, M., Sheardown, H., Al-Ahmed, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Hu, J.; Huang, Y.; Zeng, X.; Li, Q.; Ren, L.; Sun, R.; Xu, J.-B.; Wong, C.-P. Polymer composite with enhanced thermal conductivity and mechanical strength through orientation manipulating of BN. Compos. Sci. Technol. 2018, 160, 127–137. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, W.; Huang, Z.; Liu, G.; Wu, B. Tribological performances of epoxy resin composite coatings using hexagonal boron nitride and cubic boron nitride nanoparticles as additives. Chem. Phys. Lett. 2019, 732, 136646. [Google Scholar] [CrossRef]
- Dagdag, O.; El Gouri, M.; El Mansouri, A.; Outzourhit, A.; El Harfi, A.; Cherkaoui, O.; El Bachiri, A.; Hamed, O.; Jodeh, S.; Hanbali, G.; et al. Rheological and electrical study of a composite material based on an epoxy polymer containing cyclotriphosphazene. Polymers 2020, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Dagdag, O.; Berradi, M.; El Bouchti, M.; Assouag, M.; Elharfi, A. development rheological and anti-corrosion property of epoxy polymer and its composite. Heliyon 2019, 5, e02789. [Google Scholar] [CrossRef] [PubMed]
- Dagdag, O.; Essamri, A.; El Gana, L.; El Bouchti, M.; Hamed, O.; Cherkaoui, O.; Jodeh, S.; El Harfi, A. Synthesis, characterization and rheological properties of epoxy monomers derived from bifunctional aromatic amines. Polym. Bull. 2018, 76, 4399–4413. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Yang, S.; Zhang, Q.; Hu, Y.; Ding, G.; Huo, S. Aminobenzothiazole-substituted cyclotriphosphazene derivative as reactive flame retardant for epoxy resin. React. Funct. Polym. 2020, 146, 104412. [Google Scholar] [CrossRef]
- Yang, K.; Chen, W.; Zhao, Y.; He, Y.; Chen, X.; Du, B.; Yang, W.; Zhang, S.; Fu, Y. Enhancing Dielectric Strength of Epoxy Polymers by Constructing Interface Charge Traps. ACS Appl. Mater. Interfaces 2021, 13, 25850–25857. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schartel, B. Melamine poly(metal phosphates) as flame retardant in epoxy resin: Performance, modes of action, and synergy. J. Appl. Polym. Sci. 2016, 133, 43549. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-Efficiency flame retardant behavior of Bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Müller, P.; Morys, M.; Sut, A.; Jäger, C.; Illerhaus, B.; Schartel, B. Melamine poly(zinc phosphate) as flame retardant in epoxy resin: Decomposition pathways, molecular mechanisms and morphology of fire residues. Polym. Degrad. Stab. 2016, 130, 307–319. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chem. Eng. J. 2022, 428, 131173. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Yang, S.; Yang, L.; Cao, J.; Yang, K. The synthesis, curing kinetics, thermal properties and flame rertardancy of cyclotriphosphazene-containing multifunctional epoxy resin. Thermochim. Acta 2019, 680, 178348. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Yu, R.; Liao, J. Confined crystallization and degradation of six-arm star PCL with core of cyclotriphosphazene in epoxy thermosets. Eur. Polym. J. 2020, 139, 109989. [Google Scholar] [CrossRef]
- Cui, J.; Yu, H.; Li, T.; Zhu, Y.; Zhu, A.; Mao, X.; Qi, C.; Yang, B.; Guo, J.; Mu, B.; et al. Improvement of mechanical properties and flame retardancy of epoxy resin by phosphorylated cyclotriphosphazene hyperbranched polymeric flame retardants. Polymer 2022, 256, 125182. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, L.-L.; Zhao, B. A novel aminothiazole-based cyclotriphosphazene derivate towards epoxy resins for high flame retardancy and smoke suppression. Polym. Degrad. Stab. 2021, 190, 109651. [Google Scholar] [CrossRef]
- Liang, W.-J.; Zhao, B.; Zhao, P.-H.; Zhang, C.-Y.; Liu, Y.-Q. Bisphenol-S bridged penta(anilino)cyclotriphosphazene and its application in epoxy resins: Synthesis, thermal degradation, and flame retardancy. Polym. Degrad. Stab. 2017, 135, 140–151. [Google Scholar] [CrossRef]
- Usri, S.N.K.; Jamain, Z.; Makmud, M.Z.H. A review on synthesis, structural, flame retardancy and dielectric properties of hexasubstituted cyclotriphosphazene. Polymers 2021, 13, 2916. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Jamain, Z.; Khairuddean, M.; Lotus, M.; Manaff, N.; Makmud, M. Synthesis and Characterization of Hexasubstituted Cyclotriphosphazene Derivatives with Azo Linking Units. Malays. J. Chem. 2020, 22, 125–140. [Google Scholar]
