Effect of Microwave-Assisted Curing on Properties of Waterborne Silicone Antifouling Coatings
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of the Waterborne Silicone Coating
2.3. Testing and Characterization
2.3.1. Infrared Spectrum Analysis
2.3.2. Surface Morphology Analysis
2.3.3. Contact Angle Measurement
2.3.4. Tensile Test
2.3.5. Marine Bacterial Attachment Test
2.3.6. Navicular Tenera Attachment Test
3. Results
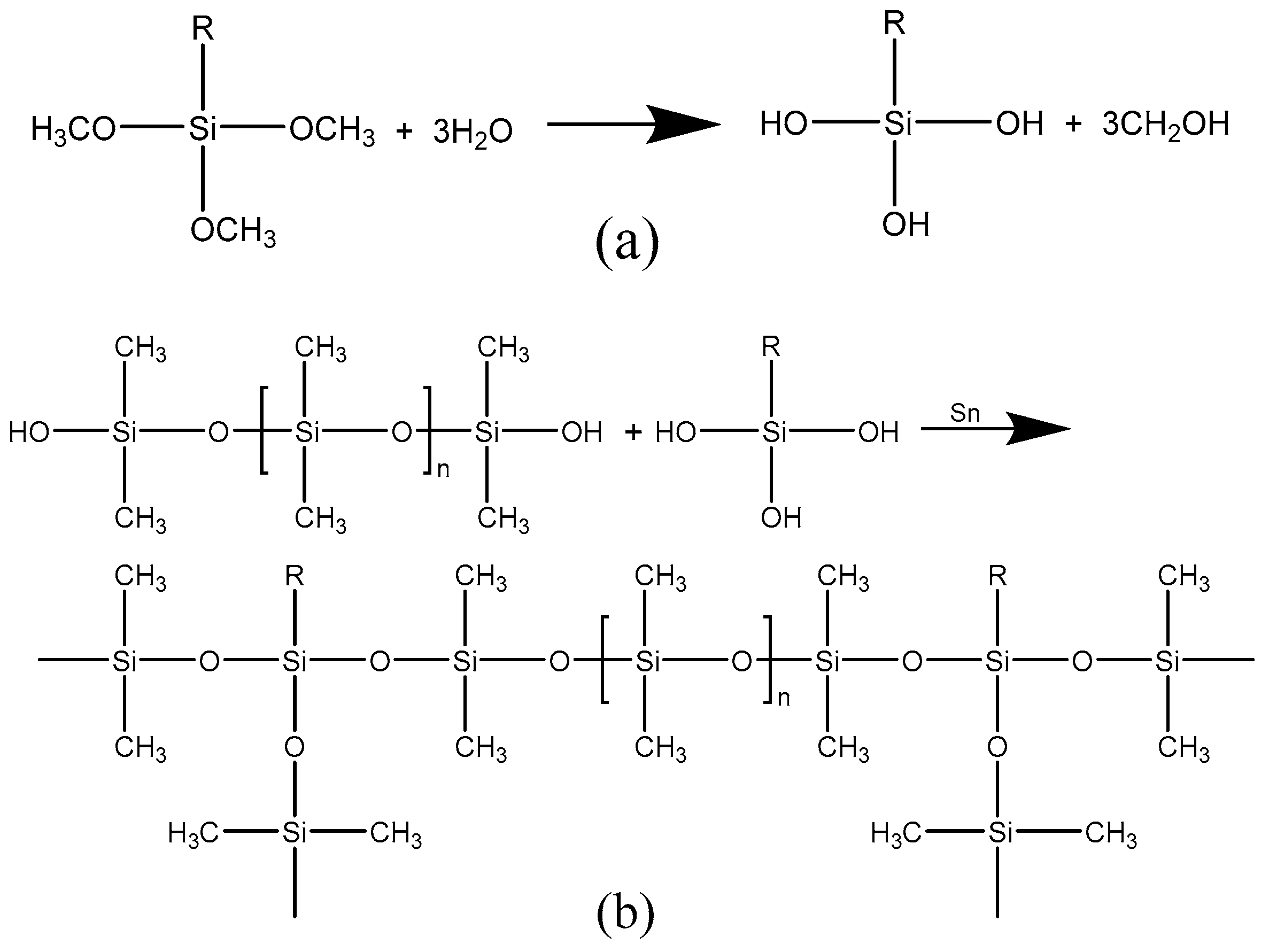
3.1. Microwave Curing Effect and Mechanism
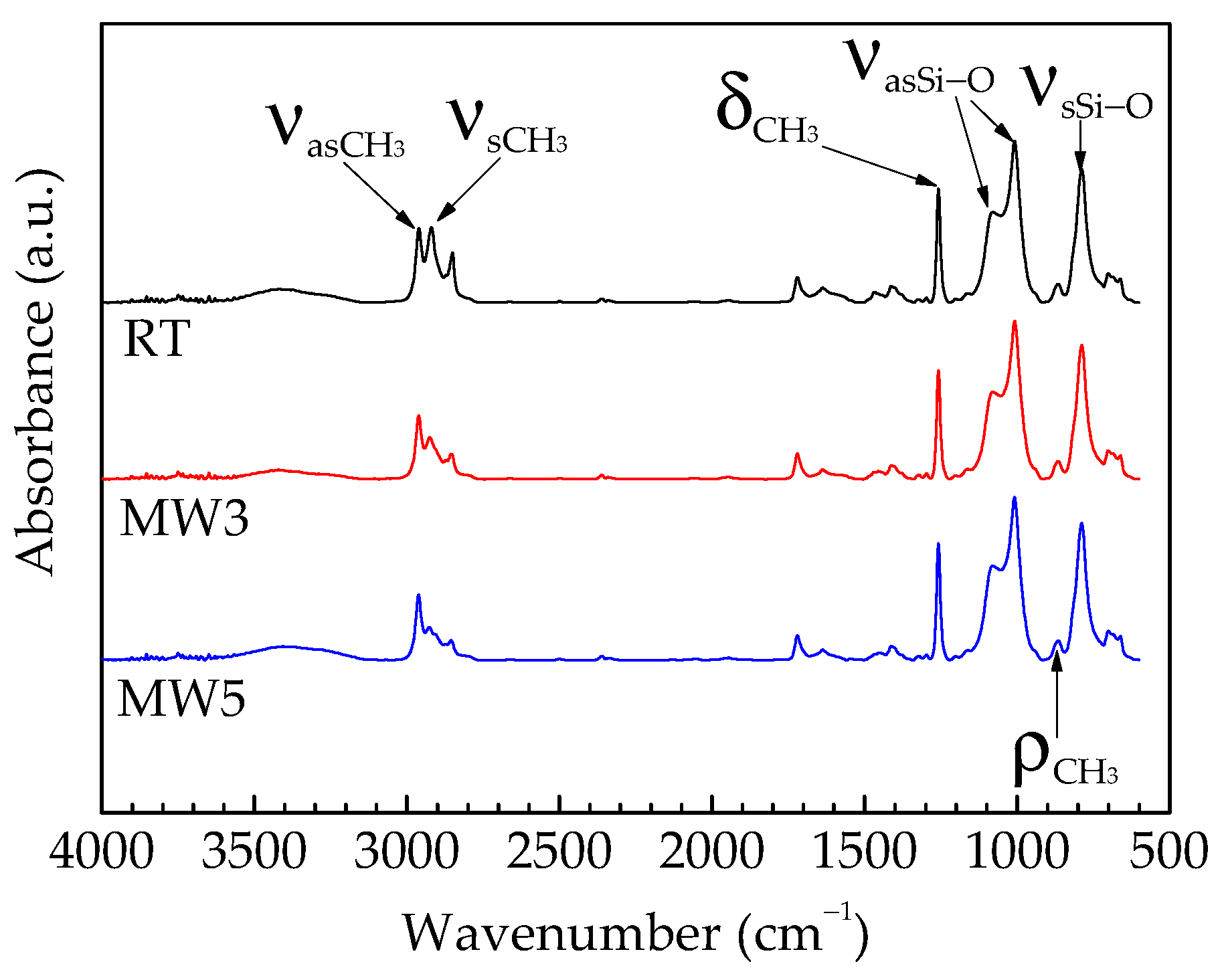
3.2. Chemical Structure
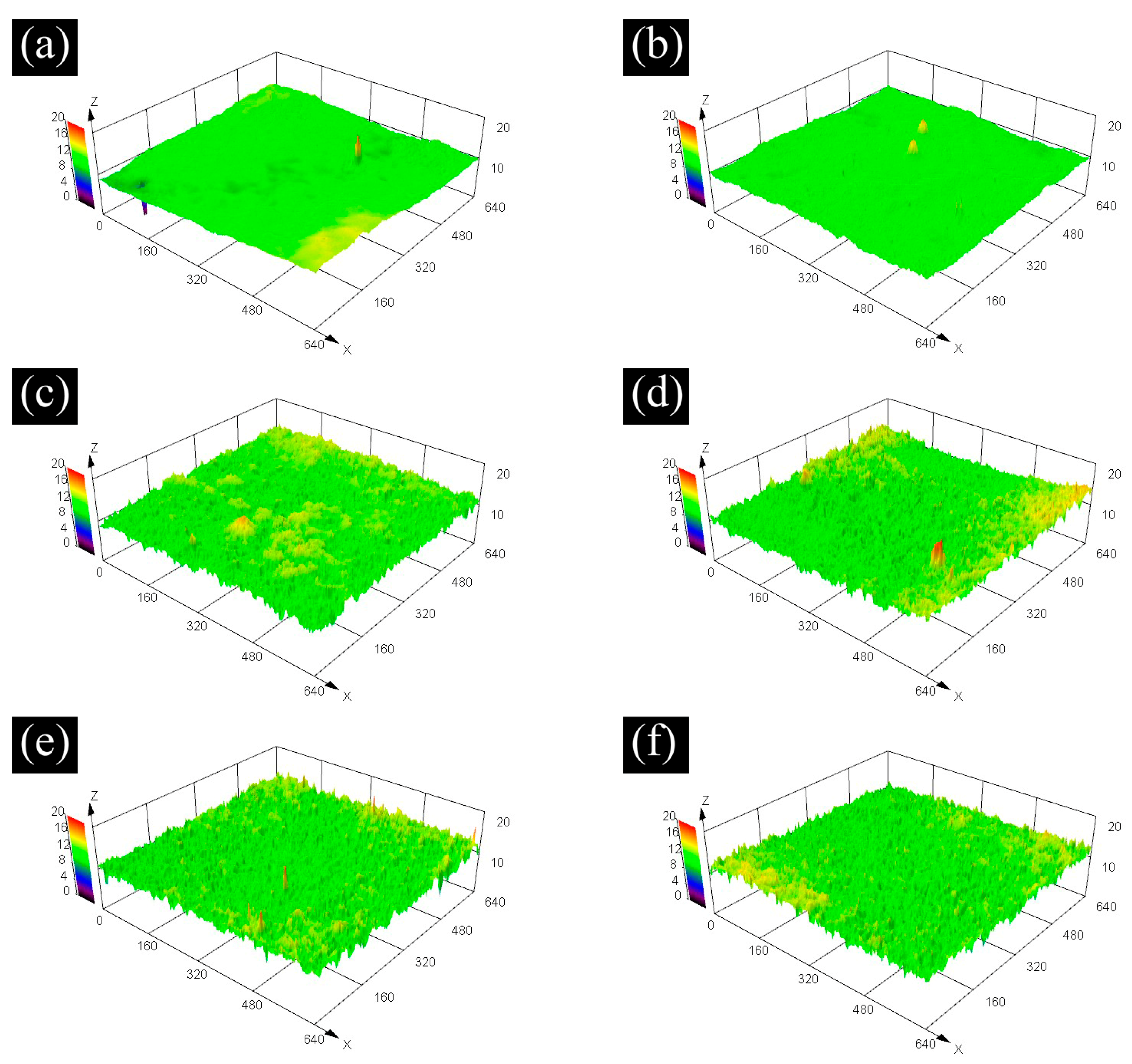
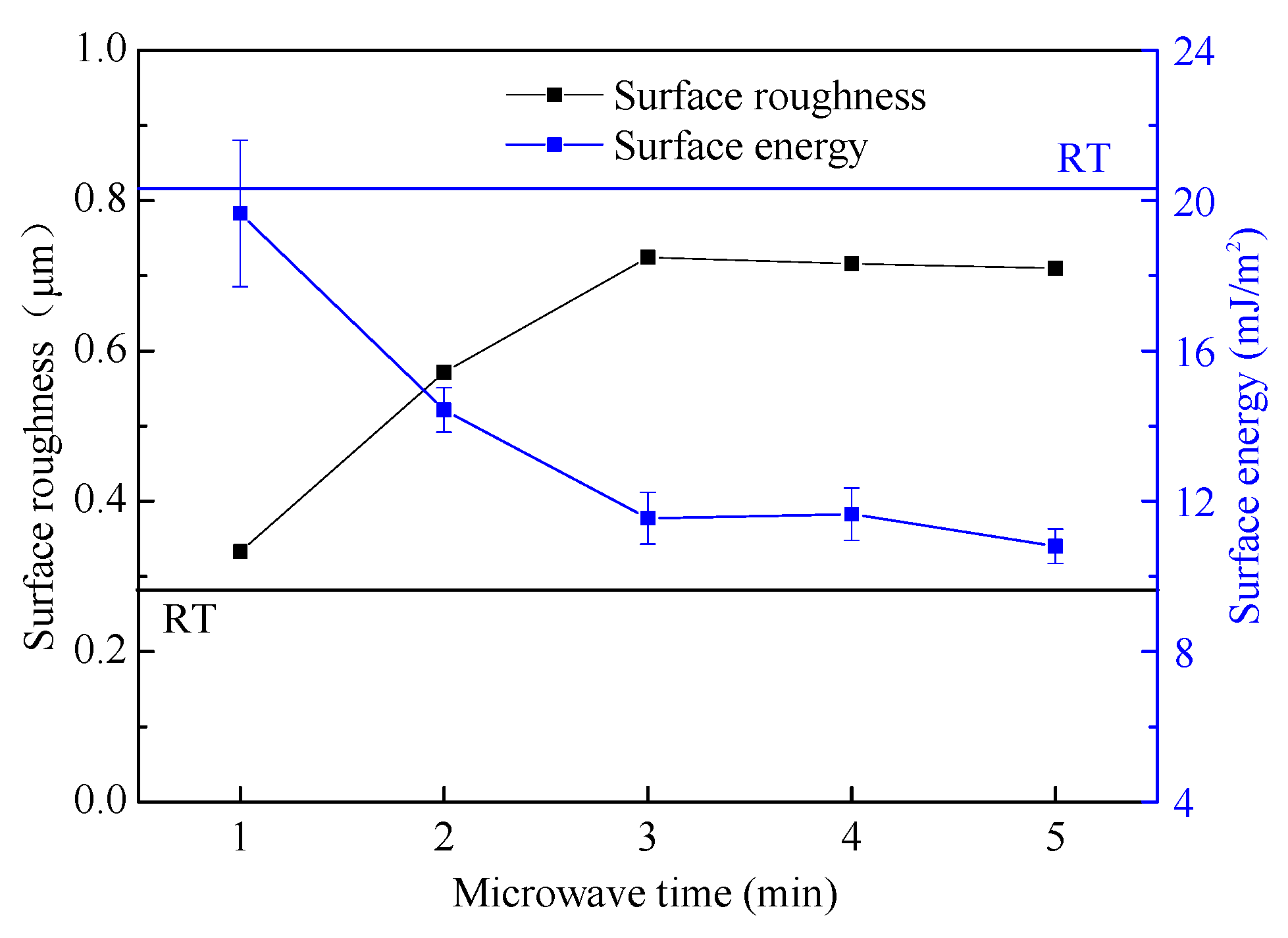
3.3. Surface Morphology and Roughness
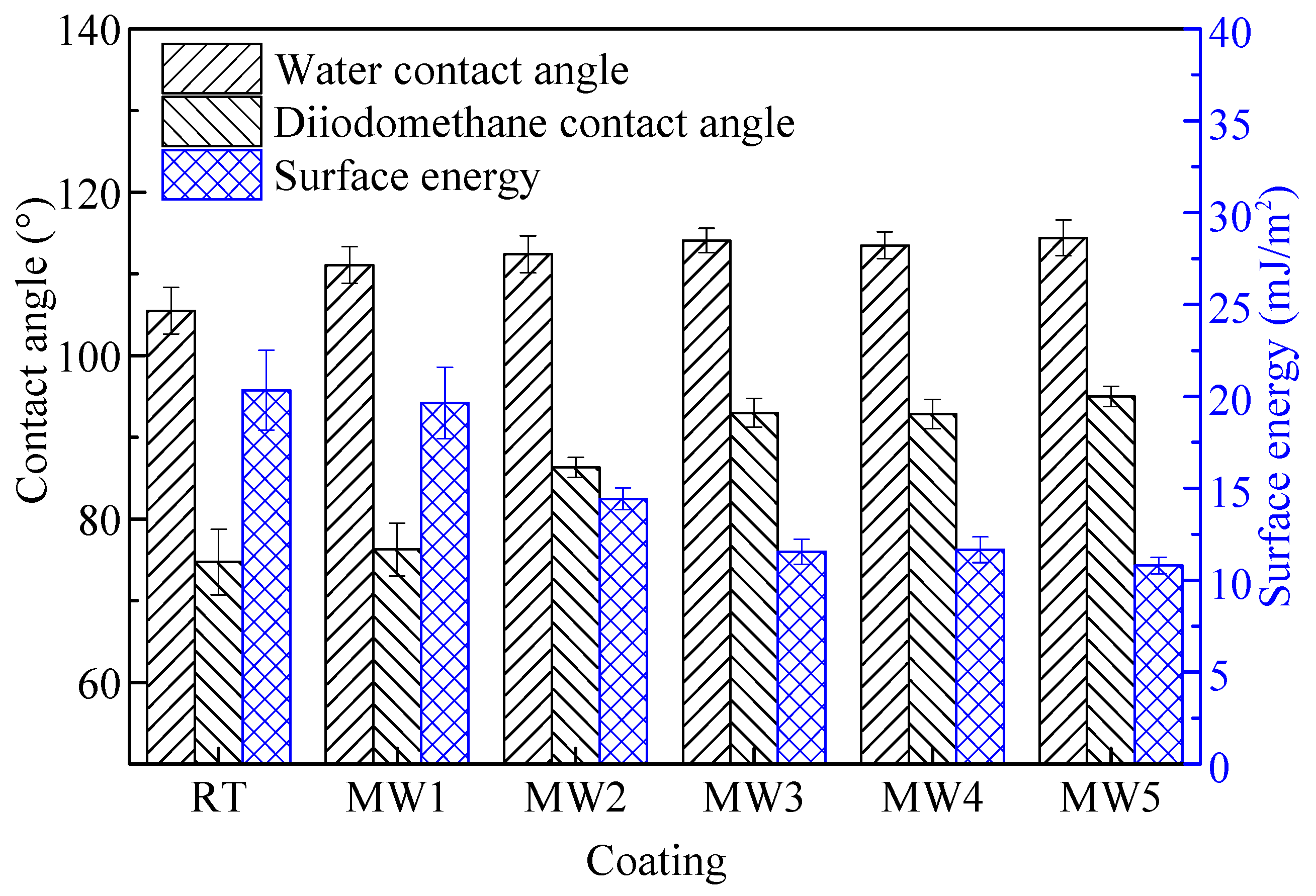
3.4. Contact Angle and Surface Energy
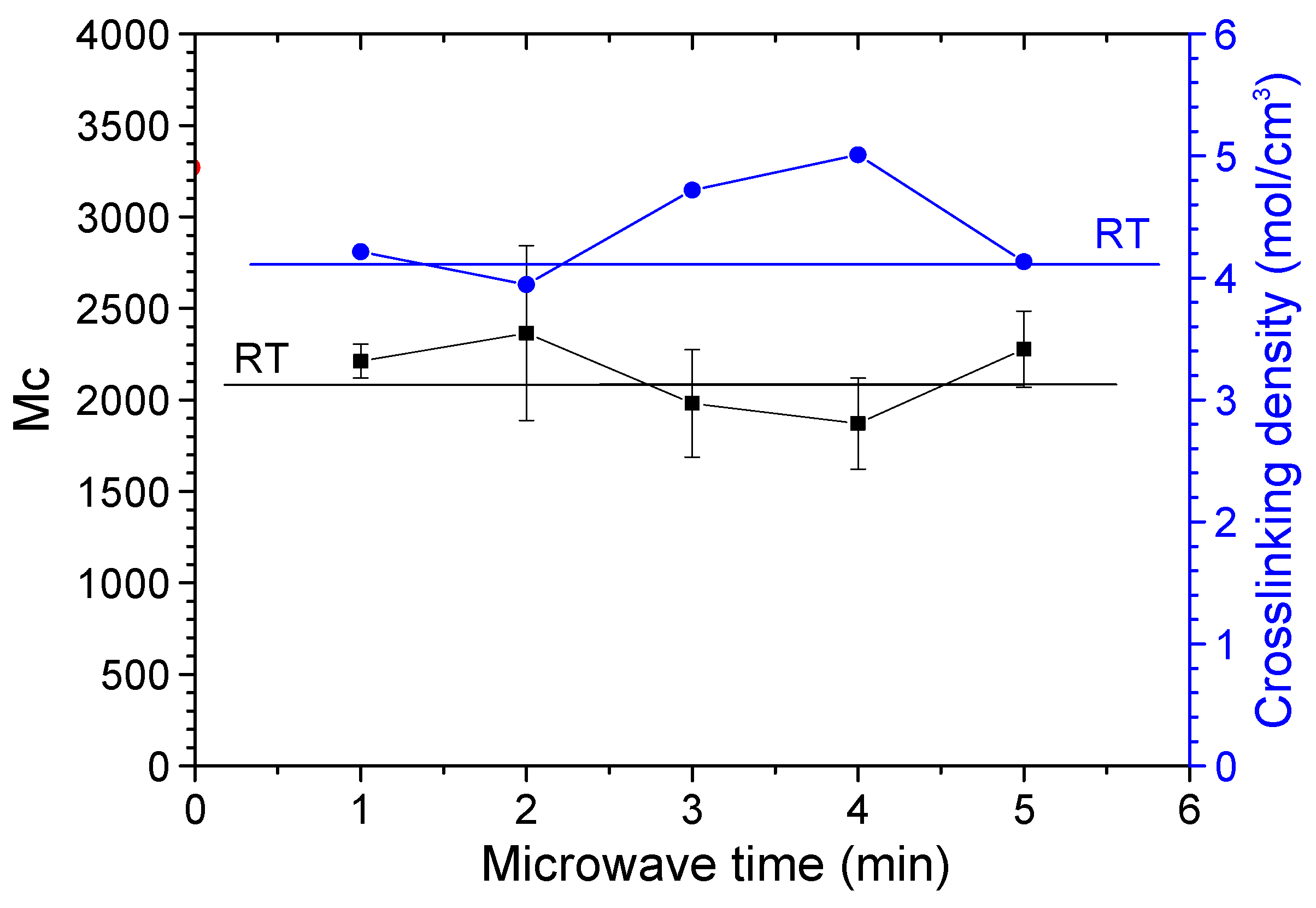
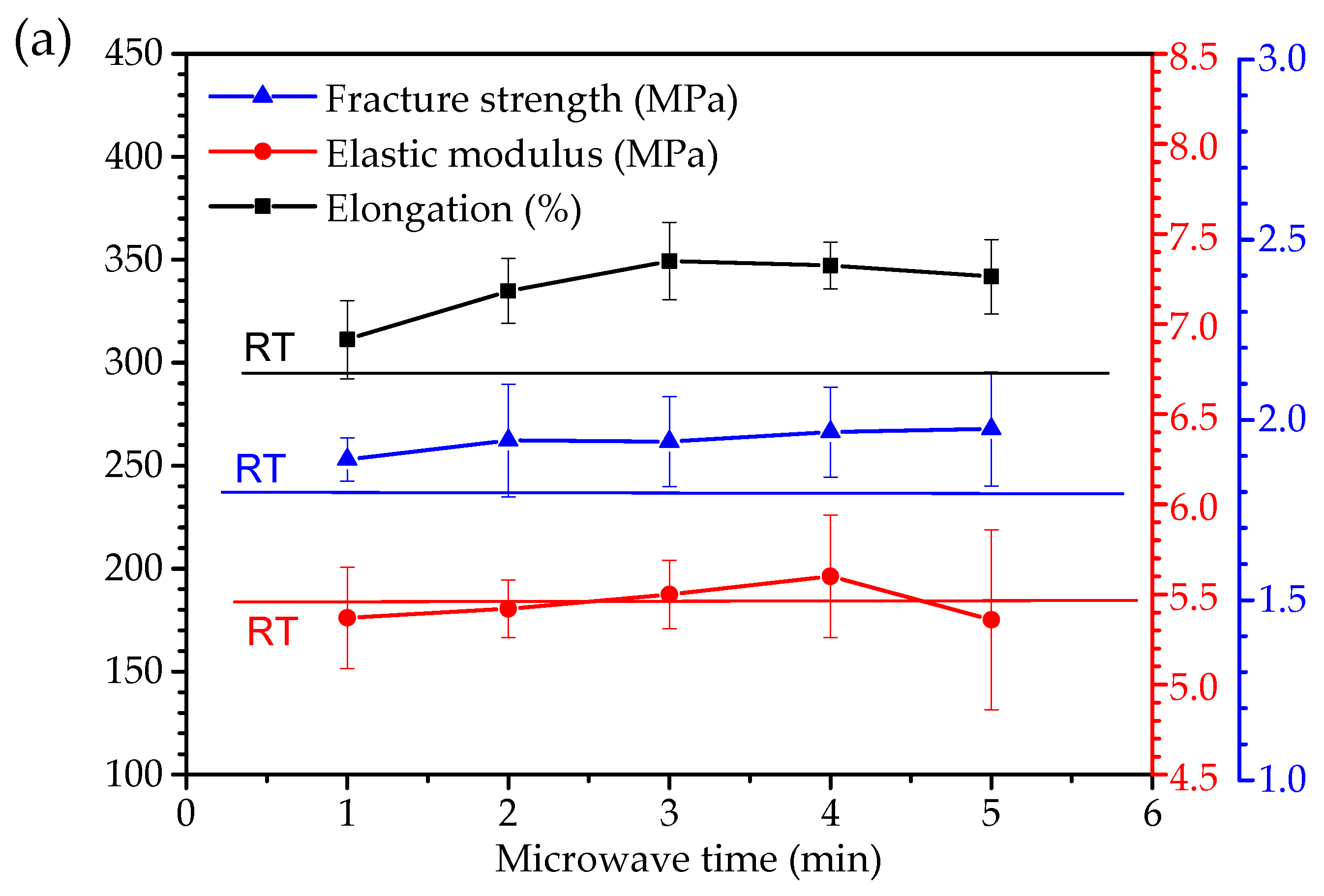
3.5. Tensile Properties
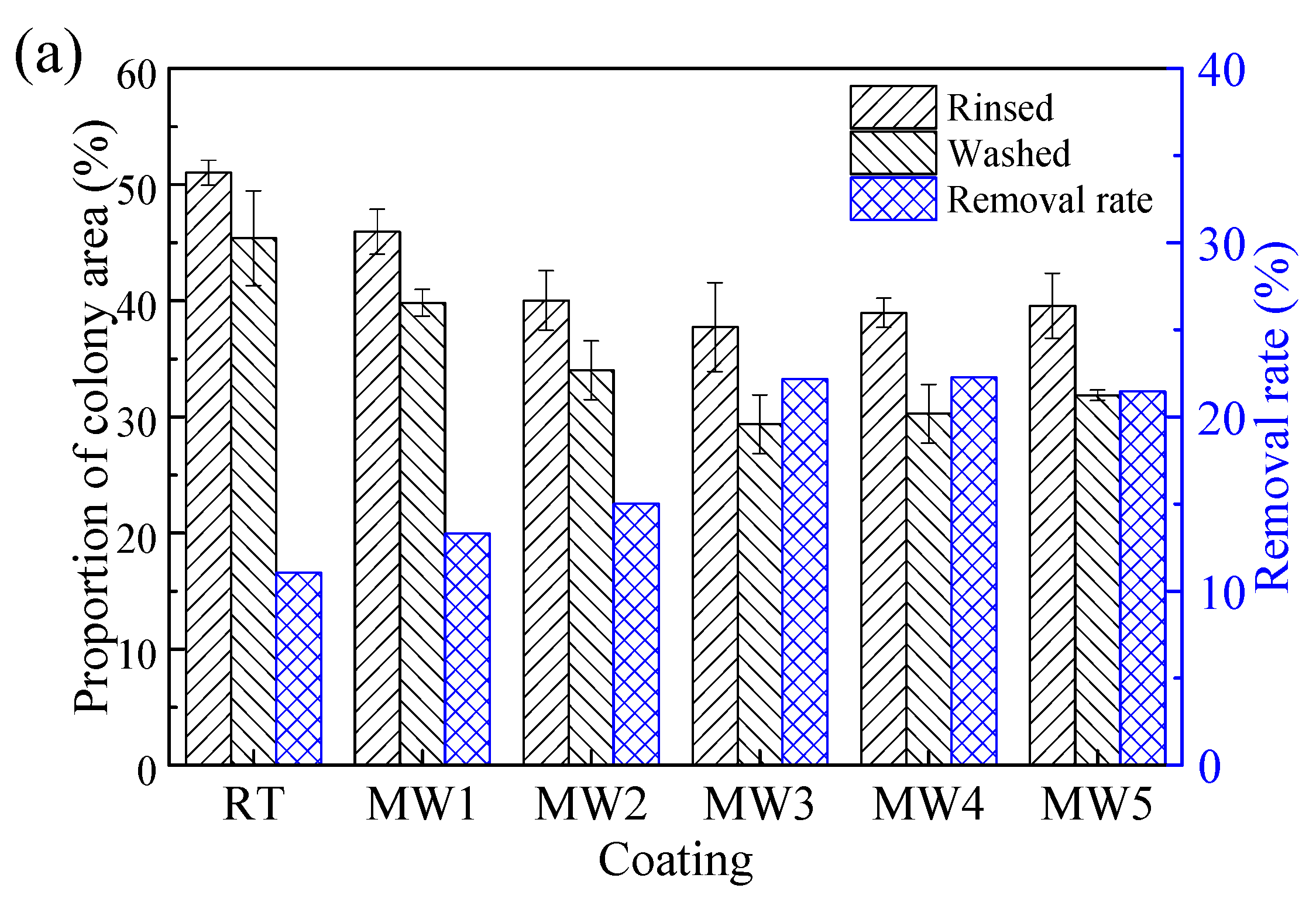
3.6. Antifouling Performance
4. Discussion
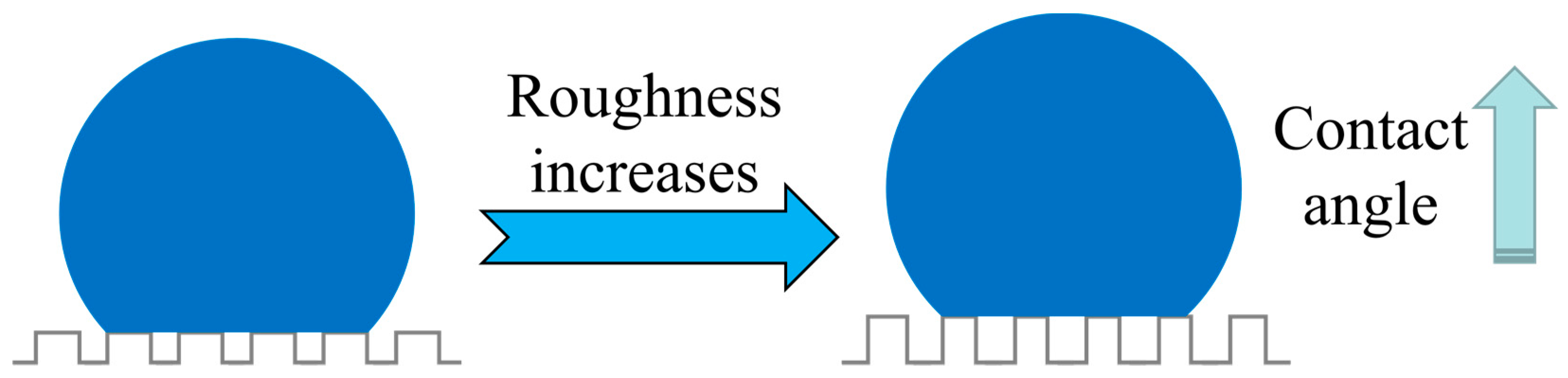
4.1. Relationship between Surface Energy and Roughness
4.2. Effect of Relative Adhesion Factor (RAF)
