Low Dielectric Properties and Transmission Loss of Polyimide/Organically Modified Hollow Silica Nanofiber Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PI/m-HSNF Composites
2.3. Characterization and Instruments
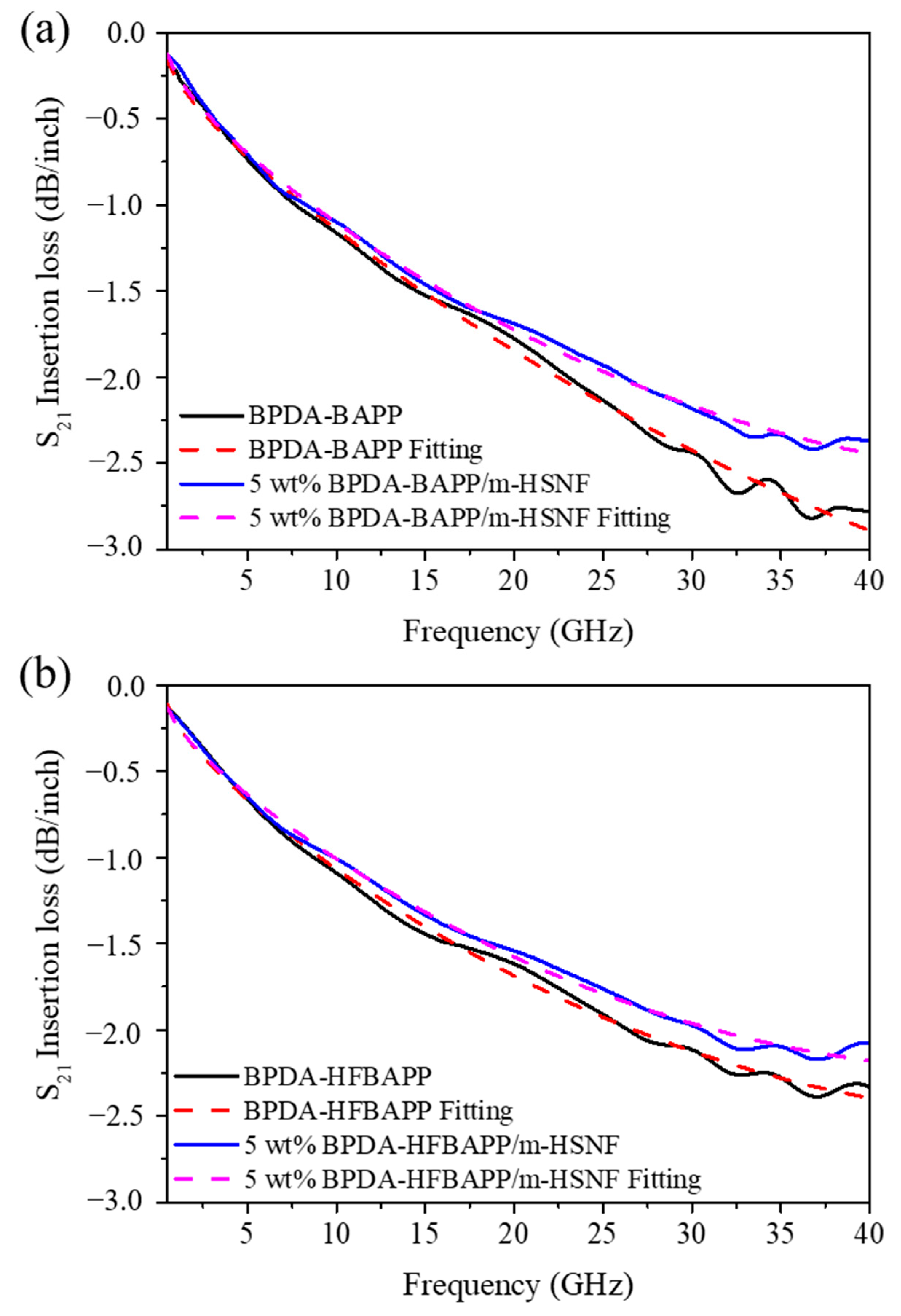
3. Results and Discussion
3.1. Structural and Morphological Characterizations of Various PI/m-HSNF Nanocomposites
3.2. Physical Properties of Various PI/m-HSNF Nanocomposites
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2020, 15, 295. [Google Scholar]
- Cao, X.; Wen, J.; Song, L.; Liu, X.; He, G. Polyimide hollow glass microspheres composite films with low dielectric constant and excellent thermal performance. J. Appl. Polym. Sci. 2021, 138, 50600. [Google Scholar] [CrossRef]
- Ogbonna, V.; Popoola, A.; Popoola, O.; Adeosun, S. A review on polyimide reinforced nanocomposites for mechanical, thermal, and electrical insulation application: Challenges and recommendations for future improvement. Polym. Bull. 2022, 79, 663. [Google Scholar] [CrossRef]
- Zhou, Y. Material foundation for future 5G technology. Acc. Mater. Res. 2022, 2, 306. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, W.; Jiao, Y.; Wu, L. Low-dielectric-constant aromatic homopolyimide and copolyimide derived from pyromellitic dianhydride, 4,4′-oxydianiline, 2,2-bis[4-(4-aminephenoxy) phenyl] propane, 1,4-bis(4-aminophenoxy) benzene, or 1,3-bis(4-aminophenoxy) benzene. J. Appl. Polym. Sci. 2019, 136, 47405. [Google Scholar] [CrossRef]
- He, Z.; Xie, J.; Liao, Z.; Ma, Y.; Zhang, M.; Zhang, W.; Yue, H.; Gao, X. Hierarchical porous structure contained composite polyimide film with enhanced dielectric and water resistance properties for dielectric material. Prog. Org. Coat. 2021, 151, 106030. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lin, Y.C.; Chen, Y.C.; Wu, P.H.; Ando, S.; Ueda, M.; Chen, W.C. Correlating the molecular structure of polyimides with the dielectric constant and dissipation factor at a high frequency of 10 GHz. ACS Appl. Polym. Mater. 2020, 3, 362. [Google Scholar] [CrossRef]
