Uniform Lithium Deposition Induced by ZnFx(OH)y for High-Performance Sulfurized Polyacrylonitrile-Based Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials Synthesis
2.1.1. Materials
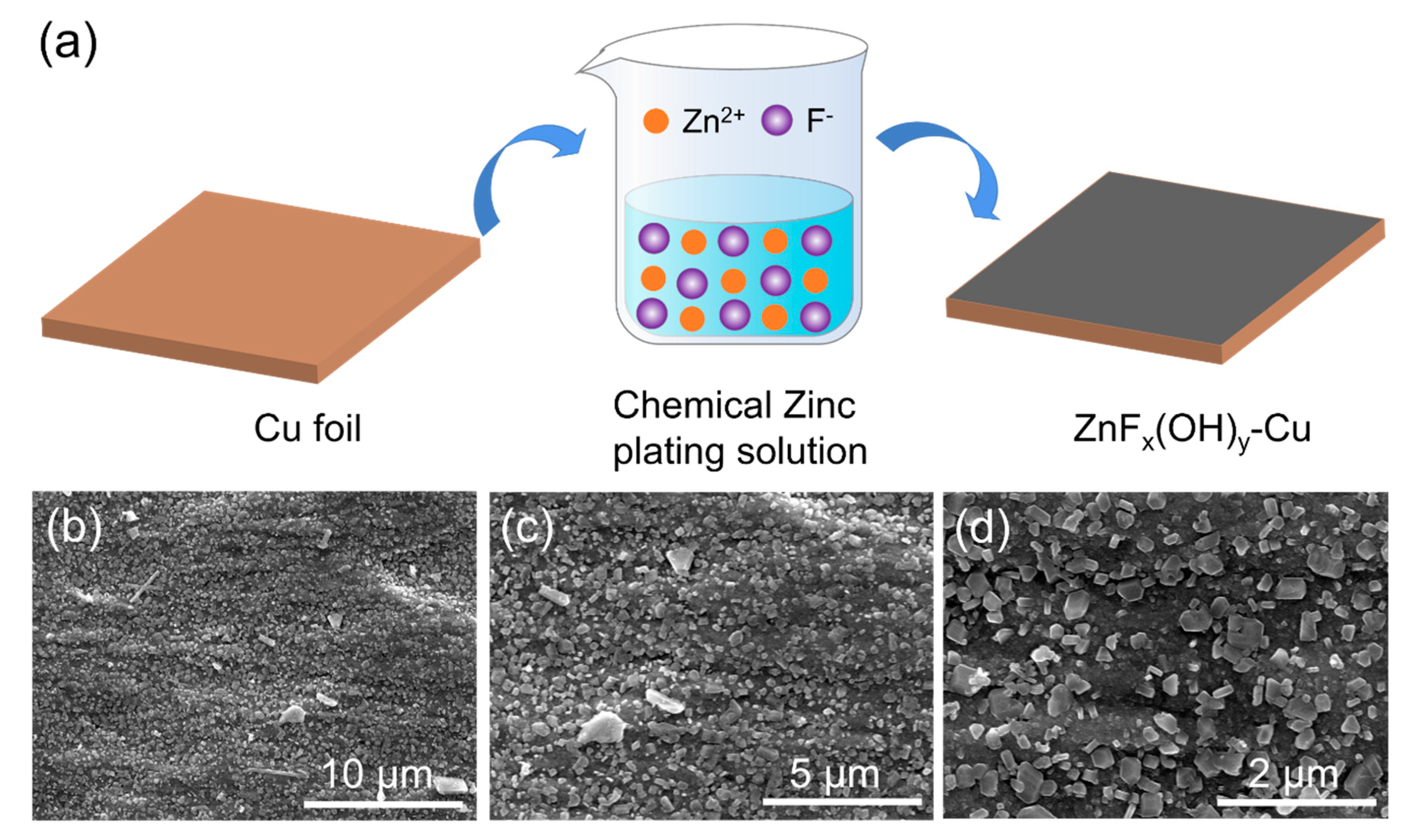
2.1.2. Preparation of ZnFx(OH)y@Cu
2.1.3. Preparation of pPAN/SeS2
2.2. Characterization
2.3. Electrochemical Measurements
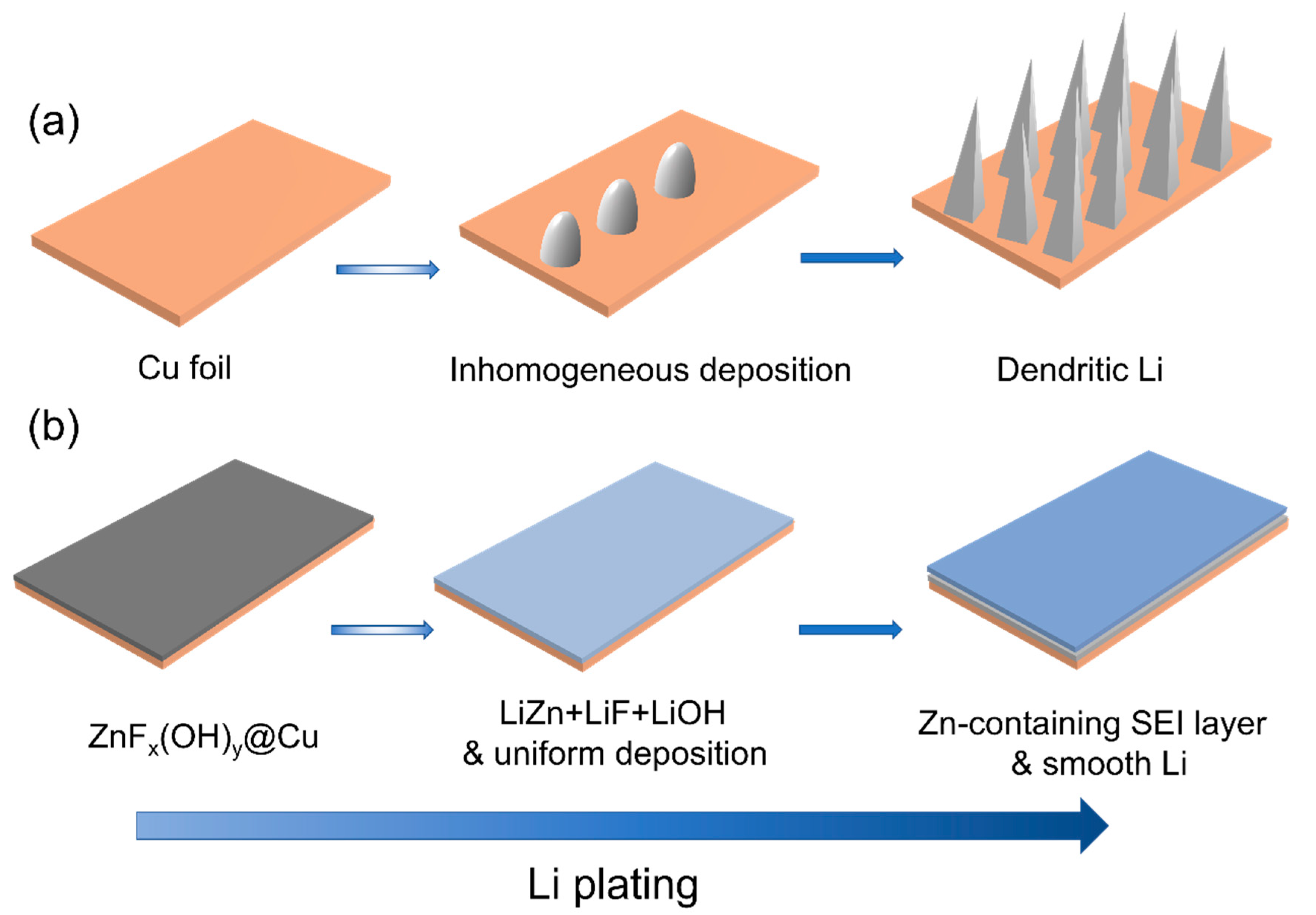
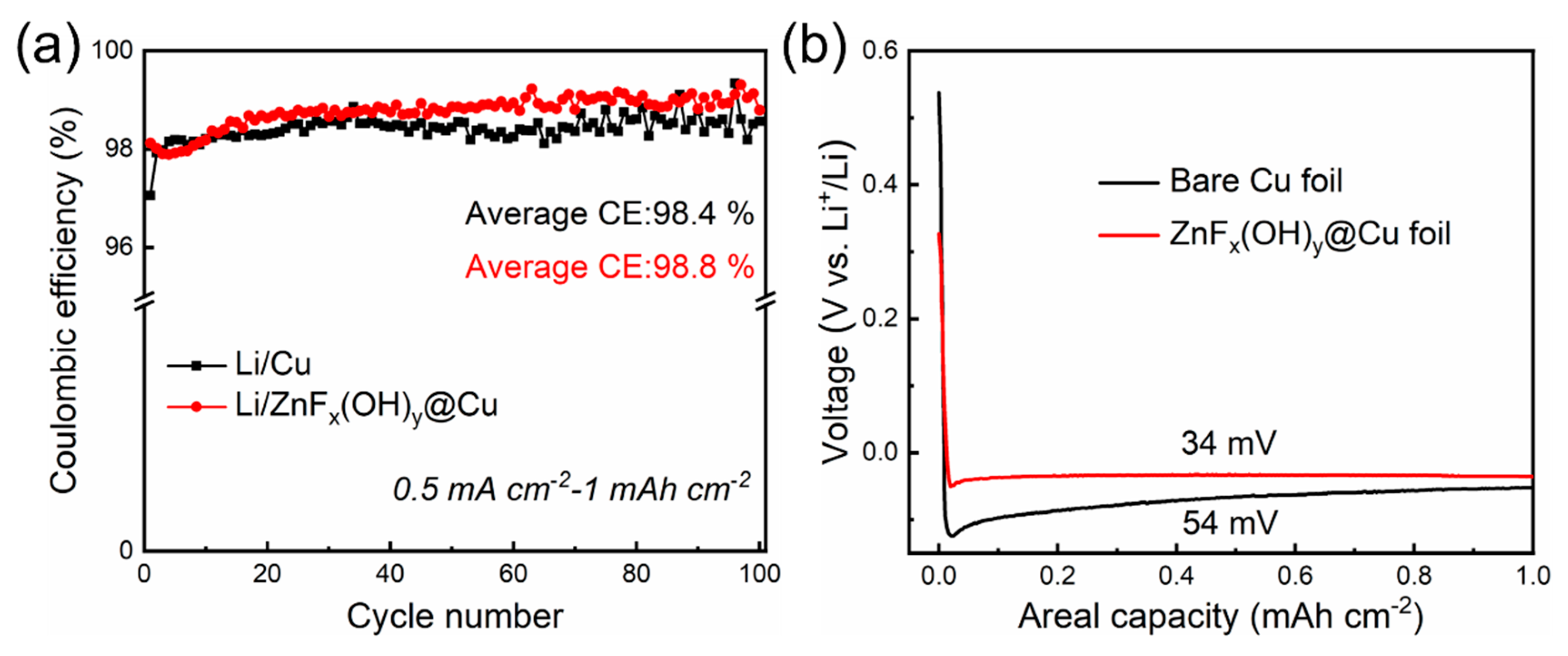
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2011, 171–179. [Google Scholar]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Lin, C.; Liang, Q.; Zhou, G.; Liu, J.; Liang, G.; Wang, M.; Li, B.; Hu, L.; Ciucci, F. Sodium-rich NASICON-structured cathodes for boosting the energy density and lifespan of sodium-free-anode sodium metal batteries. InfoMat 2022, 4, e12288. [Google Scholar] [CrossRef]
- Ahniyaz, A.; de Meatza, I.; Kvasha, A.; Garcia-Calvo, O.; Ahmed, I.; Sgroi, M.F.; Giuliano, M.; Dotoli, M.; Dumitrescu, M.A.; Jahn, M. Progress in solid-state high voltage lithium-ion battery electrolytes. Adv. Appl. Energy 2021, 4, 100070. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Chen, X.; Cheng, X.B.; Li, X.; Zhang, Q. The failure of solid electrolyte interphase on Li metal anode: Structural uniformity or mechanical strength? Adv. Energy Mater. 2020, 10, 1903645. [Google Scholar] [CrossRef]
- Liang, X.; Pang, Q.; Kochetkov, I.R.; Sempere, M.S.; Huang, H.; Sun, X.; Nazar, L.F. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2017, 2, 17119. [Google Scholar] [CrossRef]
