A Bird’s-Eye View on Polymer-Based Hydrogen Carriers for Mobile Applications
Abstract
:1. Introduction
2. Absorption-Based H2 Storage Systems
2.1. H2 Storage in Functionalized Polymers
2.2. H2 Storage in Intrinsically Conductive Polymers (ICP)
3. Adsorption-Based Storage Systems
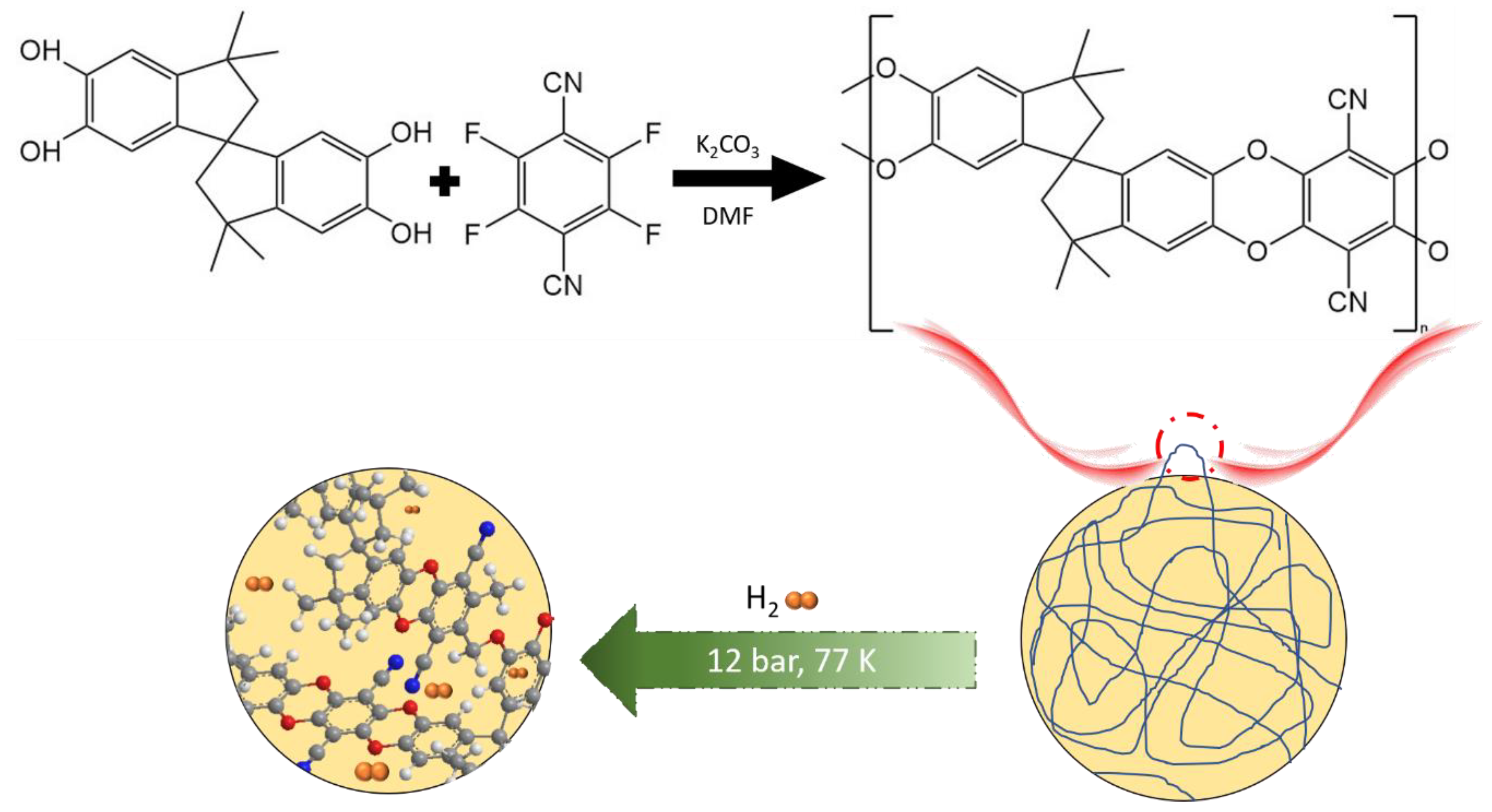
3.1. H2 Storage in Polymers of Intrinsic Microporosity (PIM)
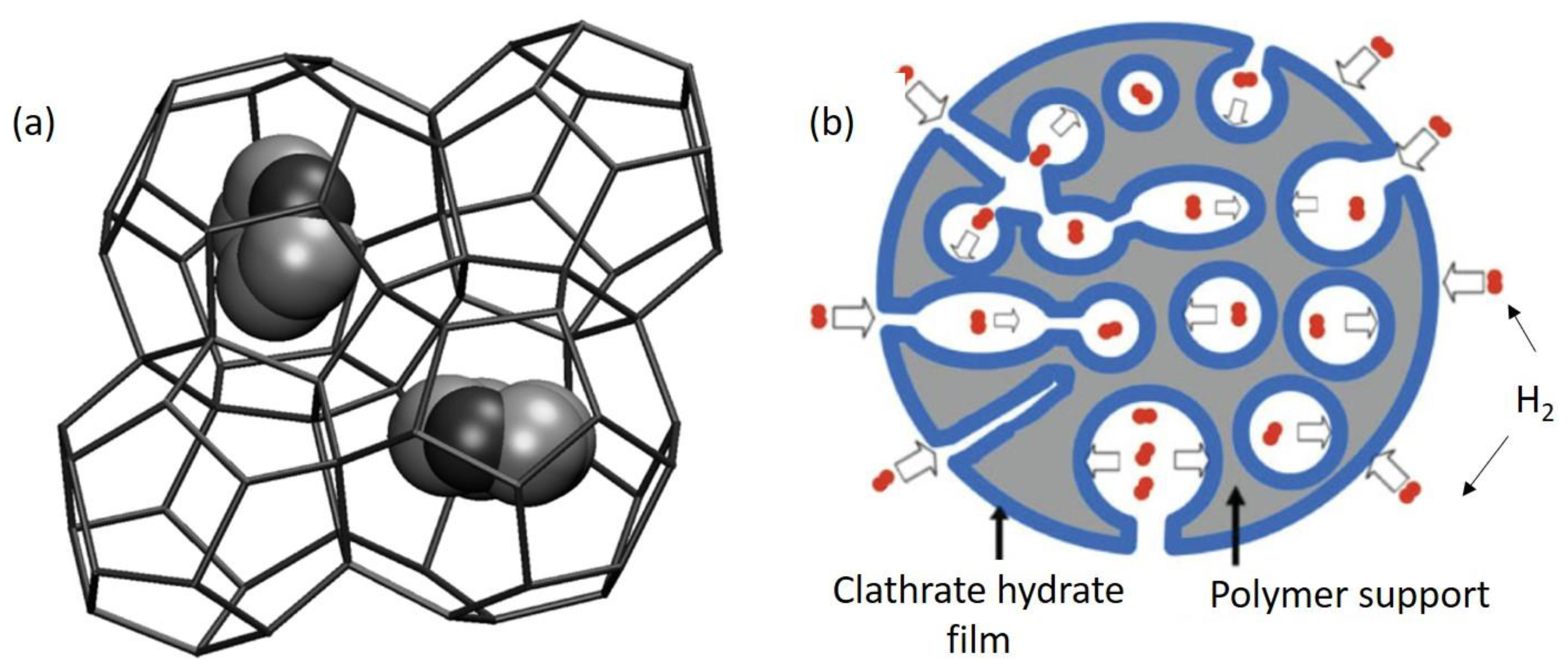
3.2. H2 Storage by Clathrates
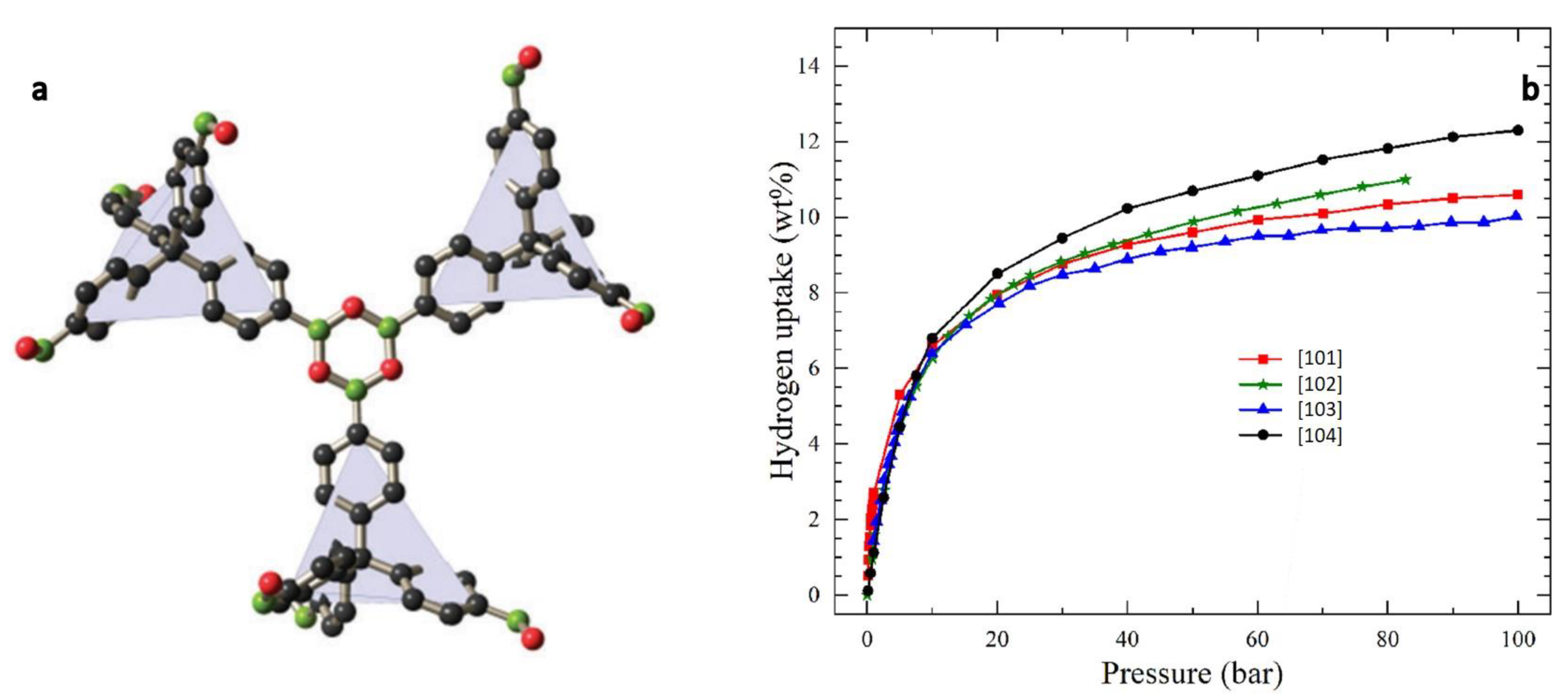
3.3. H2 Storage in Covalent Organic Frameworks (COFs)
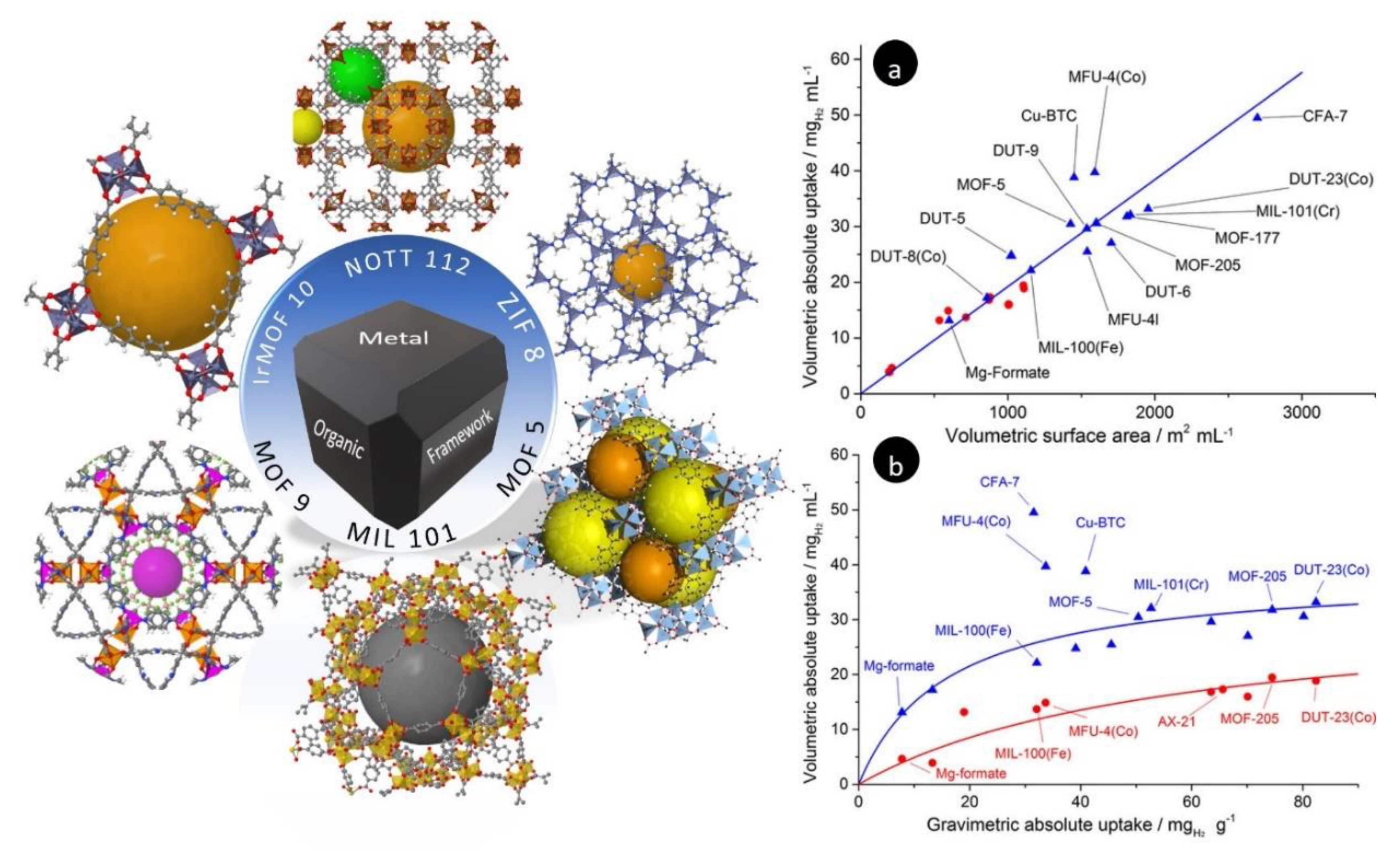
4. H2 Storage in Metal–Organic Frameworks (MOFs)
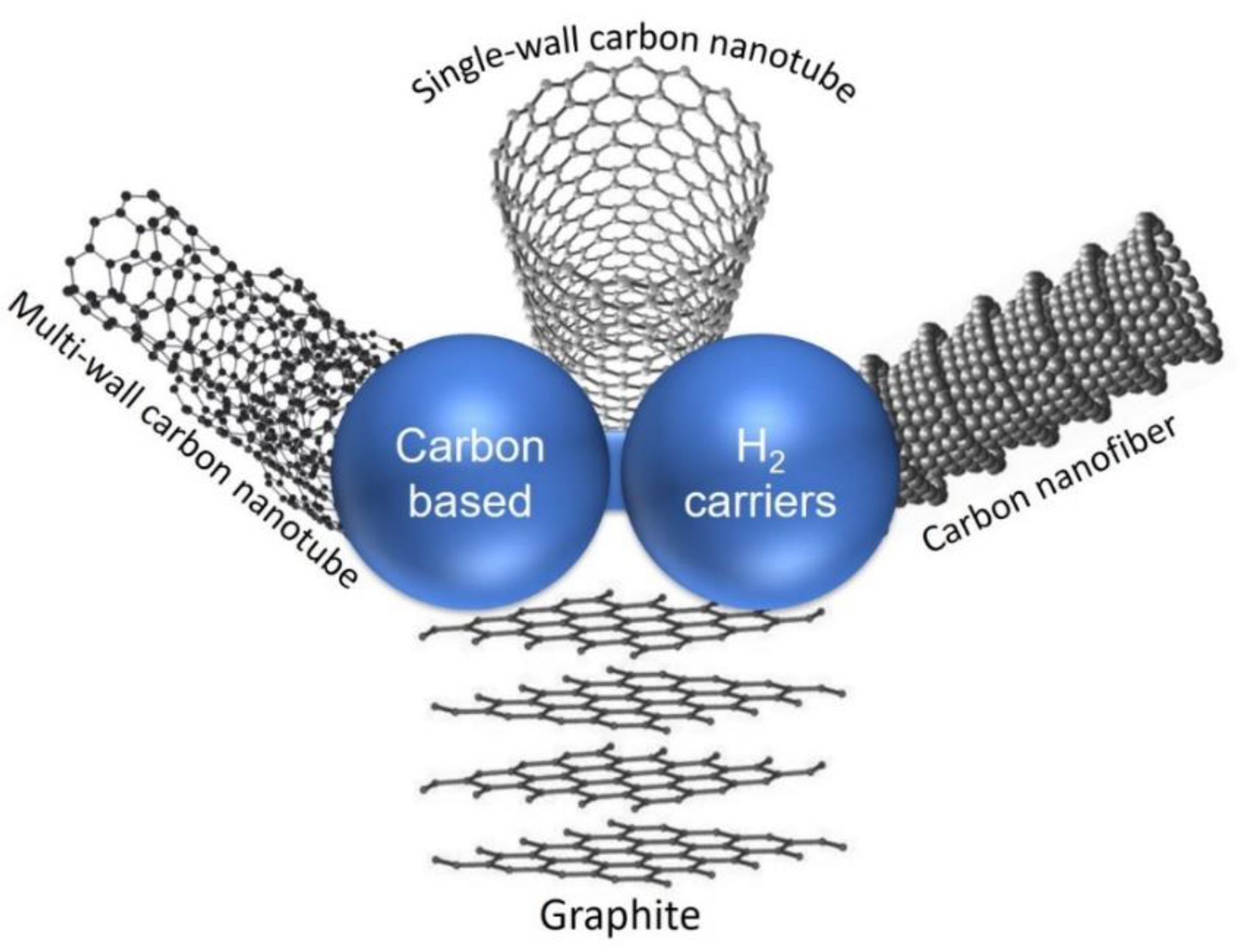