- Wu, X.; Cai, J.; Cheng, Y. Synthesis and characterization of high fluorine-containing polyimides with low-dielectric constant. J. Appl. Polym. Sci. 2022, 139, 51972. [Google Scholar] [CrossRef]
- Bei, R.; Qian, C.; Zhang, Y.; Chi, Z.; Liu, S.; Chen, X.; Xu, J.; Aldred, M.P. Intrinsic low dielectric constant polyimides: Relationship between molecular structure and dielectric properties. J. Mater. Chem. C 2017, 5, 12807. [Google Scholar] [CrossRef]
- Park, S.S.; Ha, C.S. Polyimide/hollow silica sphere hybrid films with low dielectric constant. Compos. Interfaces 2016, 23, 831. [Google Scholar] [CrossRef]
- Min, C.K.; Wu, T.B.; Yang, W.T.; Chen, C.L. Functionalized mesoporous silica/polyimide nanocomposite thin films with improved mechanical properties and low dielectric constant. Compos. Sci. Technol. 2008, 68, 1570. [Google Scholar] [CrossRef]
- Hong, Z.; Dongyang, W.; Yong, F.; Hao, C.; Yusen, Y.; Jiaojiao, Y.; Liguo, J. Dielectric properties of polyimide/SiO2 hollow spheres composite films with ultralow dielectric constant. Mater. Sci. Eng. B 2016, 203, 13. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lu, S.G.; Li, Y.Q.; Dang, Z.M.; Xin, J.H.; Fu, S.Y.; Li, G.T.; Guo, R.R.; Li, L.F. Novel silica tube/polyimide composite films with variable low dielectric constant. Adv. Mater. 2005, 17, 1056. [Google Scholar] [CrossRef]
- Li, X.H.; Shao, C.L.; Liu, Y.C. A simple method for controllable preparation of polymer nanotubes via a single capillary electrospinning. Langmuir 2007, 23, 10920. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Zhang, S.; Song, J.; Duan, H.; Zhou, M.; Gong, C.; Bao, Z.; Lu, B.; Li, X. A novel method to fabricate silica nanotubes based on phase separation effect. J. Mater. Chem. 2010, 20, 9068. [Google Scholar] [CrossRef]
- Miyaji, F.; Davis, S.A.; Charmant, J.P.; Mann, S. Organic crystal templating of hollow silica fibers. Chem. Mater. 1999, 11, 3021. [Google Scholar] [CrossRef]
- Chien, H.C.; Lin, S.Y.; Chen, E.C.; Wu, T.M. Synthesis and dielectric properties of polyimide/hollow silica nanofiber composite. J. Mater. Sci.–Mater. Electron. JMSE-D-22-02288R1.
