A Study on Doping and Compound of Zinc Oxide Photocatalysts
Abstract
1. Introduction
2. Zinc Oxide Doped with Transition Metal Ions
2.1. Doped with Divalent Metal Ions
2.2. Doped with Trivalent Metal Ions
2.3. Co-Doping of Metal Ions
3. Zinc Oxide Complex Precious Metal
3.1. Compounded with Precious Metal Silver
3.2. Compound with Other Precious Metal
4. Zinc Oxide Composite Semiconductor
4.1. Commonly Used Semiconductor Composite
4.2. Graphene Composite
5. Changes in the Structure of Zinc Oxide
5.1. Doped with Transition Metal Ions
5.2. Doped with Trivalent Metal Ions
5.3. Composite Other Semiconductor
6. Conclusions and Outlook
6.1. Summary of Proposed Processes
- Dope with transition metal ions
- 2.
- Complex precious metal
- 3.
- Composite other semiconductor
6.2. Further Improvements on Zinc Oxide Structuring
- In the field of semiconductor composites, binary composites are widely used, while ternary composites are seldom studied. Recently, some researchers [66,67] have made ternary complexes of these ZnO binary complexes, these ternary complexes show higher stability, and the separation efficiency and separation time of electron–hole pairs are greatly increased, the photocatalytic activity is higher. This is due to the excitation of the narrow-band-gap semiconductors to the electron–hole pairs in the ternary complex under the irradiation of light, and because the conducting band energy of the narrow-band-gap semiconductors is lower than that of the other two semiconductors, therefore, the electrons are transferred from the narrow band-gap semiconductor to the wide band-gap semiconductor, and the valence band energy of the narrow band-gap semiconductor is greater than the valence band energy of the other two semiconductors, the holes in the valence band of narrow-band-gap semiconductors do not move or move to the valence band of wide-band-gap semiconductors, thus the effective separation of electron–hole pairs is realized. Therefore, the ternary complex is a feasible method to improve the photocatalytic efficiency of zinc oxide, and more attempts can be made in this area in the future;
- On the basis of binary composite zinc oxide/semiconductor, doping of transition metal ions or modification of precious metals [68,69], that is, on the basis of heterojunction between zinc oxide and other materials, doping other transition metal ions or precious metals which can improve the photocatalytic efficiency of zinc oxide, the separation efficiency of photogenerated electron–hole pairs can be increased by increasing the specific surface area, so as to further improve the photocatalytic efficiency of zinc oxide.
Author Contributions
Funding
Conflicts of Interest
References
