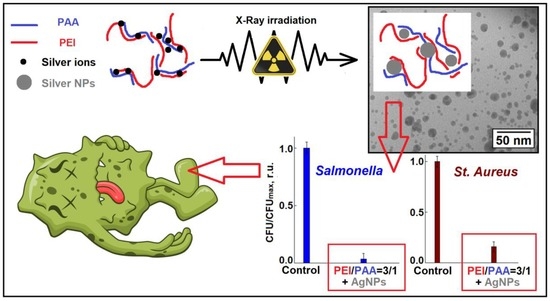
Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions
Abstract
:1. Introduction
2. Materials and Methods
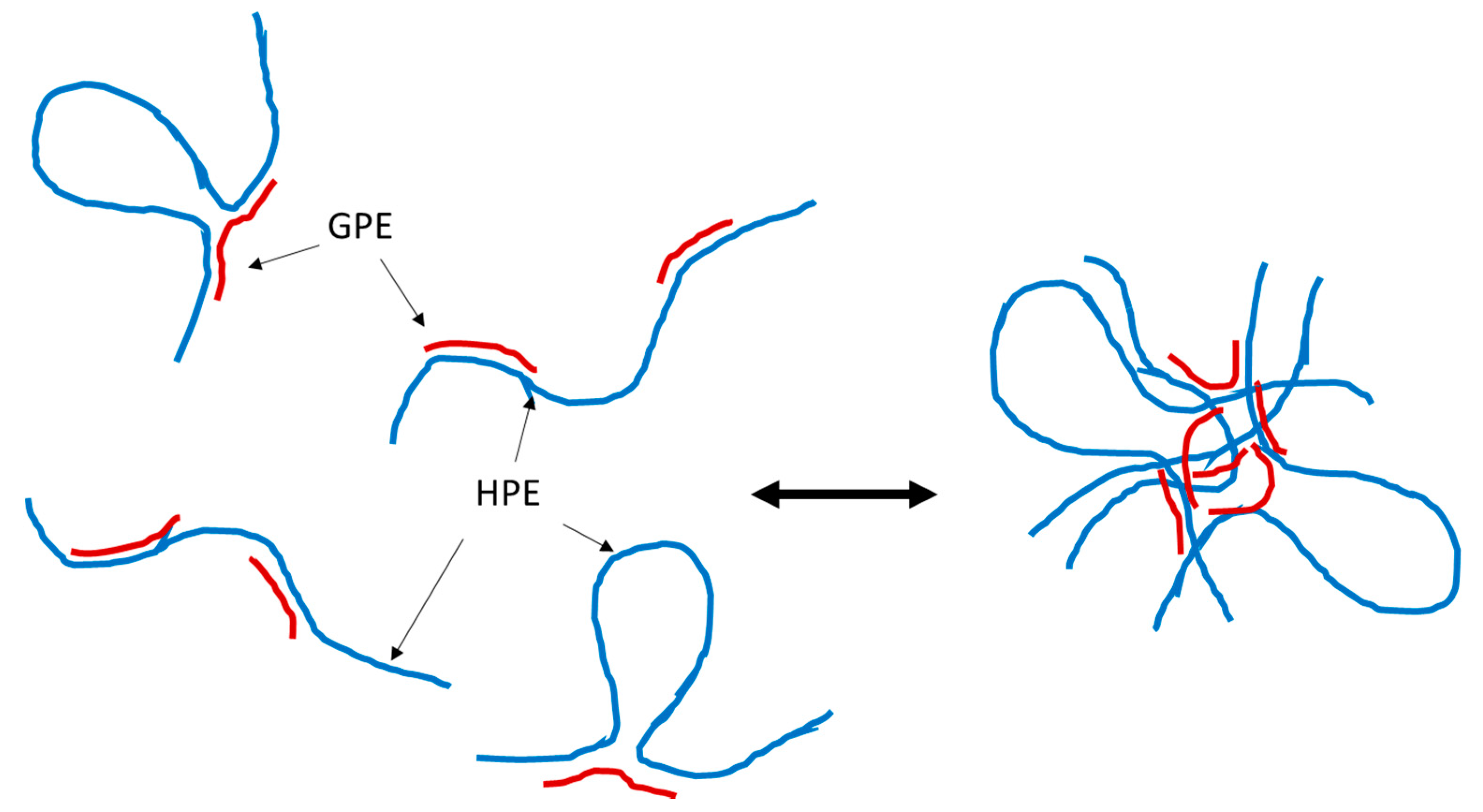
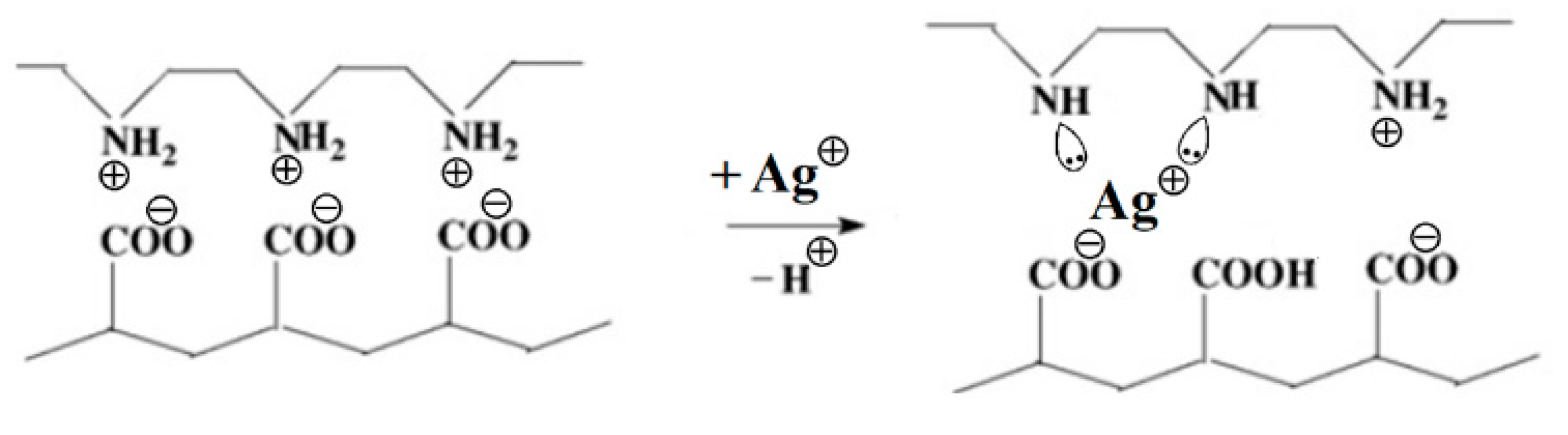
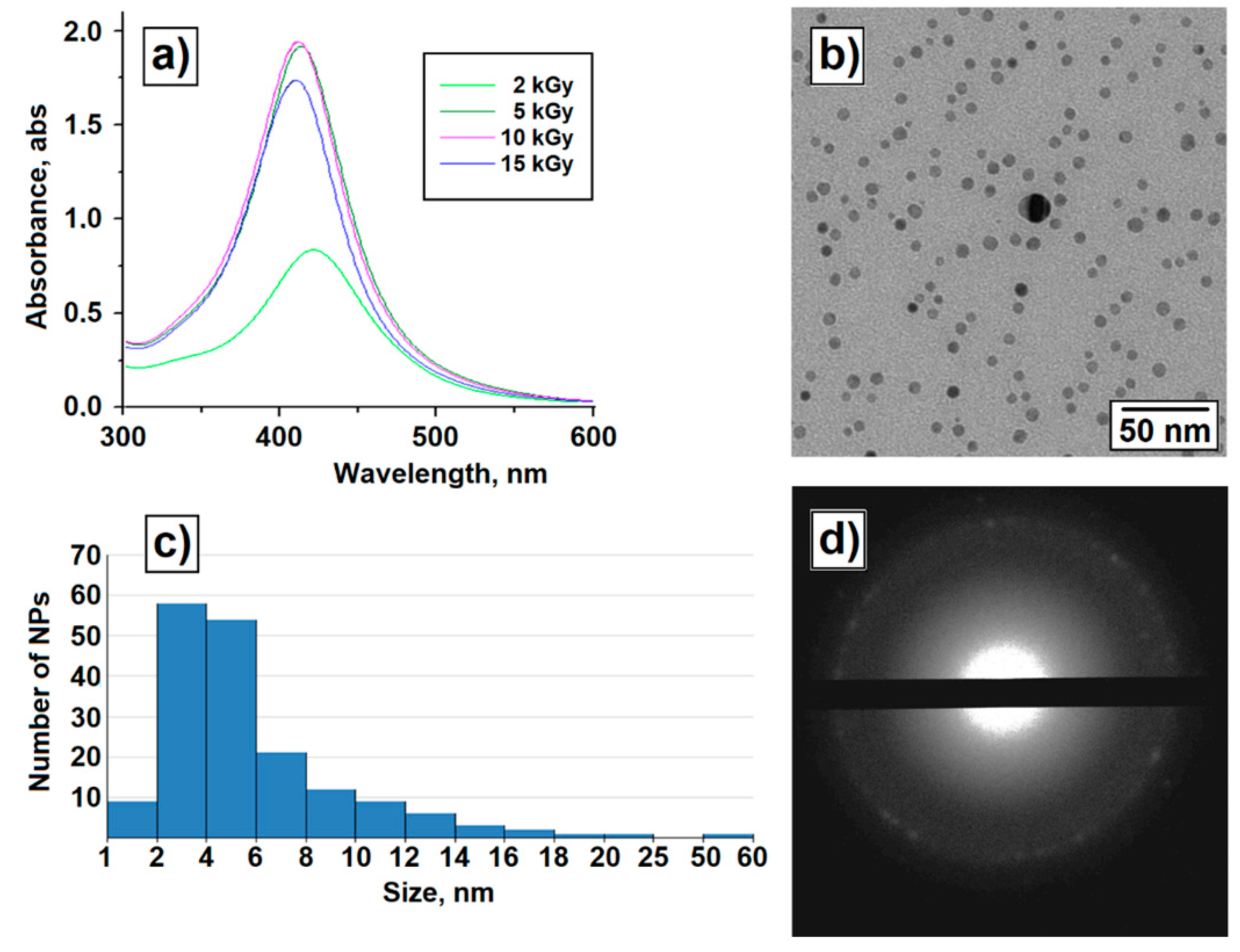
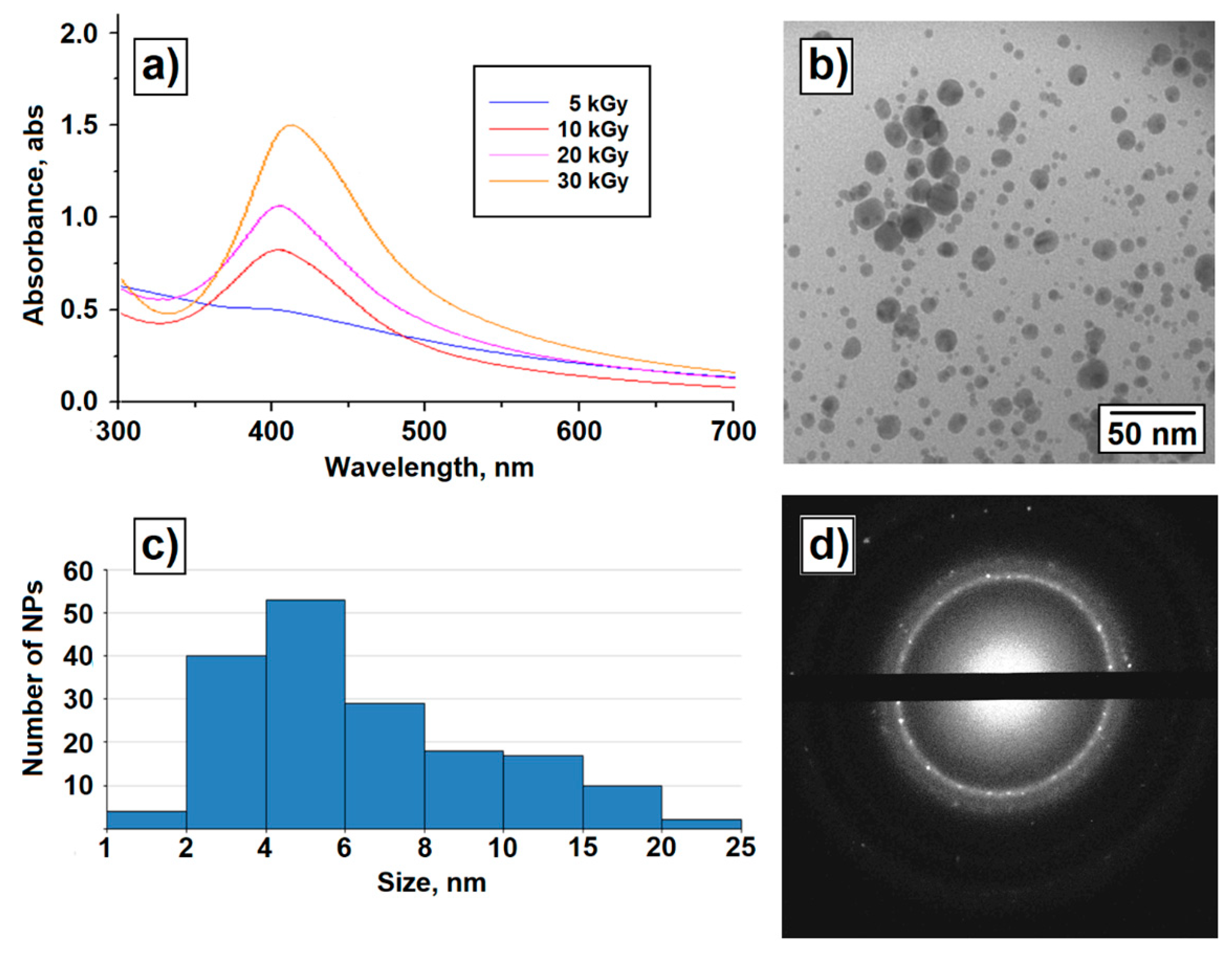
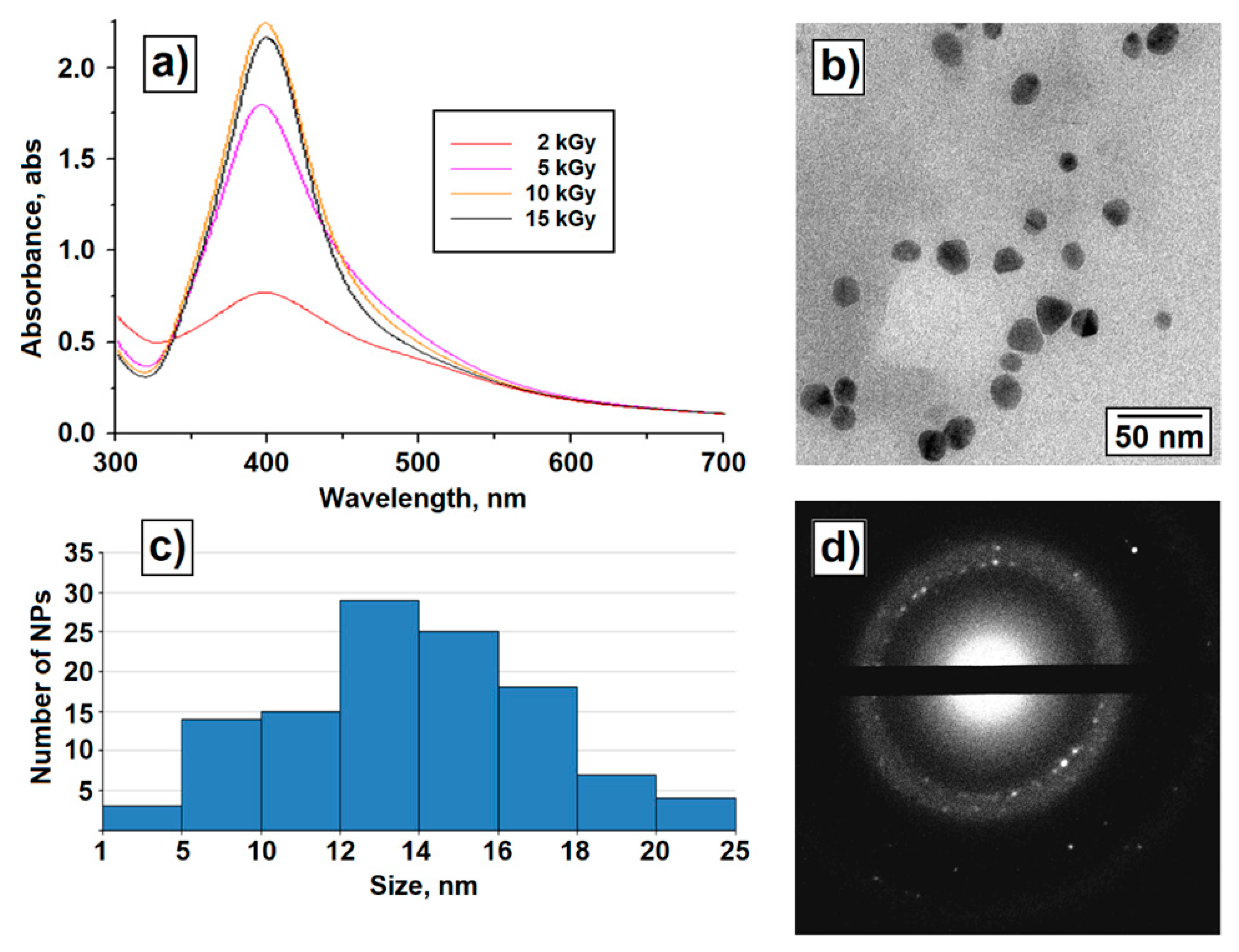
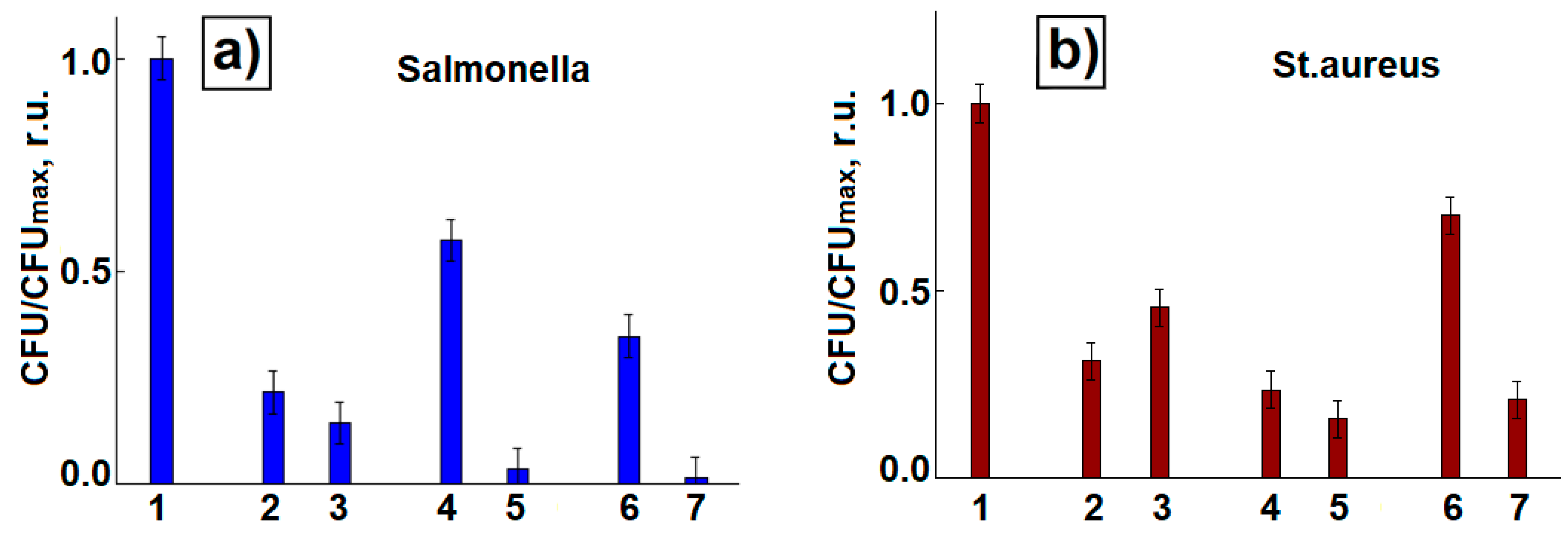
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Dai, J.H.; Bruening, M.L. Catalytic nanoparticles formed by reduction of metal ions in multilayered polyelectrolyte films. Nano Lett. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kestelman, V.N. Metallopolymer nanocomposites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 81. [Google Scholar]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current research on silver nanoparticles: Synthesis, characterization, and applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Bustos, A.R.M.; Murphy, K.E. Winchester; J.R., Vega Baudrit. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Volker, C.; Oetken, M.; Oehlmann, J. The Biological Effects and Possible Modes of Action of Nanosilver. Rev. Environ. Contam. Toxicol. 2013, 223, 81–106. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Bazzaz, B.S.F.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The role of nanotechnology in combating biofilm-based antibiotic resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 15. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef] [Green Version]
