Preparing Hydrophobic Cellulose Nanofibers-SiO2 Films and Coating by One-Step Mechanochemical Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
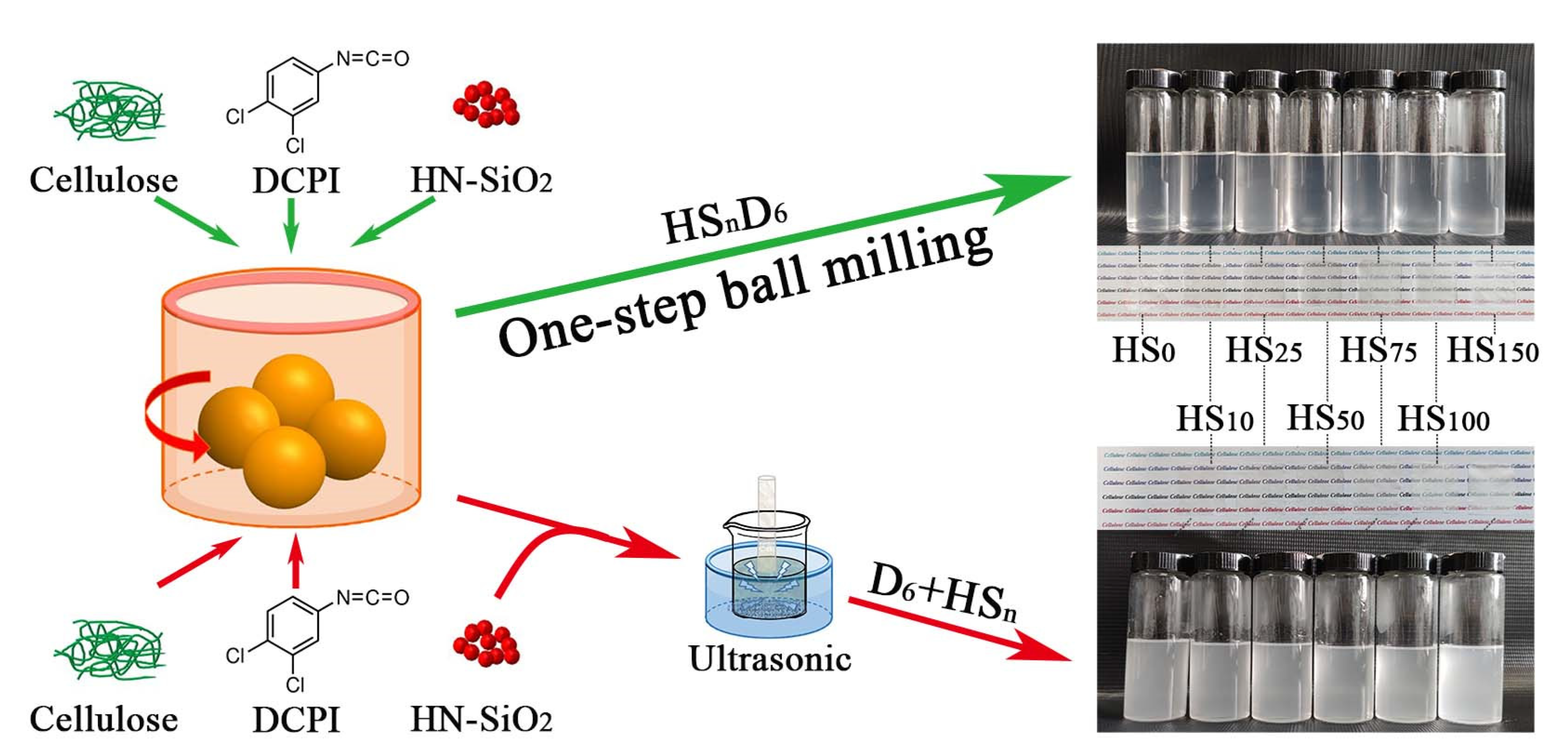
2.2. Preparation of Hydrophobic CNF/HN-SiO2 Suspension
2.3. Preparation of CNF/HN-SiO2 Films
2.4. Preparation of HSnD6 Coated Paper
2.5. Characterization
3. Results and Discussion
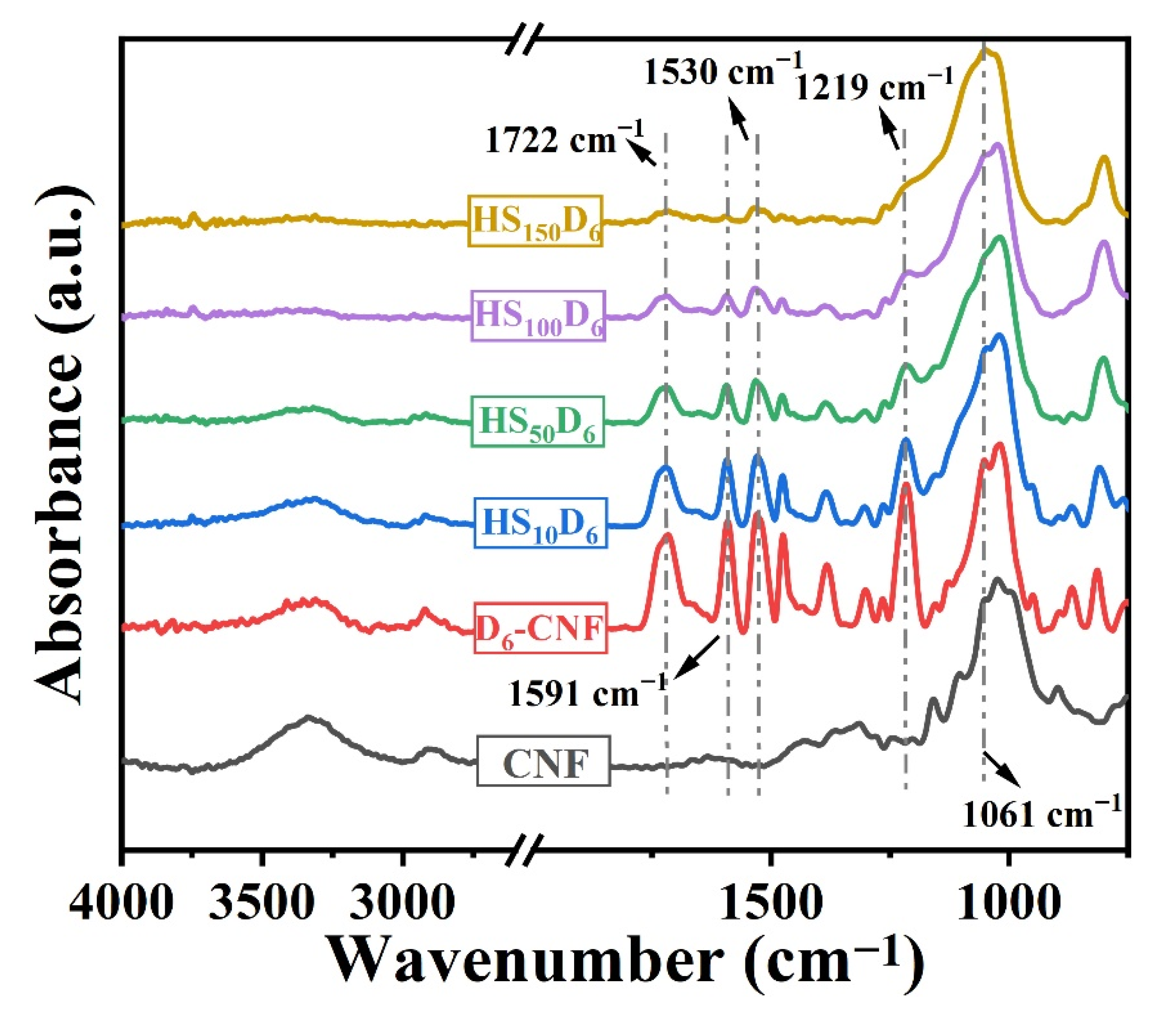
3.1. Chemical Structure of CNF in HSnD6
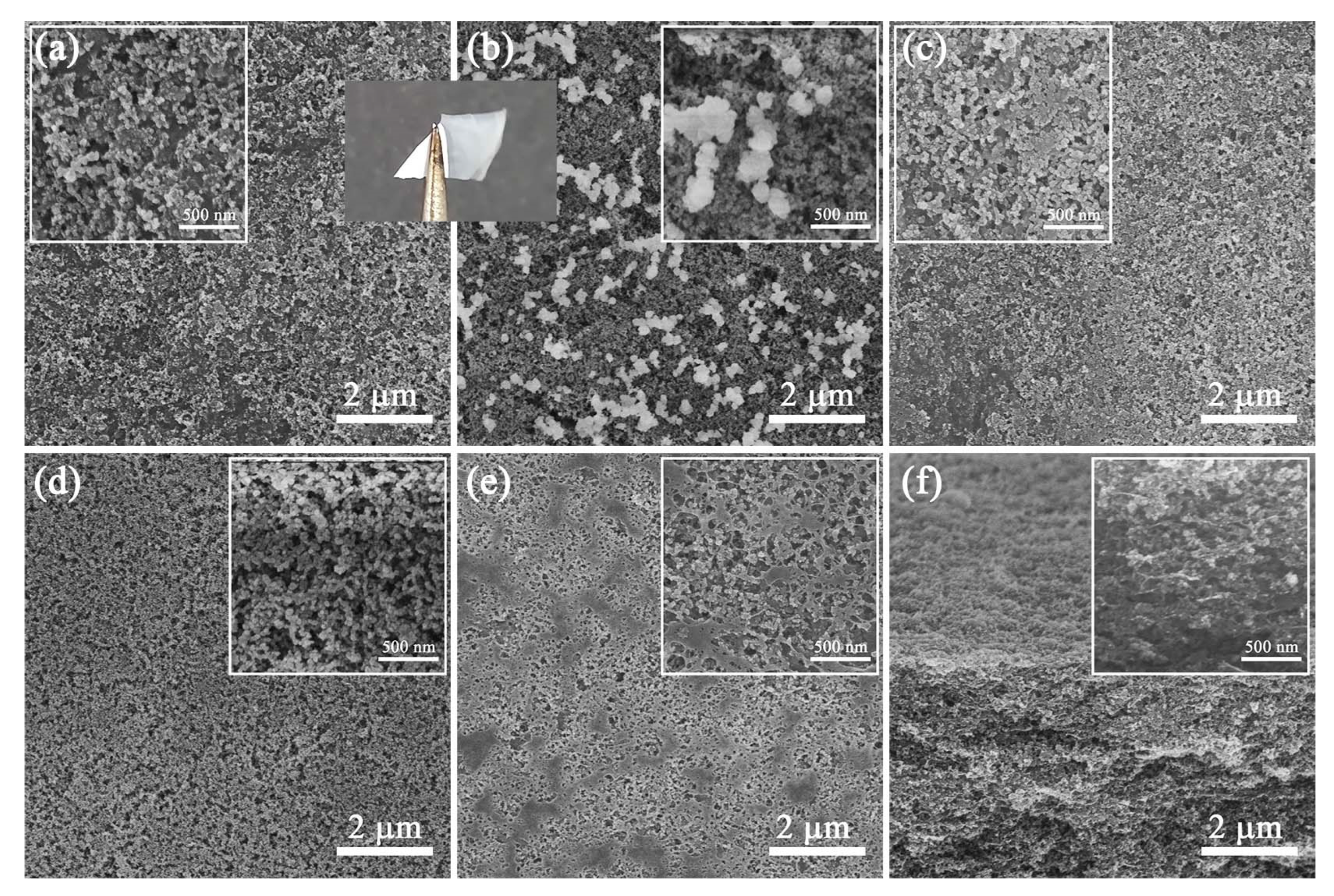
3.2. Morphology of HSnD6
3.3. Morphology of CNF/HN-SiO2 Films
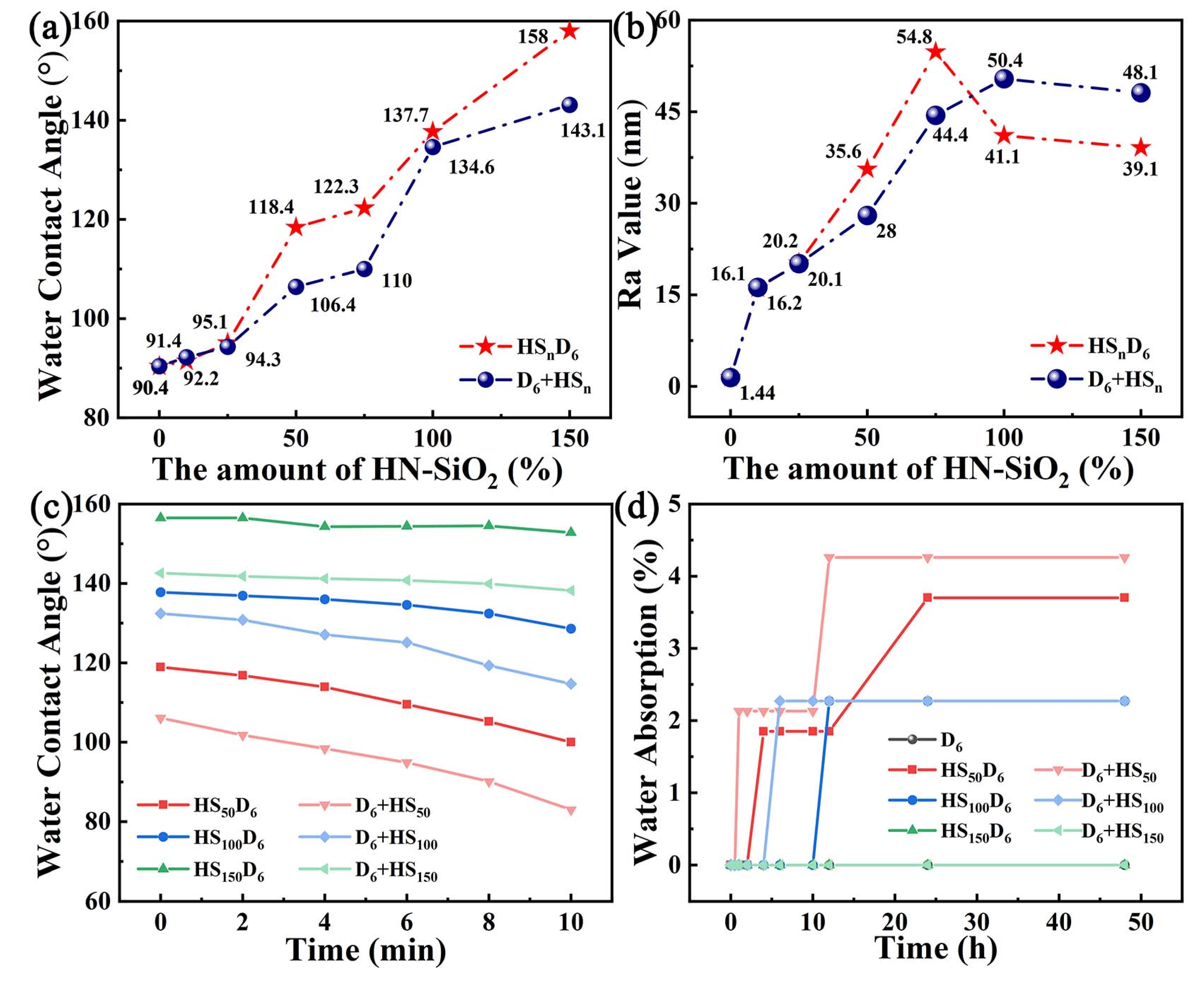
3.4. Wetting Properties of CNF/HN-SiO2 Films
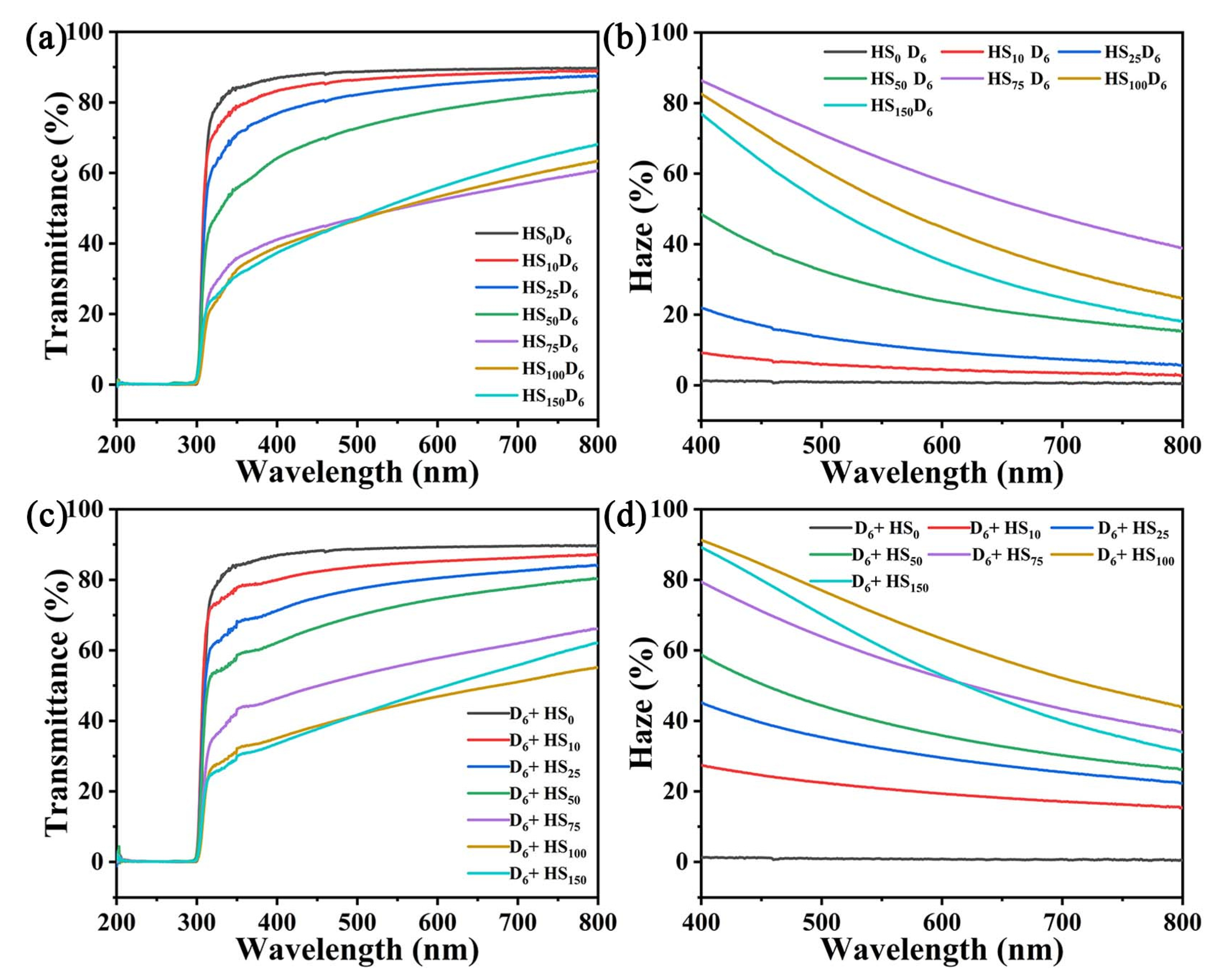
3.5. Optical Properties of CNF/HN-SiO2 Films
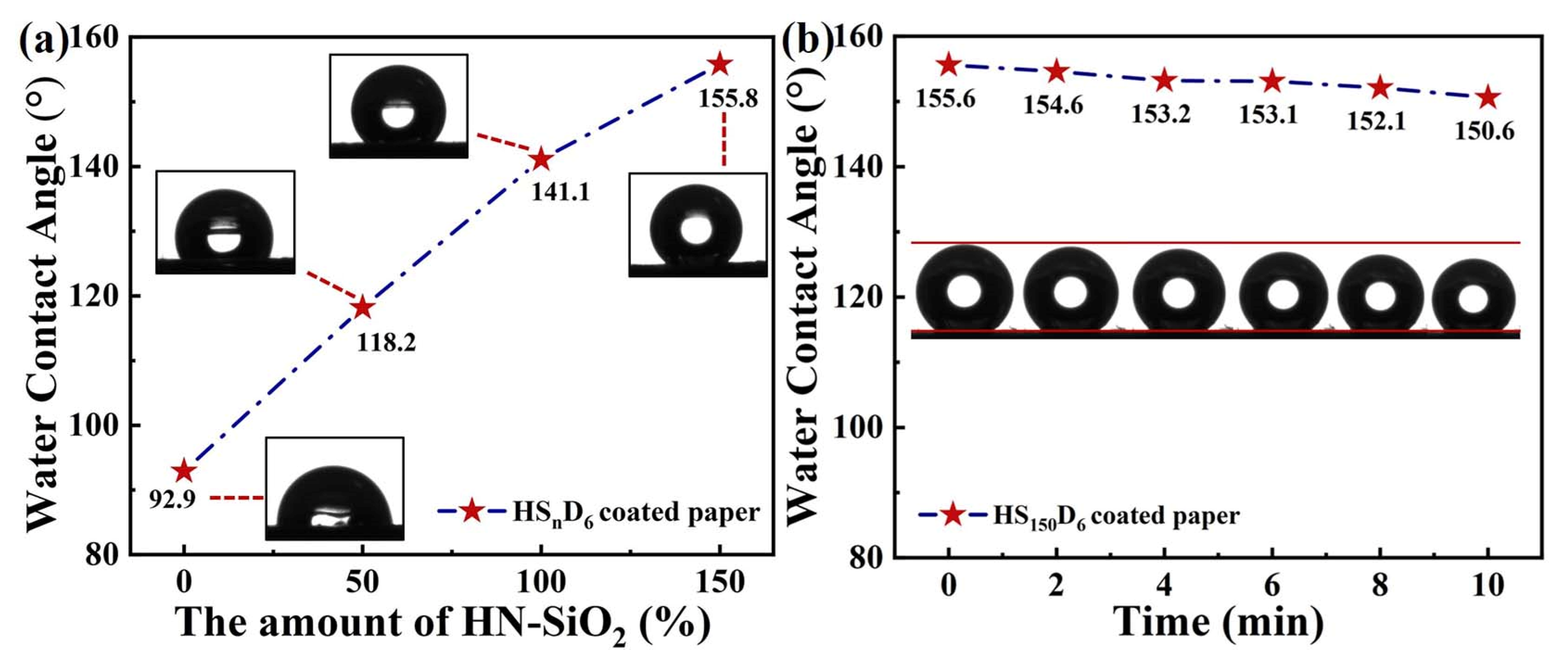
3.6. Wetting Properties of HSnD6 Coated Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically Durable Superhydrophobic Surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tang, X.; Qiu, Z.; Zhu, W.; Wang, Y.; Zhu, Y.L.; Xiao, Z.; Wang, H.; Liang, D.; Li, J.; et al. Cellulose-Based Superhydrophobic Surface Decorated with Functional Groups Showing Distinct Wetting Abilities to Manipulate Water Harvesting. ACS Appl. Mater. Interfaces 2020, 12, 40968–40978. [Google Scholar] [CrossRef] [PubMed]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, M.; Hou, Y.; Zhang, L.; Wang, D.; Shen, H.; Ding, C.; Li, C.; Fu, S. Nonfluorinated Multifunctional Superhydrophobic Cellulose Sheet with Polysaccharide B Biopolymer-Based Hierarchical Rough Composite Structure. ACS Sustain. Chem. Eng. 2020, 8, 8505–8518. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, P.; Kuang, Y.; Liu, Y.; Lin, D.; Peng, C.; Wen, Z.; Fang, Z. Durable superhydrophobic paper enabled by surface sizing of starch-based composite films. Appl. Surf. Sci. 2017, 409, 45–51. [Google Scholar] [CrossRef]
- Du, C.; Wang, J.; Chen, Z.; Chen, D. Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl. Surf. Sci. 2014, 313, 304–310. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, C.; Liu, W.; Liang, L.; Xie, Y.; Shi, H.; Zhang, F.; Pi, K. A water-rich system of constructing durable and fluorine-free superhydrophobic surfaces for oil/water separation. Appl. Surf. Sci. 2020, 507, 145165–145175. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, S. Reconstructing micro/nano hierarchical structures particle with nanocellulose for superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 171–179. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Hu, Z.; Dzapata, R.L.; Xu, Y.; Christie, P.; Guo, D.; Li, J. Facile method for the preparation of superhydrophobic cellulosic paper. Appl. Surf. Sci. 2019, 496, 143648–143657. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. A facile method for fabricating robust cellulose nanocrystal/SiO2 superhydrophobic coatings. J. Colloid Interface Sci. 2019, 536, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, X.; Xue, H.; Tang, L.; Li, R.K.Y. Facile preparation of hybrid microspheres for super-hydrophobic coating and oil-water separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Junyan, L.; Li, W.; Jingxian, B.; Ling, H. Durable superhydrophobic/highly oleophobic coatings from multi-dome SiO2 nanoparticles and fluoroacrylate block copolymers on flat substrates. J. Mater. Chem. A 2015, 3, 20134–20144. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of coral-like superhydrophobic coating on filter paper for water–oil separation. Appl. Surf. Sci. 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Gong, X. Large-Scale Spraying Fabrication of Robust Fluorine-Free Superhydrophobic Coatings Based on Dual-Sized Silica Particles for Effective Antipollution and Strong Buoyancy. Langmuir 2021, 37, 6042–6051. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Ben, K.; Chen, Z.; Wang, Y.; Guan, Z. Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl. Surf. Sci. 2015, 339, 94–101. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, L.; Liu, J.; Wang, C.; Wu, M. Cellulose nanofiber assisted dispersion of hydrophobic SiO2 nanoparticles in water and its superhydrophobic coating. Carbohydr. Polym. 2022, 290, 119504. [Google Scholar] [CrossRef]
- Le, D.; Kongparakul, S.; Samart, C.; Phanthong, P.; Karnjanakom, S.; Abudula, A.; Guan, G. Preparing hydrophobic nanocellulose-silica film by a facile one-pot method. Carbohydr. Polym. 2016, 153, 266–274. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Ewulonu, C.M.; Chen, X.; Wu, M.; Huang, Y. Fabrication of superhydrophobic and degradable cellulose paper materials for straw application. Cellulose 2021, 29, 527–540. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhuang, W.; Xu, B.; Cai, Z. Fabrication of superhydrophobic cotton fabrics by silica hydrosol and hydrophobization. Appl. Surf. Sci. 2011, 257, 5491–5498. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, Y.; Zhang, W.; Bai, S.; Zheng, X.; Huan, J.; Cao, G.; Yang, T.; Wang, M.; et al. Development of a facile and bi-functional superhydrophobic suspension and its applications in superhydrophobic coatings and aerogels in high-efficiency oil–water separation. Green Chem. 2020, 22, 7424–7434. [Google Scholar] [CrossRef]
- González Lazo, M.A.; Blank, M.; Leterrier, Y.; Månson, J.-A.E. Superhard transparent hybrid nanocomposites for high fidelity UV-nanoimprint lithography. Polymer 2013, 54, 6177–6183. [Google Scholar] [CrossRef]
- Xu, J.; Deng, X.; Dong, Y.; Zhou, Z.; Zhang, Y.; Yu, J.; Cai, J.; Zhang, Y. High-strength, transparent and superhydrophobic nanocellulose/nanochitin membranes fabricated via crosslinking of nanofibers and coating F-SiO2 suspensions. Carbohydr. Polym. 2020, 247, 116694. [Google Scholar] [CrossRef]
- Hu, N.; Liu, W.; Ding, L.; Wu, Z.; Yin, H.; Huang, D.; Li, H.; Jin, L.; Zheng, H. Removal of methylene blue from its aqueous solution by froth flotation: Hydrophobic silica nanoparticle as a collector. J. Nanoparticle Res. 2017, 19, 46–59. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple method for preparing superhydrophobic paper: Spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Hakansson, K.; Salajkova, M.; Lundell, F.; Wagberg, L.; Berglund, L.A. Highly conducting, strong nanocomposites based on nanocellulose-assisted aqueous dispersions of single-wall carbon nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef]