4.3. Relationship between Surface Roughness and Fouling Removal Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajitha, K.; Nancharaiah, Y.V.; Venugopalan, V.P. Insight into Bacterial Biofilm-Barnacle Larvae Interactions for Environmentally Benign Antifouling Strategies. Int. Biodeterior. Biodegrad. 2020, 149, 104937. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling Strategies: History and Regulation, Ecological Impacts and Mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Alshemmari, H.; Al-Awadi, M.; Karam, Q.; Talebi, L. Sedimentary Butyltin Compounds and Sediment Transport Model at the Shuwaikh Port, Kuwait Bay. Arab. J. Geosci. 2020, 13, 677. [Google Scholar] [CrossRef]
- Wrange, A.L.; Barboza, F.R.; Ferreira, J.; Eriksson-Wiklund, A.K.; Ytreberg, E.; Jonsson, P.R.; Watermann, B.; Dahlström, M. Monitoring Biofouling as a Management Tool for Reducing Toxic Antifouling Practices in the Baltic Sea. J. Environ. Manag. 2020, 264, 110447. [Google Scholar] [CrossRef]
- Pradhan, S.; Kumar, S.; Mohanty, S.; Nayak, S.K. Environmentally Benign Fouling-Resistant Marine Coatings: A Review. Polym.-Plast. Technol. Eng. 2019, 58, 498518. [Google Scholar] [CrossRef]