- Kvitek, L.; Panacek, A.; Soukupova, J.; Kolar, M.; Vecerova, R.; Prucek, R.; Holecova, M.; Zboril, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticles dispersing in chitosan solution: Preparation by gamma-ray irradiation and their antimicrobial activities. Mater. Chem. Phys. 2009, 115, 296–302. [Google Scholar] [CrossRef]
- Manikandan, A.; Sathiyabama, M. Green synthesis of copper-chitosan nanoparticles and study of its antibacterial activity. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Misin, V.M.; Zezin, A.A.; Klimov, D.I.; Sybachin, A.V.; Yaroslavov, A.A. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B 2021, 63, 459–469. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Cheng, W.; Zhou, A.; Deng, Y.; Ma, W.; Zhu, M.; Xiong, R.; Huang, C. A Prussian blue alginate microparticles platform based on gas-shearing strategy for antitumor and antibacterial therapy. Int. J. Biol. Macromol. 2022, 209, 794–800. [Google Scholar] [CrossRef]
- Rurarz, B.P.; Gibka, N.; Bukowczyk, M.; Kadłubowski, S.; Ulański, P. Radiation synthesis of poly (acrylic acid) nanogels for drug delivery applications–post-synthesis product colloidal stability. Nukleonika 2021, 66, 179–186. [Google Scholar] [CrossRef]
- Zezin, A.A. Synthesis of metal-polymer complexes and functional nanostructures in films and coatings of interpolyelectrolyte complexes. Polym. Sci. Ser. A 2019, 61, 754–764. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Zezin, A.A.; Zezin, A.B.; Müller, A.H.E. Advanced functional structures based on interpolyelectrolyte complexes. In Polyelectrolyte Complexes in the Dispersed and Solid State I; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–225. [Google Scholar]