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Xu, H.; Xu, L. Preparation of ZnO Nanomaterials and Their Photocatalytic Degradation of Organic Pollutants. Donghua Univ. Eng. Ed. 2020, 37, 271–279. [Google Scholar]
- Wang, S.; Yun, J.-H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef]
- Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Coagulation-flocculation-decantation of dye house effluents: Concentrated ef-fluents. J. Hazard. Mater. 2004, 116, 57. [Google Scholar] [CrossRef] [PubMed]
- Golob, V.; Vinder, A.; Simonic, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dye. Pigment. 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Alinsafi, A.; Khemis, M.; Pons, M.N.; Leclerc, J.P.; Yaacoubi, A.; Benhammou, A.; Nejmeddine, N. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. Process Intensif. 2005, 44, 461–470. [Google Scholar] [CrossRef]
- Papicé, S.; Koprivanac, N.; Božicé, A.L.; Meteš, A. Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dye. Pigment. 2004, 62, 291–298. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, X.; Yang, J.; Shan, X.; Yang, L.; Zhang, Y.; Li, X.; Gao, M. ZnO–graphene composite for photocatalytic degradation of methylene blue dye. Catal. Commun. 2012, 29, 29–34. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti-recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Wang, H.; Yi, G.; Tan, M.; Zu, X.; Luo, H.; Jiang, X. Initial reactant controlled synthesis of double layered TiO2, nanostructures and characterization of its spectra of absorption and photoluminescence. Mater. Lett. 2015, 148, 5–8. [Google Scholar] [CrossRef]
- Ng, K.H.; Yuan, L.S.; Cheng, C.K.; Chen, K.; Fang, C. TiO2 and ZnO photocatalytic treatment of palm oil mill effluent (POME) and feasibility of renewable energy generation: A short review. J. Clean. Prod. 2019, 233, 209–225. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Nakamura, R.; Tachibana, Y. Primary photocatalytic water reduction and oxidation at an anatase TiO2 and Pt-TiO2 nanocrystalline electrode revealed by quantitative transient absorption studies. Appl. Catal. B Environ. 2021, 296, 120226. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, S.; Kim, M.; Sinha, A.K.; Lee, S.C.; Jung, E.; Chang, W.J.; Lee, K.S.; Kim, J.H.; Cho, S.P.; et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 2019, 18, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Muller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2-ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef]
- Xie, J.; Wang, H.; Duan, M.; Zhang, L. Synthesis and photocatalysis properties of ZnO structures with different morphologies via hydrothermal method. Appl. Surf. Sci. 2011, 257, 6358–6363. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 4, 2833–2881. [Google Scholar] [CrossRef]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunligh. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047. [Google Scholar] [CrossRef]