- Xu, S.; Yu, W.; Yao, X.; Zhang, Q.; Fu, Q. Nanocellulose-assisted dispersion of graphene to fabricate poly(vinyl alcohol)/graphene nanocomposite for humidity sensing. Compos. Sci. Technol. 2016, 131, 67–76. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Zeng, H.-X.; Wu, J.; Dong, L.-Y.; Zhu, J.-T.; Xue, Z.-G.; Zhou, X.-P.; Xie, X.-L.; Mai, Y.-W. Biocompatible reduced graphene oxide sheets with superior water dispersibility stabilized by cellulose nanocrystals and their polyethylene oxide composites. Green Chem. 2016, 18, 1674–1683. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Shen, F.; Wan, J.; Lacey, S.; Fang, Z.; Dai, H.; Hu, L. Nanocellulose as green dispersant for two-dimensional energy materials. Nano Energy 2015, 13, 346–354. [Google Scholar] [CrossRef]
- Zhou, S.; You, T.; Zhang, X.; Xu, F. Superhydrophobic Cellulose Nanofiber-Assembled Aerogels for Highly Efficient Water-in-Oil Emulsions Separation. ACS Appl. Nano Mater. 2018, 1, 2095–2103. [Google Scholar] [CrossRef]
- Kuga, S.; Wu, M. Mechanochemistry of cellulose. Cellulose 2019, 26, 215–225. [Google Scholar] [CrossRef]
- Tarabanko, N.; Baryshnikov, S.V.; Kazachenko, A.S.; Miroshnikova, A.; Skripnikov, A.M.; Lavrenov, A.V.; Taran, O.P.; Kuznetsov, B.N. Hydrothermal hydrolysis of microcrystalline cellulose from birch wood catalyzed by Al2O3-B2O3 mixed oxides. Wood Sci. Technol. 2022, 56, 437–457. [Google Scholar] [CrossRef]