- Demchenko, V.; Riabov, S.; Sinelnikov, S.; Radchenko, O.; Kobylinskyi, S.; Rybalchenko, N. Novel approach to synthesis of silver nanoparticles in interpolyelectrolyte complexes based on pectin, chitosan, starch and their derivatives. Carbohydr. Polym. 2020, 242, 116431. [Google Scholar] [CrossRef]
- Demchenko, V.; Riabov, S.; Kobylinskyi, S.; Goncharenko, L.; Rybalchenko, N.; Kruk, A.; Moskalenko, O.; Shut, M. Effect of the type of reducing agents of silver ions in interpolyelectrolyte-metal complexes on the structure, morphology and properties of silver-containing nanocomposites. Sci. Rep. 2020, 10, 7126. [Google Scholar] [CrossRef]
- Schacher, F.H.; Rudolph, T.; Drechsler, M.; Müller, A.H.E. Core-crosslinked compartmentalized cylinders. Nanoscale 2011, 3, 288–297. [Google Scholar] [CrossRef]
- Dağaş, D.E.; Danelyan, G.V.; Ghaffarlou, M.; Zezina, E.A.; Abramchuk, S.S.; Feldman, V.I.; Güven, O.; Zezin, A.A. Generation of spatially ordered metal–polymer nanostructures in the irradiated dispersions of poly (acrylic acid)–poly (vinylimidazole)–Cu2+ complexes. Colloid Polym. Sci. 2020, 298, 193–202. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.; Shanmugam, M.; Kim, S.-C. Development of sustainable and antimicrobial film based on polybenzoxazine and cellulose. Int. J. Biol. Macromol. 2021, 170, 664–673. [Google Scholar] [CrossRef]