- Yeber, M.; Rodríguez, J.; Freer, J.; Baeza, J.; Durán, N.; Mansilla, H.D. Advanced oxidation of a pulp mill bleaching wastewater. Chemosphere 1999, 39, 1679–1688. [Google Scholar] [CrossRef]
- Khodja, A.A.; Sehili, T.; Pilichowski, J.F.; Boule, P. Photocatalytic degradation of 2-phenylphenol on TiO2, and ZnO in aqueous suspensions. J. Photochem. Photobiol. A Chem. 2001, 141, 231–239. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Marci, G.; Augugliaro, V.; López-Muñoz, M.J.; Martin, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.; Venezia, A.M. Preparation characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems. J. Phys. Chem. B 2001, 105, 1026–1032. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Huszla, K.; Wysokowski, M.; Zgoła-Grześkowiak, A.; Staszak, M.; Janczarek, M.; Jesionowski, T.; Wyrwas, B. UV-light photocatalytic degradation of non-ionic surfactants using ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2022, 19, 173–188. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Xie, Y.; Fan, Q.; Jimmy, C.Y.; Shu, Q.; Wang, C. Novel hollow Pt-ZnO nanocomposite micropheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142–2151. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chen, X.Y.; Ng, A.; Guo, M.Y.; Liu, F.Z.; Djurišić, A.B.; Chan, W.K.; Shi, X.Q.; Van Hove, M.A. Green emission in ZnO nanostructures-examination of the roles of oxygen and zinc vacancies. Appl. Surf. Sci. 2013, 271, 202–209. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.K.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Gabała, E.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Controlled microwave-assisted and pH-affected growth of ZnO structures and their photocatalytic performance. Powder Technol. 2021, 386, 221–235. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Q.; Lu, J.; Ma, Z. Research progress of fabrication of ZnO-based photoanode and photoelectrocatalytic water splitting performances. Chem. Ind. Eng. Prog. 2021, 40, 3. [Google Scholar]
- Commandeur, D.; Brown, G.; McNulty, P.; Dadswell, C.; Spencer, J.; Chen, Q. Yttrium-doped ZnO nanorod arrays for increased charge mobility and carrier density for enhanced solar water splitting. J. Phys. Chem. C 2019, 123, 18187–18197. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, M.I.; Adam, A.; Azad, A.M.; Qamar, M. A novel fabrication methodology for sulfur-doped ZnO nanorods as an active photoanode for improved water oxidation in visible-light regime. Nanotechnology 2016, 28, 055602. [Google Scholar] [CrossRef]