- Yaqoob, A.A.; Safian, M.T.; Rashid, M.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Chapter One—Introduction of Smart Polymer Nanocomposites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 1–25. [Google Scholar]
- Tasaki, T.; Shiotani, A.; Yamaguchi, T.; Sugimoto, K. Low Dk/Df polyimide adhesives for low transmission loss substrates. Trans. Jpn. Inst. Electron. Packag. 2018, 11, E17. [Google Scholar] [CrossRef] [Green Version]
- Yazgan, İ. NMR-based structural characterization of common aromatic poly (amic acid) polymers. Polym. Bull. 2020, 77, 1191. [Google Scholar] [CrossRef]
- Kumar, A.; Tateyama, S.; Yasaki, K.; Ali, M.A.; Takaya, N.; Singh, R.; Kaneko, T. 1H NMR and FT-IR dataset based structural investigation of poly(amic acid)s and polyimides from 4, 4′-diaminostilbene. Data Brief. 2016, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.M.; Chen, E.C.; Lin, Y.W.; Chiang, M.F.; Chang, G.Y. Preparation and characterization of melt processed polycarbonate/multi-walled carbon nanotube composites. Polym. Eng. Sci. 2008, 48, 1369. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, C.Y. Poly(ethylene 2,6-naphthalate)/layered silicate nanocomposites: Fabrication crystallization behavior and properties. Polymer 2005, 46, 5621. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, W.; Wu, L. Low dielectric constant polyimide mixtures fabricated by polyimide matrix and polyimide microsphere fillers. Polym. Int. 2020, 69, 485. [Google Scholar] [CrossRef]
- Lee, T.N.; Lau, J.H.; Ko, C.T.; Xia, T.; Lin, E.; Yang, K.M.; Lin, P.B.; Peng, C.Y.; Chang, L.; Chen, J.S.; et al. Characterization of low-loss dielectric materials for high-speed and high frequency applications. Materials 2022, 15, 2396. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, A.; Chernobryvko, M.; Ndip, I.; Kaiser, M.P.; Brottcher, M.; Scheibe, P.; Schneider-Ramelow, M.; Wieland, M.; Goetze, C.; Trewhella, J. Temperature dependent RF characterization of thin-film