- Koufakis, E.; Manouras, T.; Anastasiadis, S.H.; Vamvakaki, M. Film properties and antimicrobial efficacy of quaternized PDMAEMA brushes: Short vs long alkyl chain length. Langmuir 2020, 36, 3482–3493. [Google Scholar] [CrossRef]
- Gao, J.; White, E.M.; Liu, Q.; Locklin, J. Evidence for the phospholipid sponge effect as the biocidal mechanism in surface-bound polyquaternary ammonium coatings with variable cross-linking density. ACS Appl. Mater. Interfaces 2017, 9, 7745–7751. [Google Scholar] [CrossRef]
- Parhamifar, L.; Andersen, H.; Wu, L.P.; Hall, A.; Hudzech, D.; Moghimi, S.M. Polycation-Mediated Integrated Cell Death Processes. Adv. Genet. 2014, 88, 353–398. [Google Scholar] [CrossRef]
- Klimov, D.I.; Zezina, E.A.; Lipik, V.C.; Abramchuk, S.S.; Yaroslavov, A.A.; Feldman, V.I.; Sybachin, A.V.; Spiridonov, V.V.; Zezin, A.A. Radiation-induced preparation of metal nanostructures in coatings of interpolyelectrolyte complexes. Radiat. Phys. Chem. 2019, 162, 23–30. [Google Scholar] [CrossRef]
- Mkrtchyan, K.V.; Zezin, A.A.; Zezina, E.A.; Abramchuk, S.S.; Baranova, I.A. Formation of metal nanostructures under X-ray radiation in films of interpolyelectrolyte complexes with different silver ion content. Russ. Chem. Bull. 2020, 69, 1731–1739. [Google Scholar] [CrossRef]
- Zezin, A.A.; Klimov, D.I.; Zezina, E.A.; Mkrtchyan, K.V.; Feldman, V.I. Controlled radiation-chemical synthesis of metal polymer nanocomposites in the films of interpolyelectrolyte complexes: Principles, prospects and implications. Radiat. Phys. Chem. 2020, 169, 108076. [Google Scholar] [CrossRef]