- Cao, S.; Yan, X.; Kang, Z.; Liang, Q.; Liao, X.; Zhang, Y. Band alignment engineering for improved performance and stability of ZnFe2O4 modified CdS/ZnO nanostructured photoanode for PEC water splitting. Nano Energy 2016, 24, 25–31. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, B.; Hu, H.; Zhang, Y.; Bi, Y. ZnO nanowire arrays decorated with PtO nanowires for efficient solar water splitting. Catal. Sci. Technol. 2018, 8, 2789–2793. [Google Scholar] [CrossRef]
- Chou, C.M.; Chang, Y.C.; Lin, P.S.; Liu, F.K. Growth of Cu doped ZnO nanowires or ZnO-CuO nanowires on the sanme brass foil with high performance photocatalytic activity and stability. Mater. Chem. Phys. 2017, 201, 18–25. [Google Scholar] [CrossRef]
- Gao, Q.; Dai, Y.; Li, C.; Yang, L.; Li, X.; Cui, C. Correlation between oxygen cacancies and dopant concentration in Mn doped ZnO nanoparticles synthesized by co-Precipitation technique. J. Alloy. Compd. 2016, 684, 669–676. [Google Scholar] [CrossRef]
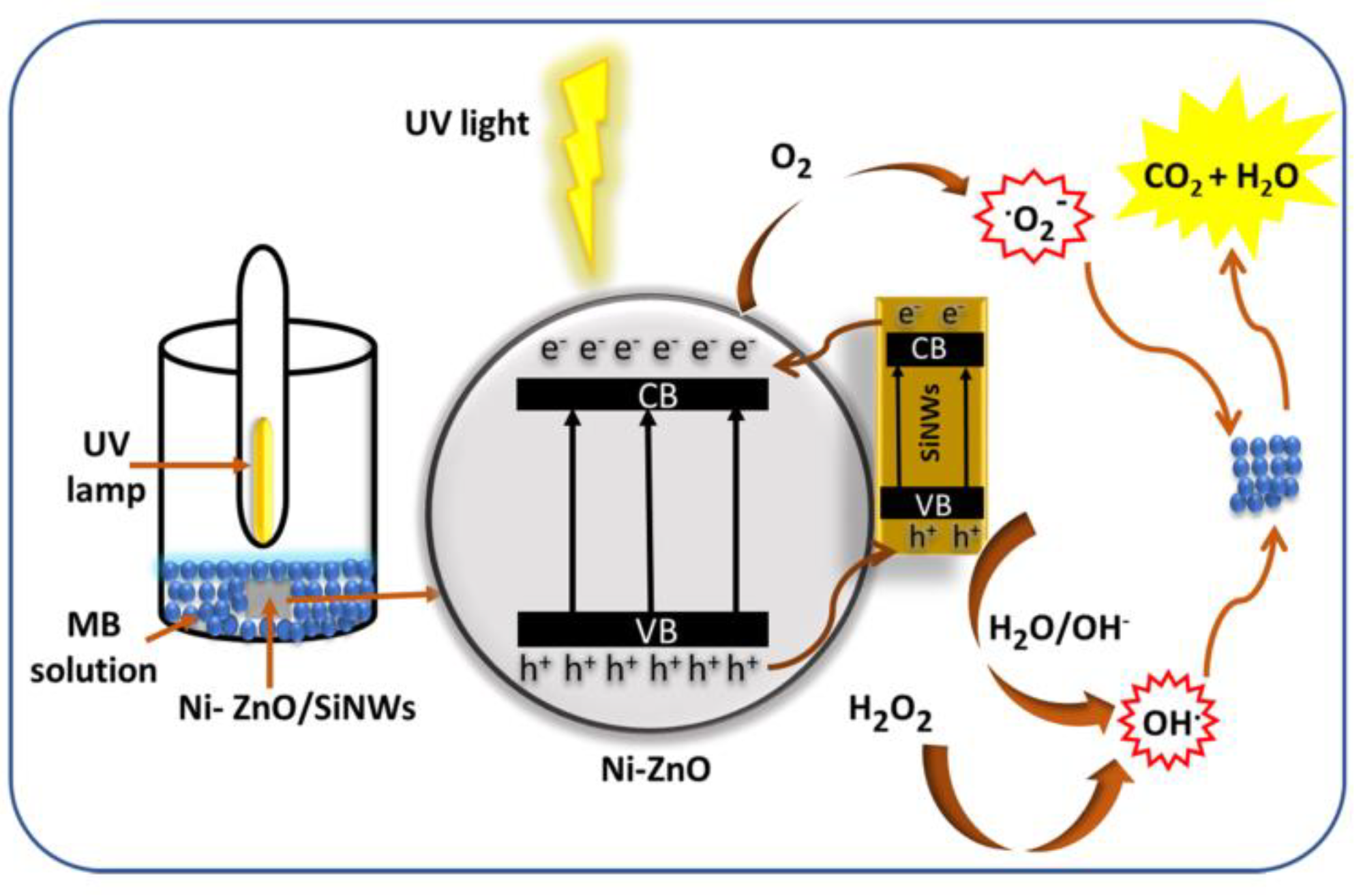
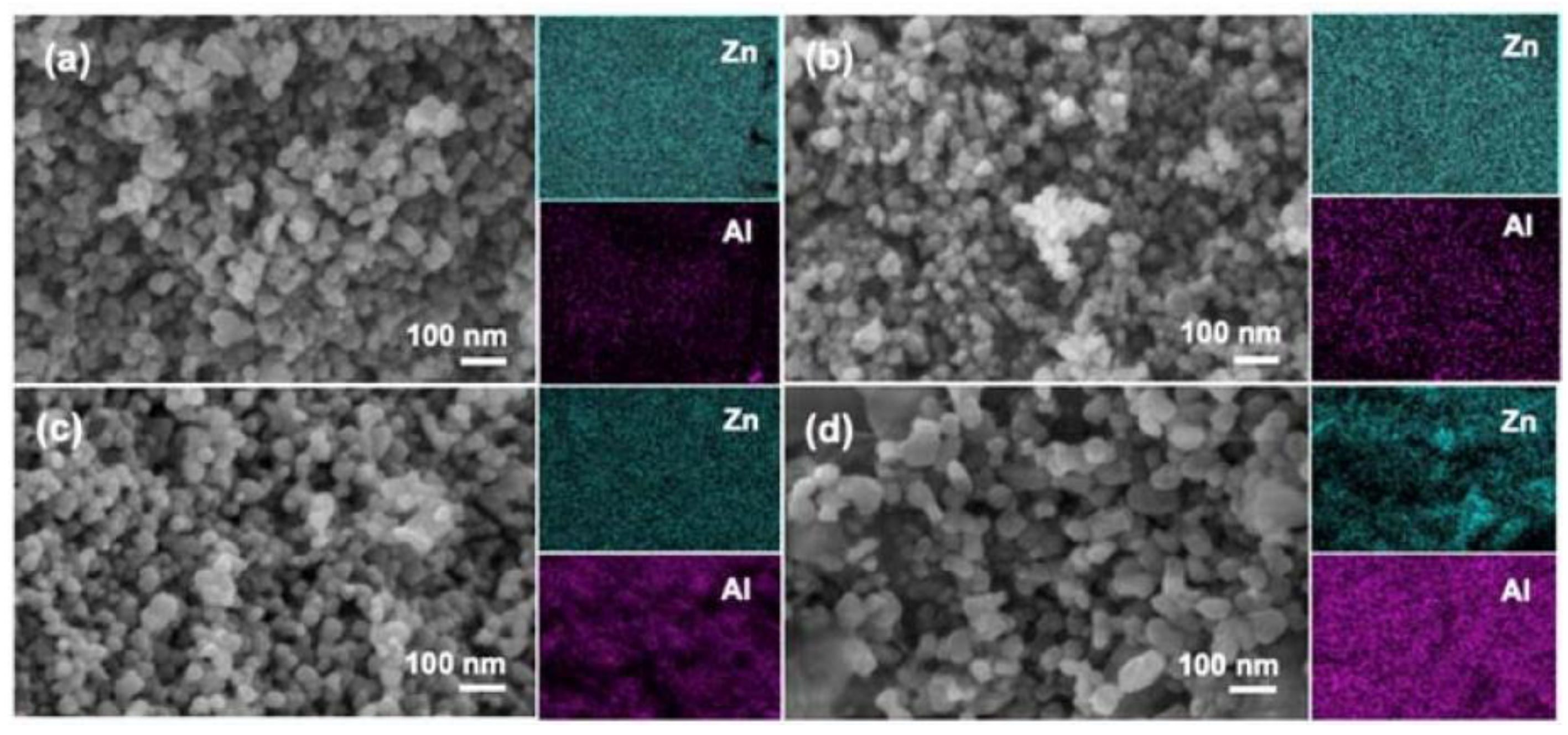
- Hammouche, J.; Daoudi, K.; Columbus, S.; Ziad, R.; Ramachandran, K.; Gaidi, M. Structural and morphological optimization of Ni doped ZnO decorated silicon nanowires for photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 131, 108763. [Google Scholar] [CrossRef]
- Tang, L.; Jia, Y.; Zhu, Z.; Hua, Y.; Wu, J.; Zou, Z.; Zhou, Y. Effects of Co Doping on the Growth and Photocatalytic Properties of ZnO Particles. Molecules 2022, 27, 833. [Google Scholar] [CrossRef]
- Šutka, A.; Käämbre, T.; Pärna, R.; Juhnevica, I.; Maiorov, M.; Joost, U.; Kisand, V. Co doped ZnO nanowires as visible light photocatalysts. Solid State Sci. 2016, 56, 54–62. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanaomaterials 2021, 11, 1059. [Google Scholar] [CrossRef]
- Roguai, S.; Djelloul, A. Structural, microstructural and photocatalytic degradation of methylene blue of zinc oxide and Fe-doped ZnO nanoparticles prepared by simple coprecipitation method. Solid State Commun. 2021, 114, 362. [Google Scholar] [CrossRef]
- Kumar, M.S.; Arunagiri, C. Efficient photocatalytic degradation of organic dyes using Fe-doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 17925–17935. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Kumar, V.; Som, S.; Bezuindenhoudt, B.C.B.; Ntwaeaborwa, O.M. Influence of ultrasonication times onthe tunable colour emission of ZnO nanophosphos for lighting applications. Ultrason. Sonochemistry 2014, 21, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non-equilibrium process: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wang, Q.P.; Xue, Z.Y. Photoluminescence of ZnO films excited with light of different wavelength. Appl. Surf. Sci. 2003, 207, 561–572. [Google Scholar] [CrossRef]