polyimide for 5G mm Wave antenna-in-package modules. In Proceedings of the 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; pp. 1–5. [Google Scholar]
- Ye, X.; Balogh, M. Physics-based fitting to improve PCB loss measurement accuracy. In Proceedings of the 2017 IEEE International Symposium on Electromagnetic Compatibility & Signal/Power Integrity (EMCSI), Washington, DC, USA, 7–11 August 2017; pp. 516–521. [Google Scholar]
- Nishimura, I.; Fujitomi, S.; Yamashita, Y.; Kawashima, N.; Miyaki, N. Development of new dielectric material to reduce transmission loss. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020; pp. 641–646. [Google Scholar]
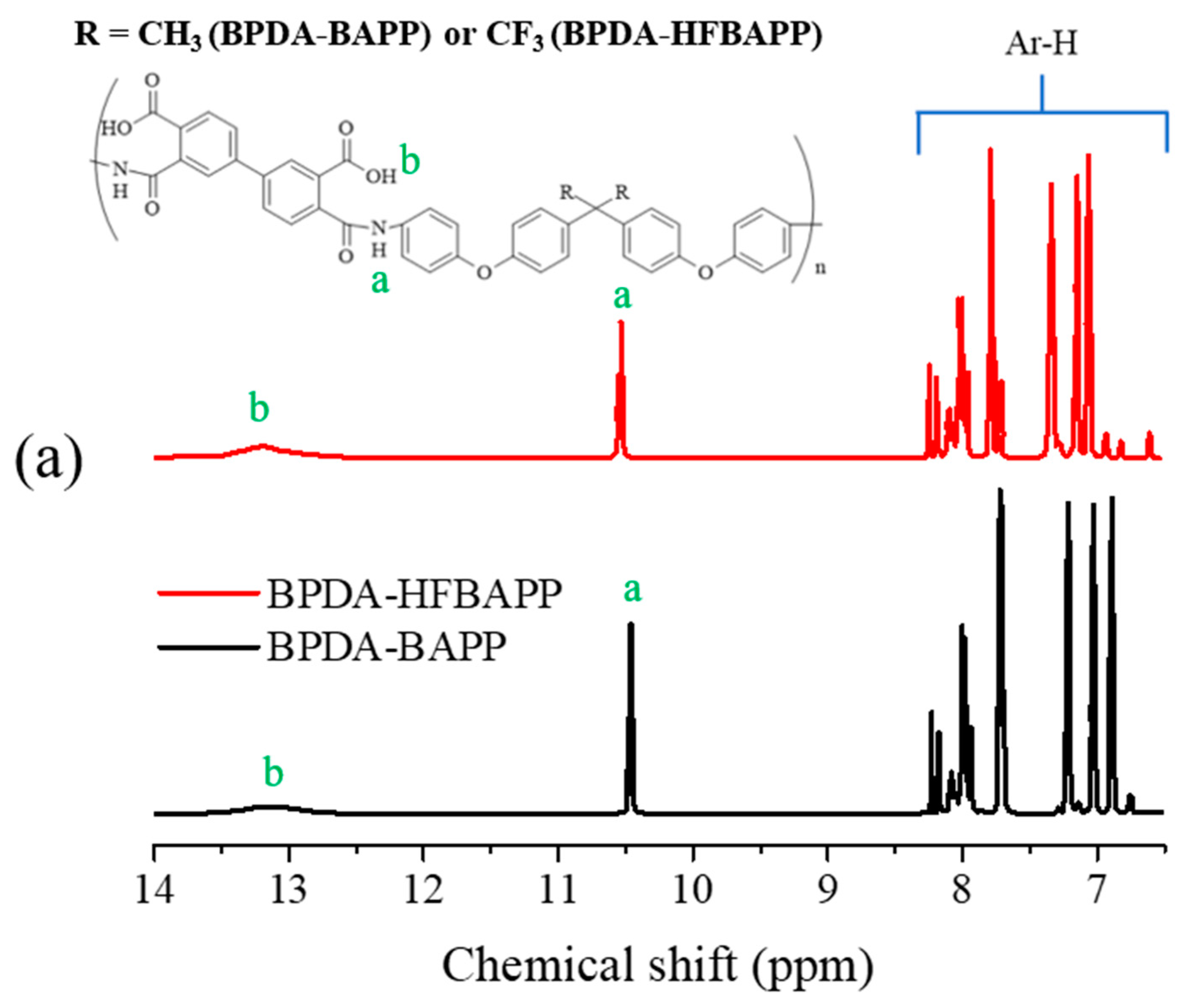

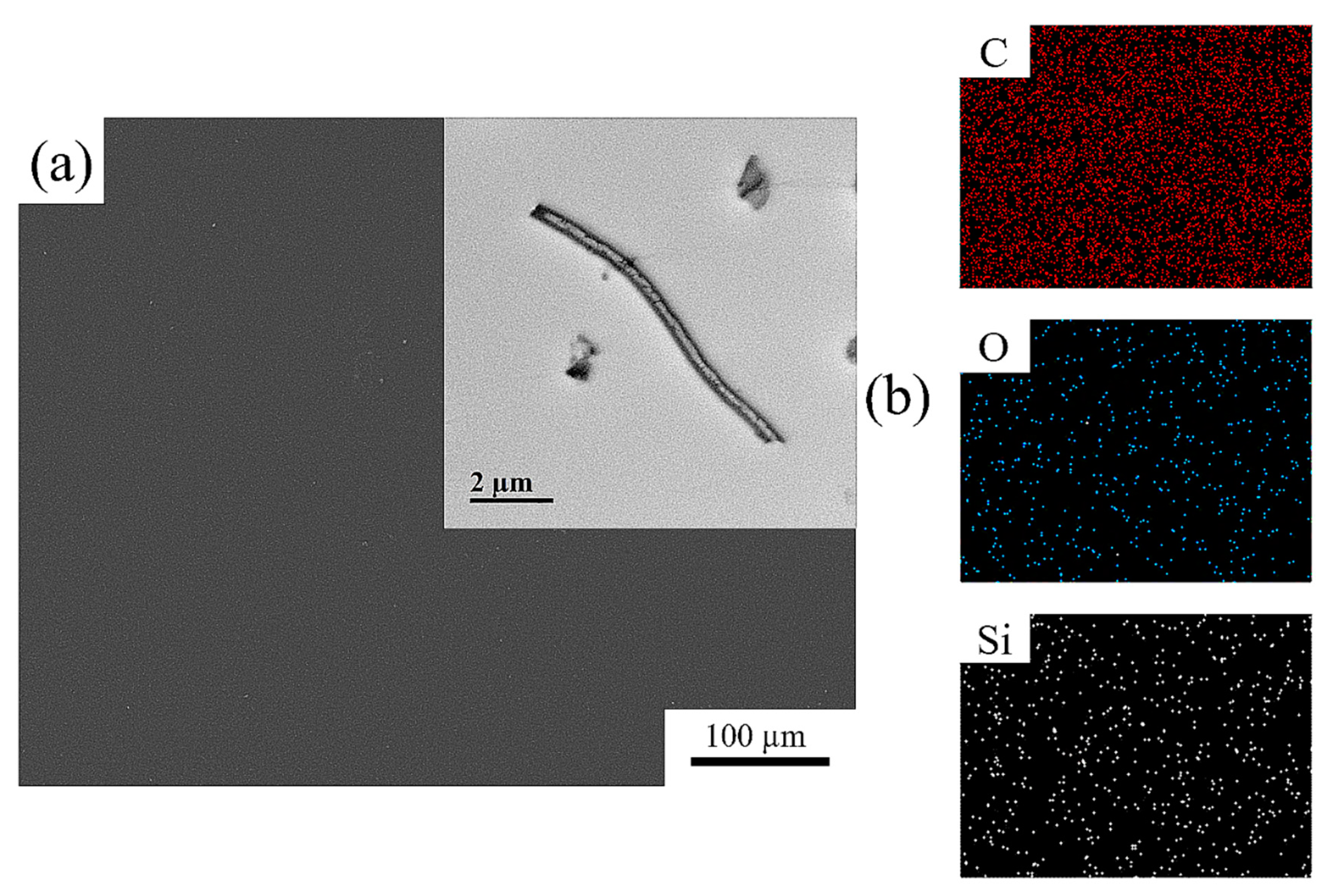
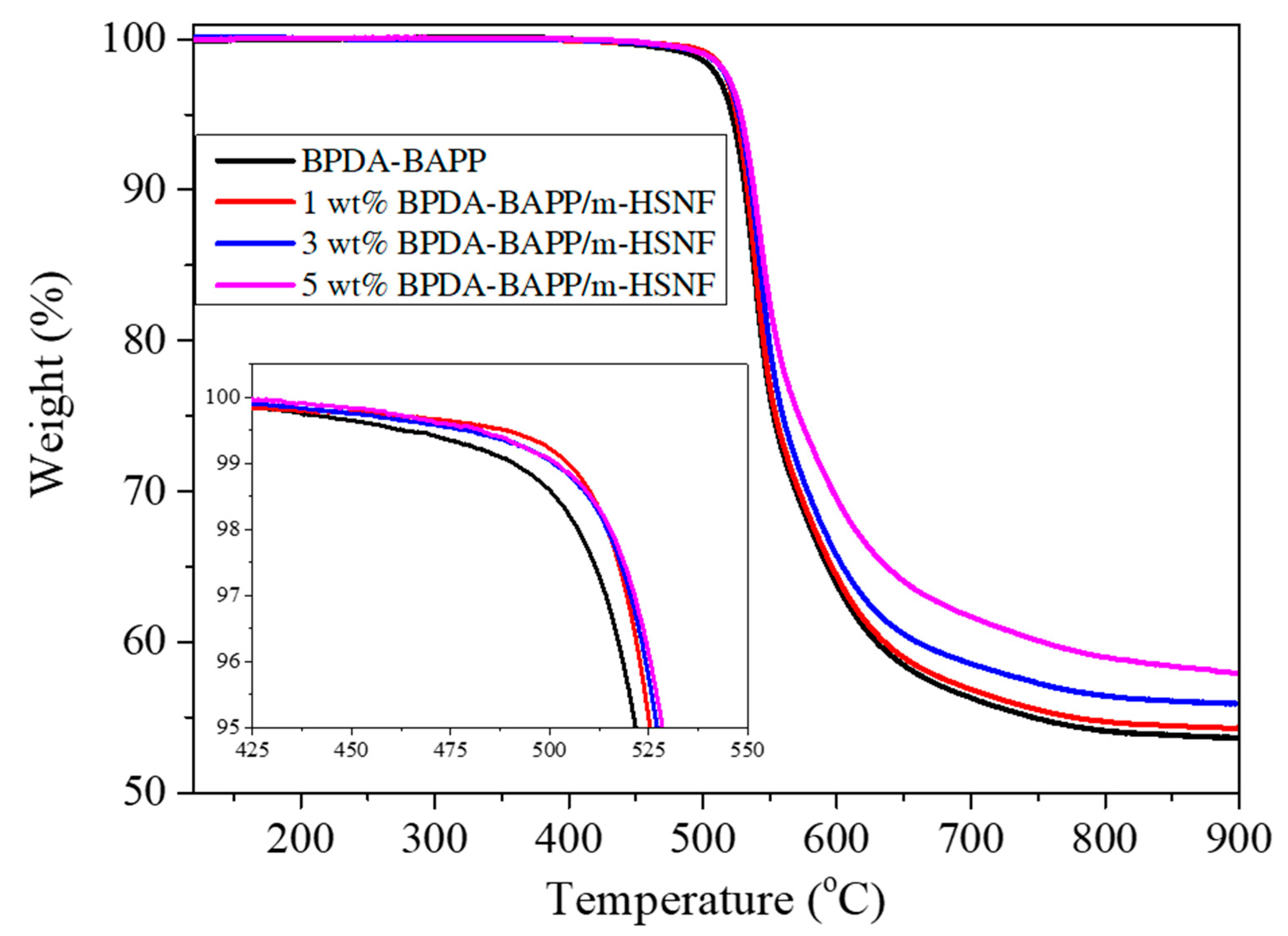

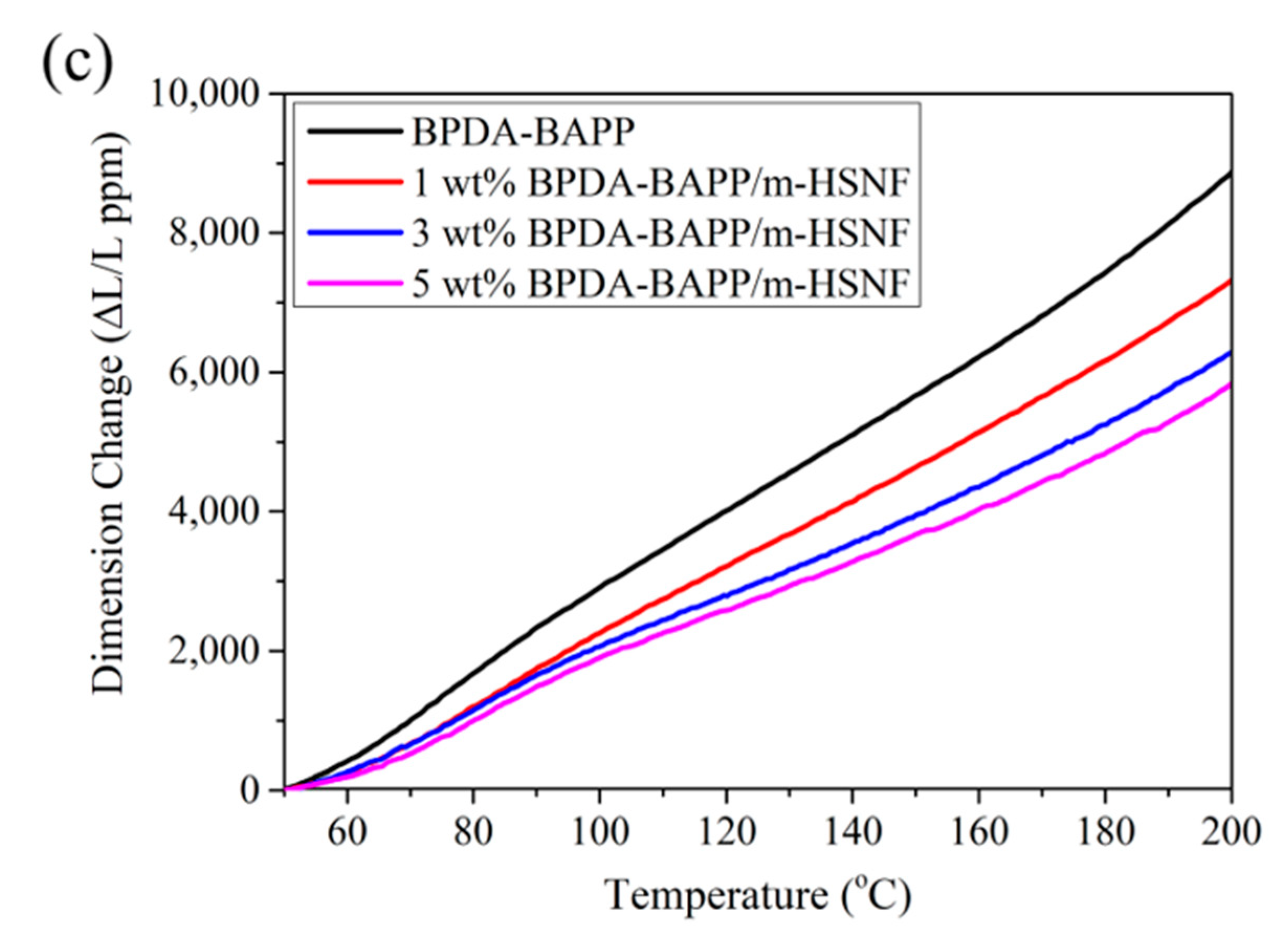


| Polymer | Mw (g/mol) × 104 | Mn (g/mol) × 104 | PDI |
|---|---|---|---|
| BPDA-BAPP | 21.95 | 16.78 | 1.307 |
| BPDA-HFBAPP | 20.87 | 16.90 | 1.235 |