- Maan, A.M.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Curr. Opin. Chem. Eng. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Song, M.; Lee, D.J.; Park, S.S. Silicone Rubber Composites Reinforced by Carbon Nanofillers and Their Hybrids for Various Applications: A Review. Polymers 2021, 13, 2322. [Google Scholar] [CrossRef]
- Selim, M.S.; Azzam, A.M.; Higazy, S.A.; El-Safty, S.A.; Shenashen, M.A. Novel graphene-based ternary nanocomposite coatings as ecofriendly antifouling brush surfaces. Prog. Org. Coat. 2022, 167, 106863. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M. Silicone/graphene oxide sheet-alumina nanorod ternary composite for superhydrophobic antifouling coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Xue, J.; Wang, L.; Fan, Y.; Xu, J.; Zhao, J.; Tian, L.; Du, W. Mechanically Enhanced Self-Stratified Acrylic/Silicone Antifouling Coatings. Coatings 2022, 12, 232. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors for VOCs Adsorption Process: A Review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Wang, X.Y.; Rong, X.J.; Tang, N. Development and Application of Waterborne Silicone Resin Elastic Exterior Wall Coating. Shandong Ind. Technol. 2014, 12, 163–164. [Google Scholar]
- Zhu, C.; Lin, W.; Chen, L.; Lv, J.; Zhang, J.; Feng, J. Deep color, Heat-Reflective, Superhydrophobic and Anti-Soiling Coatings with Waterborne Silicone Emulsion. Sol. Energy Mater. Solar Cells 2019, 199, 129–135. [Google Scholar] [CrossRef]
- Liu, S.K.; Zhang, Z.P.; Qi, Y.H. Effect of Emulsifier on the Structure and Properties of Waterborne Silicone Antifouling Coating. Coatings 2020, 10, 168. [Google Scholar] [CrossRef]
- Qiu, Y. Discussion on the coating process and the quality improvement strategy of water-based coatings. Mod. Salt Chem. Ind. 2022, 49, 3. [Google Scholar]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave Assisted Preparation of Activated Carbon from Biomass: A Review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of Microwave Ovens for Rapid Organic Synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwave Flow Chemistry as a Methodology in Organic Syntheses, Enzymatic Reactions, and Nanoparticle Syntheses. Chem. Rec. 2019, 19, 118–139. [Google Scholar] [CrossRef]
- Hiromichi, E.; Yoshitaka, H. Practical and Scalable Organic Reactions with Flow Microwave Apparatus. Chem. Rec. 2019, 19, 157–171. [Google Scholar]