- Qian, L.-J.; Ye, L.-J.; Xu, G.-Z.; Liu, J.; Guo, J.-Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011, 96, 1118–1124. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Zarybnicka, L.; Machotova, J.; Kopecka, R.; Sevcik, R.; Hudakova, M.; Pokorny, J.; Sal, J. Effect of cyclotriphosphazene-based curing agents on the flame resistance of epoxy resins. Polymers 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Synthesis of novel liquid crystalline and fire retardant molecules based on six-armed cyclotriphosphazene core containing Schiff base and amide linking units. RSC Adv. 2020, 10, 28918–28934. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. Improvement of the flame retardant and thermomechanical properties of epoxy resins by a vanillin-derived cyclotriphosphazene-cored triazole compound. Polym. Degrad. Stab. 2022, 204, 110088. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, W.-J.; Wang, J.-S.; Li, F.; Liu, Y.-Q. Synthesis of a novel bridged-cyclotriphosphazene flame retardant and its application in epoxy resin. Polym. Degrad. Stab. 2016, 133, 162–173. [Google Scholar] [CrossRef]
- Salasinska, K.; Celiński, M.; Barczewski, M.; Leszczyński, M.K.; Borucka, M.; Kozikowski, P. Fire behavior of flame retarded unsaturated polyester resin with high nitrogen content additives. Polym. Test. 2020, 84, 106379. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Liquid-Crystal and Fire-Retardant Properties of New Hexasubstituted Cyclotriphosphazene Compounds with Two Schiff Base Linking Units. Molecules 2020, 25, 2122. [Google Scholar] [CrossRef]
- Koran, K.; Özen, F.; Biryan, F.; Görgülü, A.O. Synthesis, structural characterization and dielectric behavior of new oxime-cyclotriphosphazene derivatives. J. Mol. Struct. 2016, 1105, 135–141. [Google Scholar] [CrossRef]
- Koran, K.; Özen, F.; Biryan, F.; Demirelli, K.; Görgülü, A.O. Eu+3-doped chalcone substituted cyclotriphosphazenes: Synthesis, characterizations, thermal and dielectrical properties. Inorg. Chim. Acta 2016, 450, 162–169. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M. Synthesis and Mesophase behaviour of benzylidene-based molecules containing two azomethine units. J. Phys. Conf. Ser. 2021, 1882, 012120. [Google Scholar] [CrossRef]
- Barberá, J.; Jiménez, J.; Laguna, A.; Oriol, L.; Pérez, S.; Serrano, J.L. Cyclotriphosphazene as a dendritic core for the preparation of columnar supermolecular liquid crystals. Chem. Mater. 2006, 18, 5437–5445. [Google Scholar] [CrossRef]
- Joaquín, B.; Manuel, B.; Josefina, J.; Antonio, L.; Pilar, M.M.; Luis, O.; José, L.S.; Irene, Z. Columnar mesomorphic organizations in cyclotriphosphazene. J. Am. Chem. Soc. 2005, 127, 8994–9002. [Google Scholar]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T.; Rahman, A.B.A. Synthesis, characterization and mesophase transition of hexasubstituted cyclotriphosphazene molecules with Schiff base and azo linking units and determination of their fire retardant properties. Macromol. Res. 2021, 29, 331–341. [Google Scholar] [CrossRef]
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N. Elemental analysis of organic compounds with the use of automated CHNS analyzers. J. Anal. Chem. 2008, 63, 1094–1106. [Google Scholar] [CrossRef]
- Ma, H.-X.; Li, J.-J.; Qiu, J.-J.; Liu, Y.; Liu, C.-M. Renewable Cardanol-Based Star-Shaped Prepolymer Containing a Phosphazene Core as a Potential Biobased Green Fire-Retardant Coating. ACS Sustain. Chem. Eng. 2017, 5, 350–359. [Google Scholar] [CrossRef]
- Liu, F.; Wei, H.; Huang, X.; Zhang, J.; Zhou, Y.; Tang, X. Preparation and Properties of Novel Inherent Flame-Retardant Cyclotriphosphazene-Containing Epoxy Resins. J. Macromol. Sci. Part B 2010, 49, 1002–1011. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Guo, X.; Yang, R. Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites. Polym. Degrad. Stab. 2010, 95, 2541–2546. [Google Scholar] [CrossRef]