- Long, D.; Wu, G.; Chen, S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1126–1131. [Google Scholar] [CrossRef]
- Sosulin, I.S.; Zezin, A.A.; Feldman, V.I. Effect of irradiation on poly(acrylic acid)-polyethyleneimine interpolyelectrolyte complexes: An electron paramagnetic resonance study. Rad. Phys. Chem. 2022, 167, 110198. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; National Inst. of Standards and Technology-PL: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Nastulyavichus, A.; Tolordava, E.; Rudenko, A.; Zazymkina, D.; Shakhov, P.; Busleev, N.; Romanova, Y.; Ionin, A.; Kudryashov, S. In Vitro Destruction of Pathogenic Bacterial Biofilms by Bactericidal Metallic Nanoparticles via Laser-Induced Forward Transfer. Nanomaterials 2020, 10, 2259. [Google Scholar] [CrossRef]
- Zezin, A.B.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv. Colloid Interface Sci. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Müller, A.H.E.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef]
- Synatschke, C.V.; Löbling, T.I.; Förtsch, M.; Hanisch, A.; Schacher, F.H.; Müller, A.H.E. Micellar Interpolyelectrolyte Complexes with a Compartmentalized Shell. Macromolecules 2013, 46, 6466–6474. [Google Scholar] [CrossRef]
- Müller, M.; Keßler, B.; Fröhlich, J.; Poeschla, S.; Torger, B. Polyelectrolyte Complex Nanoparticles of Poly(ethyleneimine) and Poly(acrylic acid): Preparation and Applications. Polymers 2011, 3, 762–778. [Google Scholar] [CrossRef] [Green Version]
