Pilot Scale Hybrid Organic/Inorganic Coatings on a Polyolefin Separator to Enhance Dimensional Stability for Thermally Stable Long-Life Rechargeable Batteries
Abstract
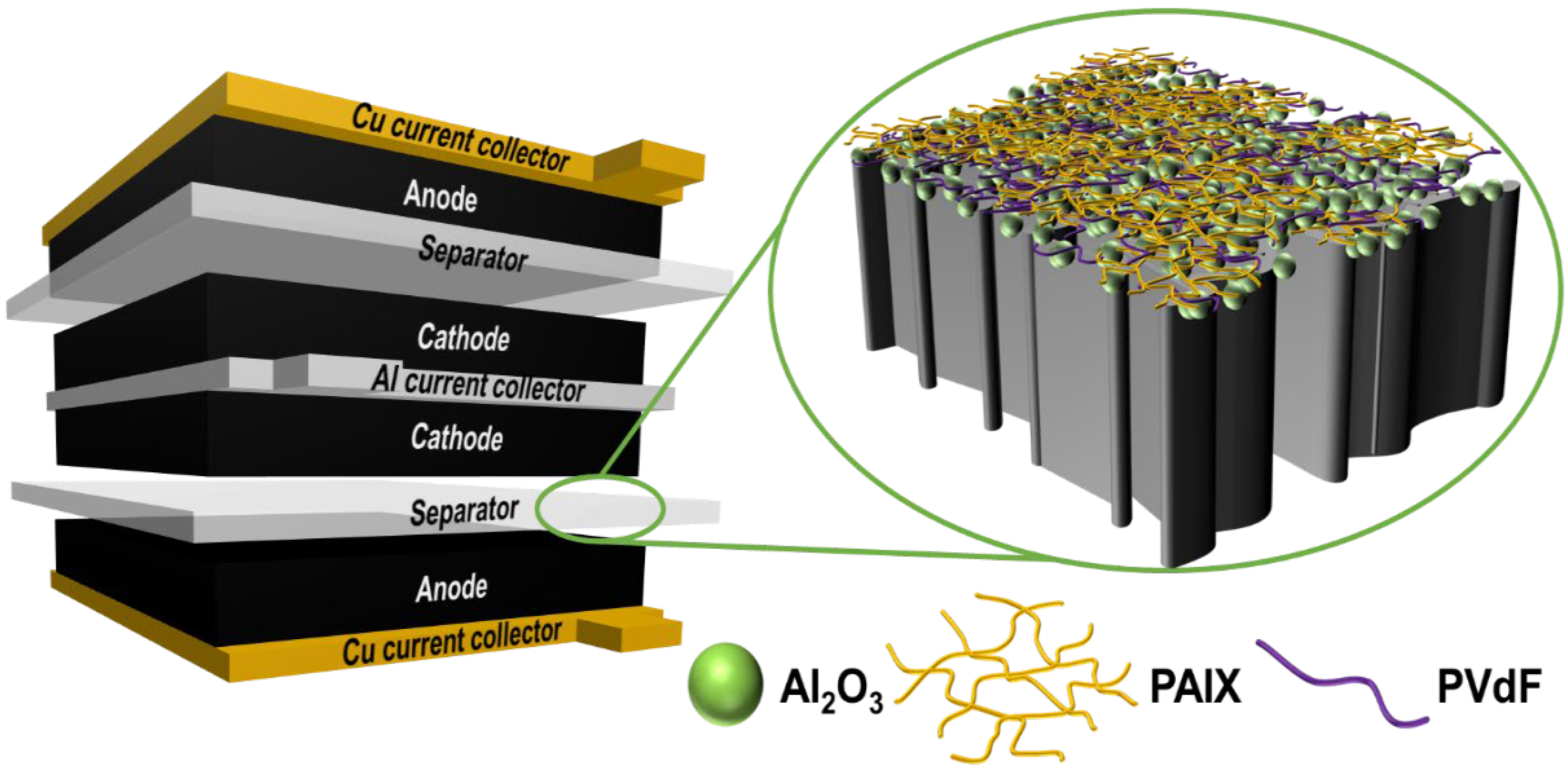
:1. Introduction
2. Experimental
2.1. Crosslinkable Polyamide-Imide Preparation
2.2. Polyethylene Separator Coating
2.3. Thermal and Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, W.; Zhang, J.; Li, H. Batteries with high theoretical energy densities. Energy Stor. Mater. 2020, 26, 46–55. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Knudsen Kær, S. Review of Parameter Determination for Thermal Modeling of Lithium Ion Batteries. Batteries 2018, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Shapter, J.G.; Wu, Q.; Yin, T.; Gao, G.; Cui, D. Nanostructured anode materials for lithium-ion batteries: Principle, recent progress and future perspectives. J. Mater. Chem. A 2017, 5, 19521–19540. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-S.; Wu, Z.; Petrova, V.; Xing, X.; Lim, H.-D.; Liu, H.; Liu, P. Analysis of Rate-Limiting Factors in Thick Electrodes for Electric Vehicle Applications. J. Electrochem. Soc. 2018, 165, A525–A533. [Google Scholar] [CrossRef] [Green Version]
- Mehrjerdi, H.; Hemmati, R. Stochastic model for electric vehicle charging station integrated with wind energy. Sustain. Energy Technol. Assess. 2020, 37, 100577. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Javaid, N.; Hussain, H.M.; Abdul, W.; Almogren, A.; Alamri, A.; Azim Niaz, I. An Optimized Home Energy Management System with Integrated Renewable Energy and Storage Resources. Energies 2017, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.; Wu, D.; Luo, J.; Li, J.; Deng, P.; Shen, Y.; Tian, X. A plasma bombing strategy to synthesize high-loading single-atom catalysts for oxygen reduction reaction. Cell Rep. Phys. Sci. 2022, 3, 100880. [Google Scholar] [CrossRef]
- Rao, P.; Wu, D.; Wang, T.-J.; Li, J.; Deng, P.; Chen, Q.; Shen, Y.; Chen, Y.; Tian, X. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404. [Google Scholar] [CrossRef]
- Rao, P.; Wang, T.-J.; Li, J.; Deng, P.-L.; Shen, Y.-J.; Chen, Y.; Tian, X.-L. Plasma induced Fe-NX active sites to improve the oxygen reduction reaction performance. Adv. Energy Mater. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Ghiji, M.; Novozhilov, V.; Moinuddin, K.; Joseph, P.; Burch, I.; Suendermann, B.; Gamble, G. A Review of Lithium-Ion Battery Fire Suppression. Energies 2020, 13, 5117. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Stor. Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Tang, X.; Zhu, J.; Chen, S.; Dai, H. Internal short circuit mechanisms, experimental approaches and detection methods of lithium-ion batteries for electric vehicles: A review. Renew. Sust. Energ. Rev. 2021, 141, 110790. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Solid Electrolytes for High-Temperature Stable Batteries and Supercapacitors. Adv. Energy Mater. 2021, 11, 2002869. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Q.; Guo, S.; Yu, L.; Hu, X. Thermoregulating Separators Based on Phase-Change Materials for Safe Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2008088. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Zhuo, D.; Lee, H.-W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the Life of Lithium-Based Rechargeable Batteries by Reaction of Lithium Dendrites with a Novel Silica Nanoparticle Sandwiched Separator. Adv. Mater. 2017, 29, 1603987. [Google Scholar] [CrossRef]
- Prasanna, K.; Kim, C.-S.; Lee, C.W. Effect of SiO2 coating on polyethylene separator with different stretching ratios for application in lithium ion batteries. Mater. Chem. Phys. 2014, 146, 545–550. [Google Scholar] [CrossRef]
- Shin, W.-K.; Kim, D.-W. High performance ceramic-coated separators prepared with lithium ion-containing SiO2 particles for lithium-ion batteries. J. Power Sources 2013, 226, 54–60. [Google Scholar] [CrossRef]
- Cho, J.; Jung, Y.-C.; Lee, Y.S.; Kim, D.-W. High performance separator coated with amino-functionalized SiO2 particles for safety enhanced lithium-ion batteries. J. Membr. Sci. 2017, 535, 151–157. [Google Scholar] [CrossRef]
- Ding, L.; Yan, N.; Zhang, S.; Xu, R.; Wu, T.; Yang, F.; Cao, Y.; Xiang, M. Low-Cost Mass Manufacturing Technique for the Shutdown-Functionalized Lithium-Ion Battery Separator Based on Al2O3 Coating Online Construction during the β-iPP Cavitation Process. ACS Appl. Mater. Interfaces 2022, 14, 6714–6728. [Google Scholar] [CrossRef]
- Lee, D.-W.; Lee, S.-H.; Kim, Y.-N.; Oh, J.-M. Preparation of a high-purity ultrafine α-Al2O3 powder and characterization of an Al2O3-coated PE separator for lithium-ion batteries. Powder Technol. 2017, 320, 125–132. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, L.; Bai, Y.; Song, S.; Liu, G.; Xue, H.; Wang, T.; Huang, X.; He, J. Amino-Functionalized Al2O3 Particles Coating Separator with Excellent Lithium-Ion Transport Properties for High-Power Density Lithium-Ion Batteries. Adv. Eng. Mater. 2020, 22, 1901545. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Wang, C.; Wang, Z.; Guo, S.; Wang, L.; Liang, X.; Xiang, H. An AlOOH-coated polyimide electrospun fibrous membrane as a high-safety lithium-ion battery separator. Ionics 2019, 25, 2677–2684. [Google Scholar] [CrossRef]