5. H2 Storage in Carbon-Based Hydrogen Carriers
5.1. Activated Carbon (AC) Hydrogen Carriers
5.2. Graphite-Based Hydrogen Carriers
5.3. Carbon Nanotubes (CNTs) as Hydrogen Carriers
5.4. Carbon Nanofibers (CNF) as Hydrogen Carriers
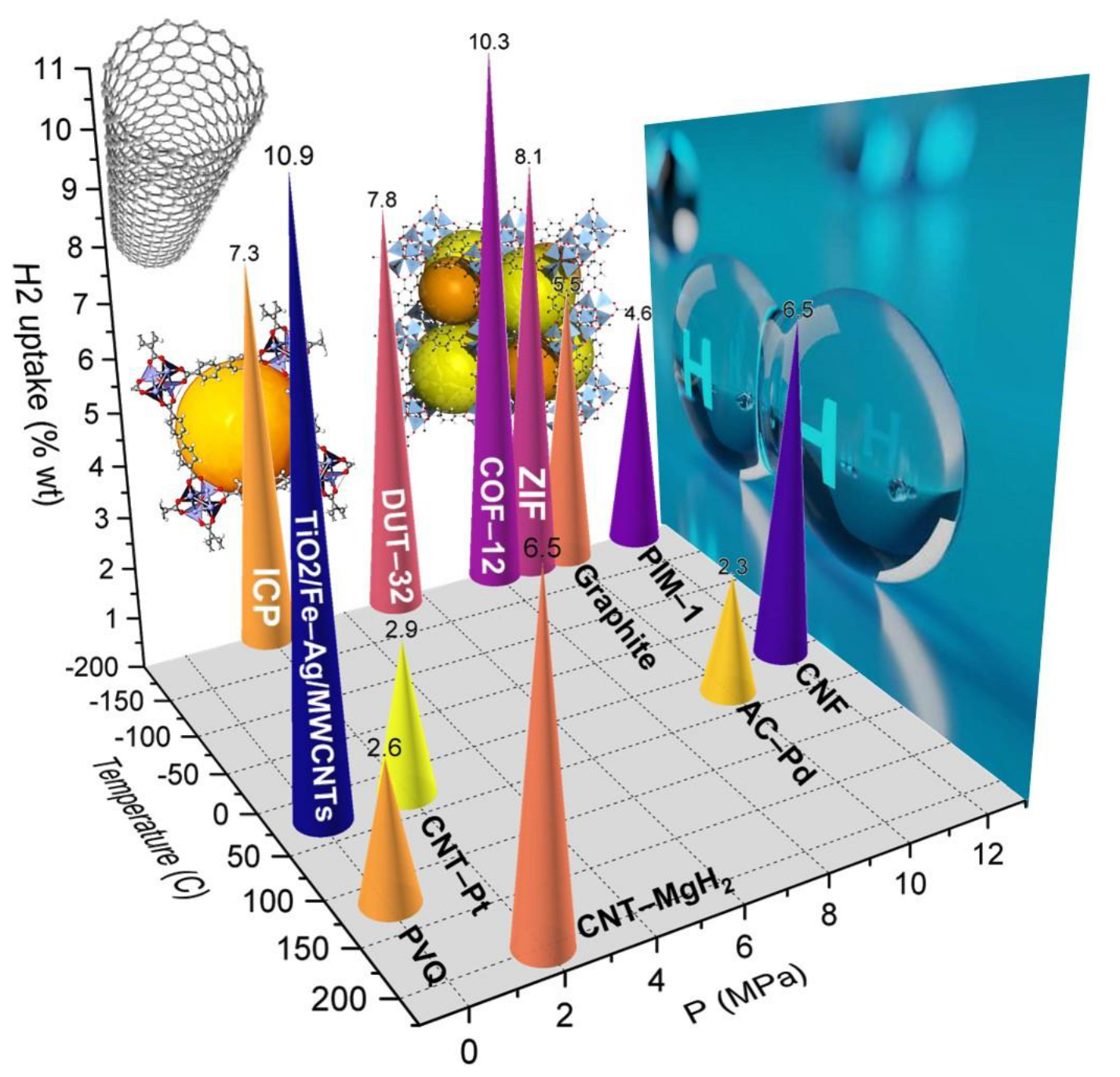
6. Summary
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Möller, D. Chemistry for Environmental Scientists. Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 11 September 2015; ISBN 9783110419337. [Google Scholar]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
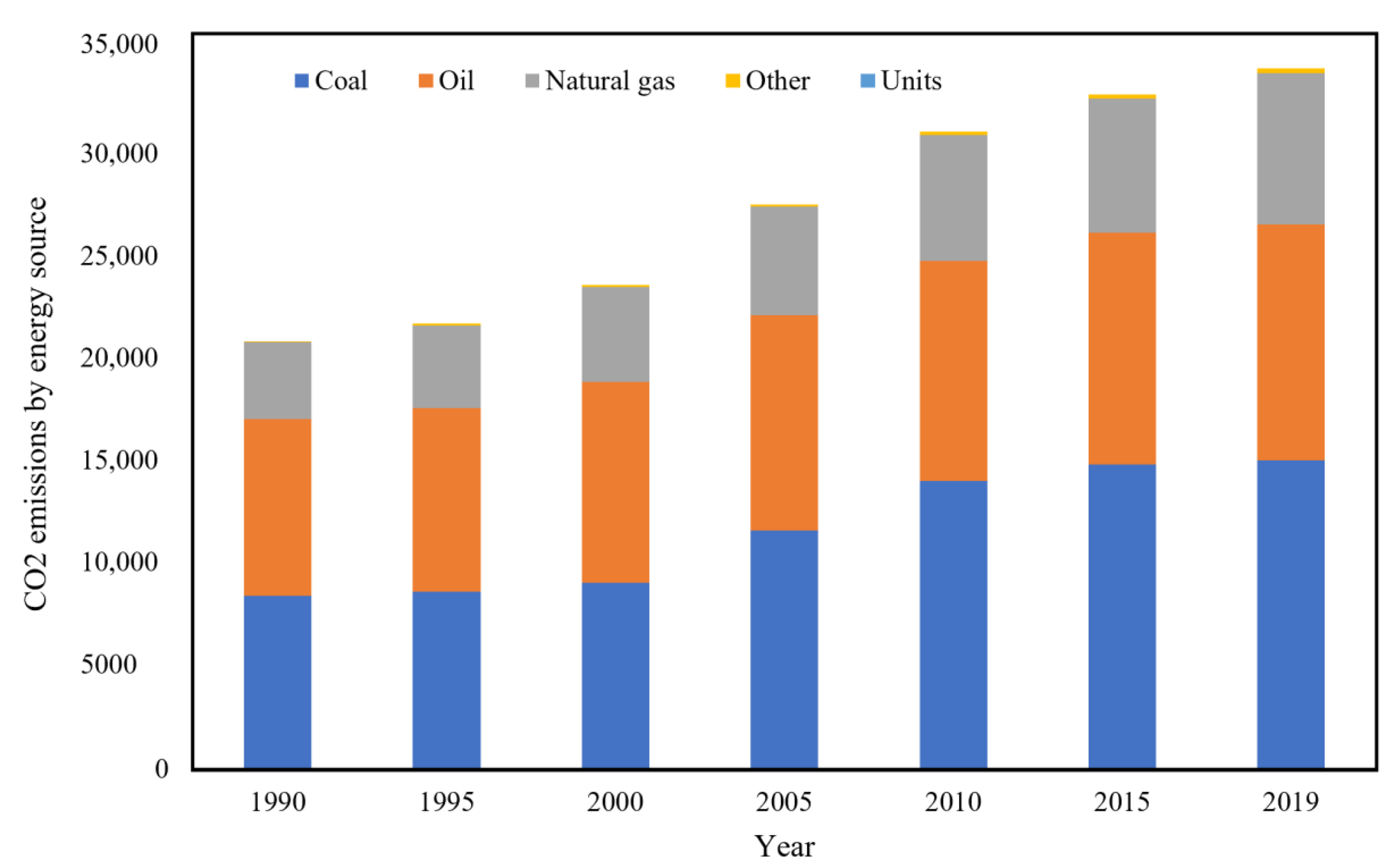
- IEA. Global Primary Energy, Electricity Generation. Final Consumption and CO2 Emissions by Fuel; IEA: Paris, France, 