| Sample | T5% (°C) a | Tg (°C) b | E′ (GPa) c | CTE (ppm/°C) d |
|---|---|---|---|---|
| BPDA-BAPP | 521.4 | 245.9 | 0.97 | 59.2 |
| 1 wt% BPDA-BAPP/m-HSNF | 525.2 | 248.9 | 1.16 | 48.7 |
| 3 wt% BPDA-BAPP/m-HSNF | 527.3 | 250.4 | 1.33 | 42.3 |
| 5 wt% BPDA-BAPP/m-HSNF | 528.4 | 251.4 | 1.57 | 38.8 |
| BPDA-HFBAPP | 539.7 | 252.7 | 0.80 | 59.1 |
| 1 wt% BPDA-HFBAPP/m-HSNF | 541.9 | 255.3 | 0.97 | 47.2 |
| 3 wt% BPDA-HFBAPP/m-HSNF | 543.2 | 256.5 | 1.18 | 44.0 |
| 5 wt% BPDA-HFBAPP/m-HSNF | 543.8 | 257.1 | 1.38 | 40.9 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| BPDA-BAPP | 86.8 ± 1.3 | 10.5 ± 1.4 |
| 1 wt% BPDA-BAPP/m-HSNF | 101.9 ± 2.5 | 9.1 ± 1.9 |
| 3 wt% BPDA-BAPP/m-HSNF | 109.2 ± 2.7 | 8.2 ± 1.4 |
| 5 wt% BPDA-BAPP/m-HSNF | 84.5 ± 3.2 | 5.9 ± 2.3 |
| BPDA-HFBAPP | 82.0 ± 2.4 | 9.8 ± 0.4 |
| 1 wt% BPDA-HFBAPP/m-HSNF | 99.1 ± 2.5 | 9.2 ± 0.9 |
| 3 wt% BPDA-HFBAPP/m-HSNF | 108.9 ± 3.5 | 6.7 ± 1.4 |
| 5 wt% BPDA-HFBAPP/m-HSNF | 84.8 ± 3.3 | 5.0 ± 0.8 |