- Dornbusch, D.A.; Hilton, R.; Lohman, S.D.; Suppes, G.J. Experimental validation of the elimination of dendrite short-circuit failure in secondary lithium-metal convection cell batteries. J. Electrochem. Soc. 2014, 162, A262. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building safe lithium-ion batteries for electric vehicles: A review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Kong, B.; Liu, W.; Sun, Y.; Song, M.S.; Chen, J.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Stretchable lithium metal anode with improved mechanical and electrochemical cycling stability. Joule 2018, 2, 1857–1865. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Y.; Cui, Y. Lithium metal anode materials design: Interphase and host. Electrochem. Energy Rev. 2019, 2, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Zhang, J.; Chen, X.; Zhang, X.D.; Li, J.Y.; Huang, L.B.; Zhang, X.; Shi, J.L.; Yin, Y.X.; Zhang, Q. Uniform nucleation of lithium in 3D current collectors via bromide intermediates for stable cycling lithium metal batteries. J. Am. Chem. Soc. 2018, 140, 18051–18057. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hu, R.; Zhang, D.; Liu, J.; Liu, F.; Cui, J.; Lin, Z.; Wu, J.; Zhu, M. Constructing Li-rich artificial SEI layer in alloy–polymer composite electrolyte to achieve high ionic conductivity for all-solid-state lithium metal batteries. Adv. Mater. 2021, 33, 2004711. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryu, J.H.; Lim, Y.R.; Nah, I.W.; Lee, K.R.; Archer, L.A.; Cho, W. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 2018, 3, 889–898. [Google Scholar] [CrossRef]