- Izumrudov, V.A.; Sybachin, A.V. Phase separation in solutions of polyelectrolyte complexes: The decisive effect of a host polyion. Polym. Sci. Ser. A 2006, 48, 1098–1104. [Google Scholar] [CrossRef]
- Zezin, A.B.; Rogacheva, V.B.; Feldman, V.I.; Afanasiev, P.; Zezin, A.A. From triple interpolyelectrolyte-metal complexes to polymer-metal nanocomposites. Adv. Colloid Interface Sci. 2010, 158, 84–93. [Google Scholar] [CrossRef]
- Kabanov, V.A. Polyelectrolyte complexes in solution and in bulk. Russ. Chem. Rev. 2005, 74, 3. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual salt and pH induced changes in polyethylenimine solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef] [PubMed]
- Zezin, A.A.; Feldman, V.I.; Abramchuk, S.S.; Danelyan, G.V.; Dyo, V.V.; Plamper, F.A.; Müller, A.H.E.; Pergushov, D.V. Efficient size control of copper nanoparticles generated in irradiated aqueous solutions of star-shaped polyelectrolyte containers. Phys. Chem. Chem. Phys. 2015, 17, 11490–11498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Ershov, B.G. Colloidal copper in aqueous solutions: Radiation-chemical reduction, mechanism of formation, and properties. Russ. Chem. Bull. 1994, 43, 16–21. [Google Scholar] [CrossRef]
- Ershov, B.G.; Janata, E.; Henglein, A. Growth of silver particles in aqueous solution: Long-lived” magic” clusters and ionic strength effects. J. Phys. Chem. 1993, 97, 339–343. [Google Scholar] [CrossRef]