| m-HSNF Content (wt%) | 28 GHz | 38 GHz | ||
|---|---|---|---|---|
| BPDA-BAPP | BPDA-HFBAPP | BPDA-BAPP | BPDA-HFBAPP | |
| 0 | 3.11 | 2.83 | 3.09 | 2.81 |
| 1 | 3.01 | 2.77 | 2.98 | 2.75 |
| 3 | 2.87 | 2.65 | 2.85 | 2.64 |
| 5 | 2.79 | 2.59 | 2.76 | 2.58 |
| Materials | Frequency (GHz) | Dielectric Constant | Dielectric Loss | Ref. |
|---|---|---|---|---|
| PI | 38.9 | 2.9 | 0.010 | [25] |
| PI | 38.6 | 3.09 | 0.010 | [26] |
| BPDA-BAPP | 38 | 3.09 | 0.0097 | This work |
| 5 wt% BPDA-BAPP/m-HSNF | 38 | 2.76 | 0.0099 | This work |
| BPDA-HFBAPP | 38 | 2.81 | 0.0088 | This work |
| 5 wt% BPDA-HFBAPP/m-HSNF | 38 | 2.58 | 0.0090 | This work |
| Sample | 28 GHz | 38 GHz | ||
|---|---|---|---|---|
| Loss (dB/inch) | Uncertainty | Loss (dB/inch) | Uncertainty | |
| BPDA-BAPP | −2.32 | 1.4% | −2.80 | 4.0% |
| 5 wt% BPDA-BAPP/m-HSNF | −2.09 | 0.2% | −2.40 | 3.6% |
| BPDA-HFBAPP | −2.05 | 1.1% | −2.35 | 2.9% |