- Zhang, J.G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium metal anodes with nonaqueous electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef]
- Teng, W.; Wu, J.; Liang, Q.; Deng, J.; Yu, X.; Liu, Q.; Wang, B.; Ma, T.; Nan, D.; Liu, J. Designing advanced liquid electrolytes for alkali metal batteries: Principles, progress, and perspectives. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; An, Y.; Tao, Y.; Feng, J.; Qian, Y. Uniform Li deposition by regulating the initial nucleation barrier via a simple liquid-metal coating for a dendrite-free Li–metal anode. J. Mater. Chem. A 2019, 7, 18861–18870. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chae, O.B.; Wu, M.; Lim, E.; Kim, G.; Hong, Y.J.; Jung, W.B.; Choi, S.; Gereige, I.; Suk, J. Extraordinary dendrite-free Li deposition on highly uniform facet wrinkled Cu substrates in carbonate electrolytes. Nano Energy 2021, 82, 105736. [Google Scholar] [CrossRef]
- Jung, Y.; Park, S.; Kim, J.K.; Kim, M.; Kang, B. Toward achieving high kinetics in anodeless Li2S battery: Surface modification of Cu current collector. Adv. Funct. Mater. 2022, 32, 2109759. [Google Scholar] [CrossRef]
- He, D.; Liao, Y.; Cheng, Z.; Sang, X.; Yuan, L.; Li, Z.; Huang, Y. Facile one-step vulcanization of copper foil towards stable Li metal anode. Sci. China Mater. 2020, 63, 1663–1671. [Google Scholar] [CrossRef]
- Yuan, H.; Nai, J.; Tian, H.; Ju, Z.; Zhang, W.; Liu, Y.; Tao, X.; Lou, X.W. An ultrastable lithium metal anode enabled by designed metal fluoride spansules. Sci. Adv. 2020, 6, eaaz3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Lv, Q.; Bao, C.; Wang, B.; Ren, P.; Zhong, H.; Yang, Y.; Liu, X.; Dong, Y.; Jin, F. Seamless alloying stabilizes solid-electrolyte interphase for highly reversible lithium metal anode. Cell Rep. Phys. Sci. 2022, 3, 100785. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, J.; Chen, X.; Yuan, L.; Li, Z.; Huang, Y. Li2S-based anode-free full batteries with modified Cu current collector. Energy Storage Mater. 2020, 30, 179–186. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Zou, P.; Fu, X.; Lin, Z.; Li, Y.; Liu, Y.; Yang, C.; Liu, M. Lithiated zinc oxide nanorod arrays on copper current collectors for robust Li metal anodes. Chem. Eng. J. 2019, 378, 122243. [Google Scholar] [CrossRef]
- Chen, K.H.; Sanchez, A.J.; Kazyak, E.; Davis, A.L.; Dasgupta, N.P. Synergistic effect of 3D current collectors and ALD surface modification for high coulombic efficiency lithium metal anodes. Adv. Energy Mater. 2019, 9, 1802534. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Xu, R.; Cheng, X.B.; Peng, H.J.; Zhang, R.; Huang, J.Q.; Zhang, Q. Columnar lithium metal anodes. Angew. Chem. Int. Ed. 2017, 129, 14395–14399. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Y.; Qu, S.; Yao, Z.; Liu, Y.; Li, C.; Wang, Z.; Li, J. ZnF2 coated three dimensional Li-Ni composite anode for improved performance. J. Mater. 2019, 5, 176–184. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.W.; Xiong, F.; Hsu, P.C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. A lithium–sulfur cell based on reversible lithium deposition from a Li2S cathode host onto a hostless-anode substrate. Adv. Energy Mater. 2018, 8, 1801556. [Google Scholar] [CrossRef]