- Dabir, F.; Esfahani, H.; Bakhtiargonbadi, F.; Khodadadi, Z. Study on microstructural and electro-optical properties of sol–gel derived pure and Al/Cu-doped ZnO thin films. J. Sol-Gel Sci. Technol. 2020, 96, 529–538. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt-doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J.; Liu, X.G.; Jia, H.S.; Xu, B.S. Worm-like Ag/ZnO core-shell heterostructural composites: Fabrication, characterization, and photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Peychev, B.; Vasileva, P. Novel starch-mediated synthesis of Au/ZnO nanocrystals and their photocatalytic properties. Heliyon 2021, 7, e07402. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, H.; Li, J.; Xia, Z.; Zuo, J. Preparation of Flower-Like Ag@AgBr/ZnO Photocatalyst and Photocatalytic Degradation Mechanism on Cefuroxime Sodium. J. Environ. 2020, 146, 04020046. [Google Scholar] [CrossRef]
- Wu, D.; Tian, J.; Xing, Y.; Jin, X.; Ni, G. Fabrication of Z-scheme ZnO/Bi2O4 heterojunction photocatalyst with superior photocatalytic nitrogen fixation under visible light irradiation. Solid State Sci. 2021, 119, 106709. [Google Scholar] [CrossRef]
- Gonullu, M.P. Design and characterization of single bilayer ZnO/Al2O3 film by ultrasonically spray pyrolysis and its application in photocatalysis. Micro Nanostructures 2022, 164, 107113. [Google Scholar] [CrossRef]
- Helaïli, N.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. P-Cu2O/n-ZnO heterojunction applied to visible light orange II degradation. Sol. Energy 2010, 84, 1187–1192. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Gao, C.; Zhong, K.; Fang, X.; Fang, D.; Zhao, H.; Wang, D.; Li, B.; Zhai, Y.; Chu, X.; Li, J.; et al. Brief Review of Photocatalysis and Photoresponse Properties of ZnO–Graphene Nanocomposites. Energies 2021, 14, 6403. [Google Scholar] [CrossRef]
- Ahmed, I.; Shi, L.; Pasanen, H.; Vivo, P.; Maity, P.; Hatamvand, M.; Zhan, Y. There is plenty of room at the top: Generation of hot charge carriers and their applications in perovskite and other semiconductor-based optoelectronic devices. Light Sci. Appl. 2021, 10, 1–28. [Google Scholar] [CrossRef]
- Verma, A.; Choudhary, R.B. Influence of CdS nanorods on the optoelectronic properties of 2-dimensional rGO decorated polyindole matrix. Mater. Sci. Semicond. Process. 2020, 110, 104948. [Google Scholar] [CrossRef]
- Wilson, K.C.; Ahamed, M.B. Influence of bath temperature on surface modification and optoelectronic properties of chemical bath deposited CdS thin film nanostructures. Mater. Sci. Eng. B 2019, 251, 114444. [Google Scholar] [CrossRef]