| 5 wt% BPDA-HFBAPP/m-HSNF | −1.90 | 0.7% | −2.14 | 3.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-Y.; Ye, Y.-M.; Chen, E.-C.; Wu, T.-M. Low Dielectric Properties and Transmission Loss of Polyimide/Organically Modified Hollow Silica Nanofiber Composites. Polymers 2022, 14, 4462. https://doi.org/10.3390/polym14204462
Lin S-Y, Ye Y-M, Chen E-C, Wu T-M. Low Dielectric Properties and Transmission Loss of Polyimide/Organically Modified Hollow Silica Nanofiber Composites. Polymers. 2022; 14(20):4462. https://doi.org/10.3390/polym14204462
Chicago/Turabian StyleLin, Shu-Yang, Yu-Min Ye, Erh-Ching Chen, and Tzong-Ming Wu. 2022. "Low Dielectric Properties and Transmission Loss of Polyimide/Organically Modified Hollow Silica Nanofiber Composites" Polymers 14, no. 20: 4462. https://doi.org/10.3390/polym14204462
APA StyleLin, S.-Y., Ye, Y.-M., Chen, E.-C., & Wu, T.-M. (2022). Low Dielectric Properties and Transmission Loss of Polyimide/Organically Modified Hollow Silica Nanofiber Composites. Polymers, 14(20), 4462. https://doi.org/10.3390/polym14204462