- Liu, Y.T.; Liu, S.; Li, G.R.; Gao, X.P. Strategy of enhancing the volumetric energy density for lithium–sulfur batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef]
- Ma, T.; Ren, X.; Hu, L.; Teng, W.; Yu, X. A diluted electrolyte for long-life sulfurized polyacrylonitrile-based anode-free Li-S batteries. Polymers 2022, 14, 3312. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Wang, L.; Yang, J.; Hao, Z.; Xiang, J.; Yuan, K.; Huang, Y.; Shan, B.; Yuan, L. Ether-compatible sulfurized polyacrylonitrile cathode with excellent performance enabled by fast kinetics via selenium doping. Nat. Commun. 2019, 10, 1021. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.; Li, Y.; Ma, T.; Ren, X.; Nan, D.; Liu, J.; Wang, X.; Yang, Q.; Deng, J. Uniform Lithium Deposition Induced by ZnFx(OH)y for High-Performance Sulfurized Polyacrylonitrile-Based Lithium-Sulfur Batteries. Polymers 2022, 14, 4494. https://doi.org/10.3390/polym14214494
Teng W, Li Y, Ma T, Ren X, Nan D, Liu J, Wang X, Yang Q, Deng J. Uniform Lithium Deposition Induced by ZnFx(OH)y for High-Performance Sulfurized Polyacrylonitrile-Based Lithium-Sulfur Batteries. Polymers. 2022; 14(21):4494. https://doi.org/10.3390/polym14214494
Chicago/Turabian StyleTeng, Wanming, Yanyan Li, Ting Ma, Xiuyun Ren, Ding Nan, Jun Liu, Xiaohu Wang, Qin Yang, and Jiaojiao Deng. 2022. "Uniform Lithium Deposition Induced by ZnFx(OH)y for High-Performance Sulfurized Polyacrylonitrile-Based Lithium-Sulfur Batteries" Polymers 14, no. 21: 4494. https://doi.org/10.3390/polym14214494