- Mostafavi, M.; Keghouche, N.; Delcourt, M.-O.; Belloni, J. Ultra-slow aggregation process for silver clusters of a few atoms in solution. Chem. Phys. Lett. 1990, 167, 193–197. [Google Scholar] [CrossRef]
- Lampre, I.; Pernot, P.; Mostafavi, M. Spectral properties and redox potentials of silver atoms complexed by chloride ions in aqueous solution. J. Phys. Chem. B 2000, 104, 6233–6239. [Google Scholar] [CrossRef]
- Henglein, A. The reactivity of silver atoms in aqueous solutions (A γ-radiolysis study). Ber. Der Bunsenges. Für Phys. Chem. 1977, 81, 556–561. [Google Scholar] [CrossRef]
- Bakar, A.; De, V.V.; Zezin, A.A.; Abramchuk, S.S.; Güven, O.; Feldman, V.I. Spatial organization of a metal–polymer nanocomposite obtained by the radiation-induced reduction of copper ions in the poly (allylamine)–poly (acrylic acid)–Cu2+ system. Mendeleev Commun. 2012, 22, 211–212. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Q.; Yang, T.; Cao, J.; Lin, Q.; Yuan, Z.; Li, L. Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents. Int. J. Environ. Res. Public Health 2016, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz, M.; Barszczewska-Rybarek, I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers 2020, 12, 2551. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Stepanova, D.A.; Bolshakova, A.V.; Marina, V.I.; Osterman, I.A.; Sybachin, A.V. Hyperbranched kaustamin as an antibacterial for surface treatment. Mendel. Commun. 2022, 32, 561–563. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Afonina, I.A.; Kraeva, L.A.; Gia, T. Bactericidal activity of colloidal silver against grampositive and gramnegative bacteria. Antibiot. i Khimioterapiia = Antibiot. Chemoterapy [Sic] 2010, 55, 11–13. [Google Scholar]