- De Conto, J.F.; Oliveira, M.R.; Oliveira, M.M.; Brandão, T.G.; Campos, K.V.; Santana, C.C.; Egues, S.M. One-Pot Synthesis and Modification of Silica Nanoparticles with 3-Chloropropyl-Trimethoxysilane Assisted by Microwave Irradiation. Chem. Eng. Commun. 2018, 205, 533–537. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wang, M.; Zhang, Y.; Bai, L.; Zhang, Y. Fine Control of Crystal Morphologies of All-Silica DDR in Ethylenediamine-Free Gel with Inorganic Base as Mineralizing Agent. Microporous Mesoporous Mater. 2019, 288, 109596. [Google Scholar] [CrossRef]
- Castagnet, T.; Ballard, N.; Billon, L.; Asua, J.M. Microwave-Assisted Ultrafast RAFT Miniemulsion Polymerization of Biobased Terpenoid Acrylates. Biomacromolecules 2020, 21, 4559–4568. [Google Scholar] [CrossRef]
- Xie, Z.K.; Guo, J.K.; Luo, Z.H. Assessment of Microwave Effect on Polymerization Conducted under ARGET ATRP Conditions. Macromol. React. Eng. 2018, 12, 1700032. [Google Scholar] [CrossRef]
- Ma, R.; Chang, X.; Zhang, X.; Fang, P.; Long, B.; Liu, W. Effect of Curing Method on Mechanical and Morphological Properties of Carbon Fiber Epoxy Composites for Solid Rocket Motor. Polym. Compos. 2015, 36, 1703–1711. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M.; Toselli, M.; Veronesi, P.; Rocha, J.; Pilati, F. Enhancing the Scratch Resistance of Polycarbonate with Poly (Ethylene Oxide)-Silica Hybrid Coatings. Adv. Polym. Technol. 2010, 27, 117–126. [Google Scholar] [CrossRef]
- Lee, H.K.; Fang, C.Y.; Pantano, C.G.; Kang, W.H. Microwave Effect on Curing of Waterborne Polyurethane. Bull.-Korean Chem. Soc. 2011, 32, 961–963. [Google Scholar] [CrossRef][Green Version]
- Luo, W.-L.; Xu, L.-W.; Xu, L.-L. Microwave Repair of Metal Structure with Damages for Composite Materials. J. Nanjing Univ. Aeronaut. Astronaut. 2005, 37, 736–740. [Google Scholar] [CrossRef]
- Ma, S.-N.; Sun, X.-F.; Zhu, N.-S. Research and Development of Microwave Repair Technology. China Surf. Eng. 2010, 23, 100–105. [Google Scholar] [CrossRef]
- Oliver, J.F.; Mason, S.G. Liquid Spreading on Rough Metal Surfaces. J. Mater. Sci. 1980, 15, 431–437. [Google Scholar] [CrossRef]
- ISO 37:2005-Rubber; Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2005.
- Zhao, R.K.; Zhang, Z.P.; Qi, Y.H. Influence of Epoxy Content on the Properties and Marine Bacterial Adhesion of Epoxy Modified Silicone Coatings. Coatings 2020, 10, 126. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.P.; Qi, Y.H. Experimental Method for Evaluation of Benthic Diatom Adhesion on the Surface of Nontoxic Antifouling Coatings. Mar. Environ. Sci. 2006, 25, 89–92. [Google Scholar]
- Lin, Y.C.; Shangdiar, S.; Chen, S.C.; Chou, F.C.; Lin, Y.C.; Cho, C.A. Microwave Irradiation with Dilute Acid Hydrolysis Applied to Enhance the Saccharification Rate of Water Hyacinth (Eichhornia crassipes). Renew. Energy 2018, 125, 511–517. [Google Scholar] [CrossRef]
- Fan, J.; Santomauro, F.; Budarin, V.L.; Whiffin, F.; Abeln, F.; Chantasuban, T.; Gore-Lloyd, D.; Henk, D.; Scott, R.J.; Clark, J.; et al. The Additive Free Microwave Hydrolysis of Lignocellulosic Biomass for Fermentation to High Value Products. J. Clean. Prod. 2018, 198, 776–784. [Google Scholar] [CrossRef]
- Chen, J.; Ding, S.; Ji, Y.; Ding, J.; Yang, X.; Zou, M.; Li, Z. Microwave-Enhanced Hydrolysis of Poultry Feather to Produce Amino Acid. Chem. Eng. Process. Process Intensif. 2015, 87, 104–109. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.P.; Qi, Y.H. Research Progress of Silicone Antifouling Paints. Surf. Technol. 