- Nau, M.; Herzog, N.; Schmidt, J.; Meckel, T.; Andrieu-Brunsen, A.; Biesalski, M. Janus-Type Hybrid Paper Membranes. Adv. Mater. Interfaces 2019, 6, 1900892. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhao, H.F.; Sha, L.Z.; Li, J.; Li, Y.; Ma, C.; Hu, H.W. Ternary Flame Retardant System of Ammonium Polyphosphate-Diatomite-Nano-SiO2 and Its Application in Fibrous Materials. Dig. J. Nanomater. Biostructures 2020, 15, 85–92. [Google Scholar]
- Paul, B.; Mahmud-Ali, A.; Lenninger, M.; Eberle, S.; Bernt, I.; Mayer, D.; Bechtold, T. Silica incorporated cellulose fibres as green concept for textiles with reduced flammability. Polym. Degrad. Stab. 2022, 195, 10980–10989. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Jiang, B.; Ma, Z. Flame-retardant paper with robust hydrophobicity enabled by perfluorodecane doped SiO2 nanofibers. J. Sol-Gel Sci. Technol. 2019, 93, 309–314. [Google Scholar] [CrossRef]
- Shi, J.J.; Lu, L.B.; Guo, W.T.; Zhang, J.Y.; Cao, Y. Heat insulation performance, mechanics and hydrophobic modification of cellulose-SiO2 composite aerogels. Carbohydr. Polym. 2013, 98, 282–289. [Google Scholar] [CrossRef]
- He, F.; Chao, S.; Gao, Y.; He, X.; Li, M. Fabrication of hydrophobic silica–cellulose aerogels by using dimethyl sulfoxide (DMSO) as solvent. Mater. Lett. 2014, 137, 167–169. [Google Scholar] [CrossRef]