- Pigareva, V.A.; Senchikhin, I.N.; Bolshakova, A.V.; Sybachin, A.V. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkrtchyan, K.V.; Pigareva, V.A.; Zezina, E.A.; Kuznetsova, O.A.; Semenova, A.A.; Yushina, Y.K.; Tolordava, E.R.; Grudistova, M.A.; Sybachin, A.V.; Klimov, D.I.; et al. Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions. Polymers 2022, 14, 4417. https://doi.org/10.3390/polym14204417
Mkrtchyan KV, Pigareva VA, Zezina EA, Kuznetsova OA, Semenova AA, Yushina YK, Tolordava ER, Grudistova MA, Sybachin AV, Klimov DI, et al. Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions. Polymers. 2022; 14(20):4417. https://doi.org/10.3390/polym14204417
Chicago/Turabian StyleMkrtchyan, Kristina V., Vladislava A. Pigareva, Elena A. Zezina, Oksana A. Kuznetsova, Anastasia A. Semenova, Yuliya K. Yushina, Etery R. Tolordava, Maria A. Grudistova, Andrey V. Sybachin, Dmitry I. Klimov, and et al. 2022. "Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions" Polymers 14, no. 20: 4417. https://doi.org/10.3390/polym14204417
APA StyleMkrtchyan, K. V., Pigareva, V. A., Zezina, E. A., Kuznetsova, O. A., Semenova, A. A., Yushina, Y. K., Tolordava, E. R., Grudistova, M. A., Sybachin, A. V., Klimov, D. I., Abramchuk, S. S., Yaroslavov, A. A., & Zezin, A. A. (2022). Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions. Polymers, 14(20), 4417. https://doi.org/10.3390/polym14204417