2017, 46, 8. [Google Scholar]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired Marine Antifouling Coatings: Status, Prospects, and Future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent Progress in Marine Foul-Release Polymeric Nanocomposite Coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Brady, R.F., Jr.; Singer, I.L. Mechanical Factors Favoring Release from Fouling Release Coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Z.; Qi, Y. Effect of Microwave-Assisted Curing on Properties of Waterborne Silicone Antifouling Coatings. Polymers 2022, 14, 4493. https://doi.org/10.3390/polym14214493
Li M, Zhang Z, Qi Y. Effect of Microwave-Assisted Curing on Properties of Waterborne Silicone Antifouling Coatings. Polymers. 2022; 14(21):4493. https://doi.org/10.3390/polym14214493
Chicago/Turabian StyleLi, Meng, Zhanping Zhang, and Yuhong Qi. 2022. "Effect of Microwave-Assisted Curing on Properties of Waterborne Silicone Antifouling Coatings" Polymers 14, no. 21: 4493. https://doi.org/10.3390/polym14214493
APA StyleLi, M., Zhang, Z., & Qi, Y. (2022). Effect of Microwave-Assisted Curing on Properties of Waterborne Silicone Antifouling Coatings. Polymers, 14(21), 4493. https://doi.org/10.3390/polym14214493