- Li, F.; Xing, L.; Xiang, J.H.; Zhao, C.L.; Li, Z.Y.; Zhang, T.; Fu, R. Hydrophobic Cellulose-SiO2 Composite Aerogel Prepared by a Non-Supercritical Drying Process. Rare Met. Mater. Eng. 2015, 44, 647–650. [Google Scholar]
- Medina-Sandoval, C.F.; Valencia-Dávila, J.A.; Combariza, M.Y.; Blanco-Tirado, C. Separation of asphaltene-stabilized water in oil emulsions and immiscible oil/water mixtures using a hydrophobic cellulosic membrane. Fuel 2018, 231, 297–306. [Google Scholar] [CrossRef]
- Huang, J.; Li, M.; Ren, C.; Huang, W.; Wu, Q.; Li, Q.; Zhang, W.; Wang, S. Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping [Online]. Coatings 2021, 11, 1147. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Li, J.; Ying, G.; Chen, K. High-strength and super-hydrophobic multilayered paper based on nano-silica coating and micro-fibrillated cellulose. Carbohydr. Polym. 2022, 288, 119371. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.L.; Liaw, I.I.; Wu, A.H.F.; Lamb, R.N. Influence of Roughness on a Transparent Superhydrophobic Coating. J. Phys. Chem. C 2010, 114, 11228–11233. [Google Scholar] [CrossRef]
- Kosak Soz, C.; Trosien, S.; Biesalski, M. Superhydrophobic Hybrid Paper Sheets with Janus-Type Wettability. ACS Appl. Mater. Interfaces 2018, 10, 37478–37488. [Google Scholar] [CrossRef]


| Sample | Final Weight (%) | Theoretical Weight of HN-SiO2 (%) | Actual Weight of HN-SiO2 (%) | Deviation (%) |
|---|---|---|---|---|
| D6-CNF | 0 | 0 | - | - |
| HN-SiO2 | 89.73 | 100 | - | - |
| HS10D6 | 13.21 | 9.1 | 14.7 | 5.6 |
| HS50D6 | 30.35 | 33.3 | 33.8 | 0.5 |