- Yang, C.; Tong, H.; Luo, C.; Yuan, S.; Chen, G.; Yang, Y. Boehmite particle coating modified microporous polyethylene membrane: A promising separator for lithium ion batteries. J. Power Sources 2017, 348, 80–86. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Lan, Y.; Song, Z.; Luo, J.; Wei, X.; Sun, F.; Yue, Z.; Yin, C.; Zhou, L.; et al. Aqueous aluminide ceramic coating polyethylene separators for lithium-ion batteries. Solid State Ion. 2020, 345, 115188. [Google Scholar] [CrossRef]
- Zhi, Y.; Sun, X.; Li, N.; Yuan, S.; Wang, Z.; Jin, L.; Hang, J.; Shi, L. UV curable organic-inorganic hybrid coatings on microporous polyethylene separator for enhancing mechanical and electrochemical performance. J. Alloys Compd. 2018, 743, 756–762. [Google Scholar] [CrossRef]
- Mizutani, Y.; Matsuda, H.; Ishiji, T.; Furuya, N.; Takahashi, K. Improvement of electrochemical NO2 sensor by use of carbon-fluorocarbon gas permeable electrode. Sens. Actuators B Chem. 2005, 108, 815–819. [Google Scholar] [CrossRef]
- Wang, Y.; Travas-Sejdic, J.; Steiner, R. Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery. Solid State Ion. 2002, 148, 443–449. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, H.; Xiang, H.; Xia, R.; Liang, D.; Shi, P.; Dai, S.; Wang, H. Enhancement on the wettability of lithium battery separator toward nonaqueous electrolytes. J. Membr. Sci. 2016, 503, 25–30. [Google Scholar] [CrossRef]
- Xu, R.; Huang, X.; Lin, X.; Cao, J.; Yang, J.; Lei, C. The functional aqueous slurry coated separator using polyvinylidene fluoride powder particles for Lithium-ion batteries. J. Electroanal. Chem. 2017, 786, 77–85. [Google Scholar] [CrossRef]
- Xue, C.; Jin, D.; Nan, H.; Wei, H.; Chen, H.; Zhang, C.; Xu, S. A novel polymer-modified separator for high-performance lithium-ion batteries. J. Power Sources 2020, 449, 227548. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Q.; Wang, J.; Zhang, P.; Zhu, Y.; Wu, G.; Lv, Y.; Lv, L.; Zhao, Y.; Yang, M. Effects of Island-Coated PVdF-HFP Composite Separator on the Performance of Commercial Lithium-ion Batteries. Coatings 2018, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Erkoc, P.; Odeh, Y.N.; Alrifai, N.; Zirhli, O.; Gunduz Akdogan, N.; Yildiz, B.; Misirlioglu, I.B.; Akdogan, O. Photocurable pentaerythritol triacrylate/lithium phenyl-2,4,6-trimethylbenzoylphosphinate-based ink for extrusion-based 3D printing of magneto-responsive materials. J. Appl. Polym. Sci. 2020, 137, 49043. [Google Scholar] [CrossRef]
- Jung, B.; Lee, B.; Jeong, Y.-C.; Lee, J.; Yang, S.R.; Kim, H.; Park, M. Thermally stable non-aqueous ceramic-coated separators with enhanced nail penetration performance. J. Power Sources 2019, 427, 271–282. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Chen, W.-H. High glass transitions of novel organosoluble polyamide-imides based on noncoplanar and rigid diimide-dicarboxylic acid. Polym. Degrad. Stab. 2006, 91, 1731–1739. [Google Scholar] [CrossRef]