- Yang, M.Q.; Xu, Y.J. Basic principles for observing the photosensitizer role of graphene in the graphene-semiconductor composite photocatalyst from a case study on graphene-ZnO. J. Phys. Chem. C 2013, 117, 21724–21734. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Rehim, M.H.A. Synergistic effect of zinc oxide nanorods on the photocatalytic performance and the biological activity of graphene nano sheets. Heliyon 2020, 6, e03283. [Google Scholar] [CrossRef]
- Quan, W.-L.; Zhang, J.-M.; Shen, J.-H.; Li, L.-C.; Li, J.-J. Hierarchical ZnO: Architecture, Morphological Control and Photocatalytic Activity. Chin. J. Inorg. Chem. 2015, 30, 8. [Google Scholar]
- Boughelout, A.; Macaluso, R.; Kechouane, M.; Trari, M. Photocatalysis of rhodamine B and methyl orange degradation under solar light on ZnO and Cu2O thin films. Kinet. Mech. Catal 2020, 129, 1115–1130. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2, nanoparticles. Appl. Catal. B Environ. 2005, 57, 23–30. [Google Scholar] [CrossRef]
- Li, J.; Lv, S.; Liu, Y.; Bai, J.; Zhou, B.; Hu, X. Photoeletrocatalytic activity of an n-ZnO/p-Cu2O/n-TNA ternary heterojunction electrode for tetracycline degradation. J. Hazard. Mater. 2013, 262, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Khoa, N.T.; Kim, S.W.; Yoo, D.H.; Cho, S.; Kim, E.J.; Hahn, S.H. Fabrication of Au/Graphene-wrapped ZnO-nanoparticle-assembled hollow spheres with effective photoinduced charge transfer for photocatalysis. Appl. Mater. Interfaces 2015, 7, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, K.; Zhou, Y.; Li, L. Synthesis and photocatalytic property of a Ag doped ZnO/ZnSnO3 composite photocatalyst. Funct. Mater. 2011, 42, 435–437. [Google Scholar]
- Li, X.; Meng, A. Study on Preparation by Hydrothermal Method and Photocatalytic Acitivity of Fe-doped ZnO/Ag Nano composite. J. Qiangdao Univ. Sci. Technol. Nat. Sci. Ed. 2013, 34, 448–451. [Google Scholar]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A. Ultrasonic-assisted preparation of novel ternary ZnO/AgI/Fe3O4 nanocomposites as magnetically separable visible-light-driven photocatalysts with excellent activity. J. Colloid Interface Sci. 2016, 461, 144–153. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Liu, M.; Lin, L.; Cheng, Y.; Fang, C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers 2022, 14, 4484. https://doi.org/10.3390/polym14214484
Mao T, Liu M, Lin L, Cheng Y, Fang C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers. 2022; 14(21):4484. https://doi.org/10.3390/polym14214484
Chicago/Turabian StyleMao, Tan, Mengchen Liu, Liyuan Lin, Youliang Cheng, and Changqing Fang. 2022. "A Study on Doping and Compound of Zinc Oxide Photocatalysts" Polymers 14, no. 21: 4484. https://doi.org/10.3390/polym14214484
APA StyleMao, T., Liu, M., Lin, L., Cheng, Y., & Fang, C. (2022). A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers, 14(21), 4484. https://doi.org/10.3390/polym14214484