| HS100D6 | 48.40 | 50.0 | 53.9 | 3.9 |
| HS150D6 | 58.81 | 60.0 | 65.5 | 5.5 |
| Year | Materials | Method | WCA (°) | Ref |
|---|---|---|---|---|
| 2013 | aerogels | the freeze-drying and cold plasma modification technology. | 132 | [40] |
| 2014 | aerogels | the sol–gel process and the supercritical drying method by DMSO | 138 | [41] |
| 2015 | aerogels | the sol-gel and freeze-drying method. | 146 | [42] |
| 2016 | film | one-pot method | 137 | [20] |
| 2018 | film | a two-step method involving a SiO2 sol-gel process | 121 | [43] |
| 2021 | coated fabric | double coated construction by a simple multi-step dipping | 156.6 | [44] |
| 2022 | coated paper | the laminated process followed by spraying approach | 151.2 | [45] |
| this work | film and coated paper | one-step mechanochemical by ball milling | 158 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, L.; Wu, M.; Huang, Y. Preparing Hydrophobic Cellulose Nanofibers-SiO2 Films and Coating by One-Step Mechanochemical Method. Polymers 2022, 14, 4413. https://doi.org/10.3390/polym14204413
Chen X, Zhang L, Wu M, Huang Y. Preparing Hydrophobic Cellulose Nanofibers-SiO2 Films and Coating by One-Step Mechanochemical Method. Polymers. 2022; 14(20):4413. https://doi.org/10.3390/polym14204413
Chicago/Turabian StyleChen, Xi, Lijiaqi Zhang, Min Wu, and Yong Huang. 2022. "Preparing Hydrophobic Cellulose Nanofibers-SiO2 Films and Coating by One-Step Mechanochemical Method" Polymers 14, no. 20: 4413. https://doi.org/10.3390/polym14204413