- Wang, S.; Yang, G.; Wu, S.; Ren, G.; Yang, W.; Liu, X. Preparation of solution-processable colorless polyamide-imides with extremely low thermal expansion coefficients through an in-situ silylation method for potential space optical applications. e-Polymers 2016, 16, 395–402. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Ooms, F.G.B.; Kelder, E.M.; Schoonman, J.; Gerrits, N.; Smedinga, J.; Calis, G. Performance of Solupor® separator materials in lithium ion batteries. J. Power Sources. 2001, 97–98, 598–601. [Google Scholar] [CrossRef]
- Gong, W.; Gu, J.; Ruan, S.; Shen, C. A high-strength electrospun PPESK fibrous membrane for lithium-ion battery separator. Polym. Bull. 2019, 76, 5451–5462. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Xu, L.; Wang, J. Design of A High Performance Zeolite/Polyimide Composite Separator for Lithium-Ion Batteries. Polymers 2020, 12, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Lin, C.; Wang, Z.; Jin, J. Polymer of intrinsic microporosity-based macroporous membrane with high thermal stability as a Li-ion battery separator. RSC Adv. 2019, 9, 21539–21543. [Google Scholar] [CrossRef] [PubMed]





| Sample Code | Solid Content (wt %) | Organic to Al2O3 Ratio | Organic Content (wt %) | Gurley Number (s/ 100 cc) | ||
|---|---|---|---|---|---|---|
| PAIX | Polyurethane Acrylate | PVdF | ||||
| Acrylate | 10 | 1/2 | 0 | 80 | 20 | 1770 |
| PAIX12 | 10 | 1/2 | 100 | 0 | 0 | 226 |
| PAIX1291 | 10 | 1/2 | 90 | 0 | 10 | 582 |
| PAIX1282 | 10 | 1/2 | 80 | 0 | 20 | 820 |
| PAIX1382 | 10 | 1/3 | 80 | 0 | 20 | 326 |
| PAIX1373-12 | 12 | 1/3 | 70 | 0 | 30 | 278 |
| Sample Code | Solid Content (wt %) | Organic to Al2O3 Ratio | PAIX to PVdF Ratio | Density (g cc−1) | Thickness (μm) | Gurley Number (s/100 cc) |
|---|---|---|---|---|---|---|
| PAIX1373-12 | 12 | 1/3 | 7/3 | 2.15 | 2.500 | 278 |
| PAIX_DI1 | 1.84 | 3.000 | 260 | |||
| PAIX_DI2 | 1.48 | 3.875 | 245 | |||
| PAIX_EG3 | 2.04 | 4.000 | 207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, B.-S. Pilot Scale Hybrid Organic/Inorganic Coatings on a Polyolefin Separator to Enhance Dimensional Stability for Thermally Stable Long-Life Rechargeable Batteries. Polymers 2022, 14, 4474. https://doi.org/10.3390/polym14214474
Choi H, Lee B-S. Pilot Scale Hybrid Organic/Inorganic Coatings on a Polyolefin Separator to Enhance Dimensional Stability for Thermally Stable Long-Life Rechargeable Batteries. Polymers. 2022; 14(21):4474. https://doi.org/10.3390/polym14214474
Chicago/Turabian StyleChoi, Hyoungwoo, and Byoung-Sun Lee. 2022. "Pilot Scale Hybrid Organic/Inorganic Coatings on a Polyolefin Separator to Enhance Dimensional Stability for Thermally Stable Long-Life Rechargeable Batteries" Polymers 14, no. 21: 4474. https://doi.org/10.3390/polym14214474