- Taip, N.A.M.; Jamain, Z.; Palle, I. Fire-Retardant property of hexasubstituted cyclotriphosphazene derivatives with Schiff base linking unit applied as an additives in polyurethane coating for wood fabrication. Polymers 2022, 14, 3768. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, K.; Hagibeygi, M. New aromatic polyamide with azo and phosphine oxide groups in the main chain. Turk. J. Chem. 2007, 31, 65–73. [Google Scholar]
- Liu, Y.-L.; Chiu, Y.-C.; Chen, T.-Y. Phosphorus-containing polyaryloxydiphenylsilanes with high flame retardance arising from a phosphorus-silicon synergistic effect. Polym. Int. 2003, 52, 1256–1261. [Google Scholar] [CrossRef]
- Zhang, X.; Akram, R.; Zhang, S.; Ma, H.; Wu, Z.; Wu, D. Hexa(eugenol)cyclotriphosphazene modified bismaleimide resins with unique thermal stability and flame retardancy. React. Funct. Polym. 2017, 113, 77–84. [Google Scholar] [CrossRef]
- Abd El-Wahab, H. Synthesis and characterization of the flame retardant properties and corrosion resistance of Schiff’s base compounds incorporated into organic coating. Pigment Resin Technol. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- De-Yi, W. Novel fire retardant polymers and composite materials. In Woodhead Publishing Series in Composites Science and Engineering: Number 73; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Synthesis of new star-shaped liquid crystalline cyclotriphosphazene derivatives with fire retardancy bearing amide-azo and azo-azo linking units. Int. J. Mol. Sci. 2020, 21, 4267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.H.; Yu, S. Effect of chlorine-containing polymer additive on dielectric performance of polymer dielectric films. Electron. Lett. 2014, 50, 357–358. [Google Scholar] [CrossRef]
- Madhura, V.; Kulkarni, M.V.; Badami, S.; Yenagi, J.; Tonannavar, J. Effect of nitro groups on the photo physical properties of benzimidazolone: A solvatochromic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 137–143. [Google Scholar]




| Element | Compound 4a | Compound 4b | Compound 4c | |||
|---|---|---|---|---|---|---|
| Theoretical (%) | Experimental (%) | Theoretical (%) | Experimental (%) | Theoretical (%) | Experimental (%) | |
| C | 64.36 | 64.27 | 62.59 | 62.50 | 67.70 | 67.53 |
| H | 3.51 | 3.48 | 3.41 | 3.35 | 3.98 | 3.88 |
| N | 5.63 | 5.58 | 9.12 | 9.06 | 5.92 | 5.87 |
| Compounds | Temperature of 5% Weight Loss (°C) | Temperature of most Rapid Weight Loss (°C) | Residue at 700 (%) |
|---|---|---|---|
 Compound 4a | 160.89 | 162.13 | 22.1 |
 Compound 4b | 192.88 | 189.78 | 34.2 |
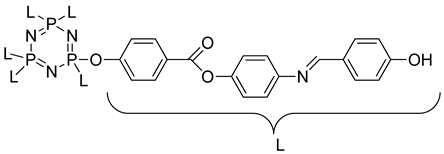 Compound 4c | 141.01 | 186.21 | 19.07 |
| Compound | Limiting Oxygen Index (%) | Side Arm |
|---|---|---|
| Pure Epoxy Resin | 22.75 (±0.00) | - |
| Epoxy Resin + HCCP | 24.71 (±0.00) | - |
| Epoxy Resin + 1 wt.% Compound 4a | 26.71 (±0.00) | –Cl |
| Epoxy Resin + 1 wt.% Compound 4b | 26.90 (±0.00) | –NO2 |
| Epoxy Resin + 1 wt.% Compound 4c | 26.53 (±0.00) | –OH |
| Compound | Breakdown Voltage (kV/mm−1) | Side Arm |
|---|---|---|
| Pure Epoxy Resin | 19.54 | - |
| Epoxy Resin + 1 wt.% Compound 4a | 23.51 | –Cl |
| Epoxy Resin + 1 wt.% Compound 4b | 24.31 | −NO2 |
| Epoxy Resin + 1 wt.% Compound 4c | 23.56 | −OH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usri, S.N.K.; Jamain, Z.; Makmud, M.Z.H. Fire Retardancy and Dielectric Strength of Cyclotriphosphazene Compounds with Schiff Base and Ester Linking Units Attached to the Electron-Withdrawing Side Arm. Polymers 2022, 14, 4378. https://doi.org/10.3390/polym14204378
Usri SNK, Jamain Z, Makmud MZH. Fire Retardancy and Dielectric Strength of Cyclotriphosphazene Compounds with Schiff Base and Ester Linking Units Attached to the Electron-Withdrawing Side Arm. Polymers. 2022; 14(20):4378. https://doi.org/10.3390/polym14204378
Chicago/Turabian StyleUsri, Siti Nur Khalidah, Zuhair Jamain, and Mohamad Zul Hilmey Makmud. 2022. "Fire Retardancy and Dielectric Strength of Cyclotriphosphazene Compounds with Schiff Base and Ester Linking Units Attached to the Electron-Withdrawing Side Arm" Polymers 14, no. 20: 4378. https://doi.org/10.3390/polym14204378
APA StyleUsri, S. N. K., Jamain, Z., & Makmud, M. Z. H. (2022). Fire Retardancy and Dielectric Strength of Cyclotriphosphazene Compounds with Schiff Base and Ester Linking Units Attached to the Electron-Withdrawing Side Arm. Polymers, 14(20), 4378. https://doi.org/10.3390/polym14204378