2018; Available online: https://www.iea.org/data-and-statistics/charts/global-primary-energy-electricity-generation-final-consumption-and-co2-emissions-by-fuel-2018 (accessed on 17 January 2020).
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen Storage Technologies for Stationary and Mobile Applications: Review, Analysis and Perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- IEA. Energy Statistics Data Browser. Available online: https://www.iea.org/reports/hydrogen (accessed on 10 September 2022).
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The Survey of Key Technologies in Hydrogen Energy Storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. In Materials for Sustainable Energy; Co-Published with Macmillan Publishers Ltd.: New York, NY, UK, 2010; Volume 414, pp. 265–270. [Google Scholar]
- Thomas, C.E. Fuel Cell and Battery Electric Vehicles Compared. Int. J. Hydrogen Energy 2009, 34, 6005–6020. [Google Scholar] [CrossRef] [Green Version]
- Eberle, D.U.; von Helmolt, D.R. Sustainable Transportation Based on Electric Vehicle Concepts: A Brief Overview. Energy Environ. Sci. 2010, 3, 689. [Google Scholar] [CrossRef]
- Toyota. Mirai. Available online: https://www.toyota.com/mirai/ (accessed on 14 April 2022).
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent Progress in Conducting Polymers for Hydrogen Storage and Fuel Cell Applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous Polymers for Hydrogen Storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Hulvey, Z.; Gennett, T.; Ahmed, A.; Autrey, T.; Camp, J.; Seon Cho, E.; Furukawa, H.; Haranczyk, M.; Head-Gordon, M.; et al. An Assessment of Strategies for the Development of Solid-State Adsorbents for Vehicular Hydrogen Storage. Energy Environ. Sci. 2018, 11, 2784–2812. [Google Scholar] [CrossRef] [Green Version]
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 7 March 2014).
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.; Mane, R.; Sharma, P. Heat Transfer Techniques in Metal Hydride Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2017, 42, 30661–30682. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH 4 for Hydrogen Storage. Adv. Energy Mater. 2017, 7, 1700299. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Huang, Z.; Zhu, M. Closing the Loop for Hydrogen Storage: Facile Regeneration of NaBH 4 from Its Hydrolytic Product. Angew. Chem. Int. Ed. 2020, 59, 8623–8629. [Google Scholar] [CrossRef]
- Chen, K.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Zhang, Y.; Zhu, M. Converting H+ from Coordinated Water into H− Enables Super Facile Synthesis of LiBH 4. Green Chem. 2019, 21, 4380–4387. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (Lohc) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A Review on Current Trends in Potential Use of Metal-Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Fischer, R.A. Covalent Organic Frameworks and Their Metal Nanoparticle Composites: Prospects for Hydrogen Storage. Phys. Status Solidi B Basic Res. 2013, 250, 1119–1127. [Google Scholar] [CrossRef]
- Cao, S.; Li, B.; Zhu, R.; Pang, H. Design and Synthesis of Covalent Organic Frameworks towards Energy and Environment Fields. Chem. Eng. J. 2019, 355, 602–623. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous Carbon-Based Materials for Hydrogen Storage: Advancement and Challenges. J. Mater. Chem. A 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Annamalai, P.; Musyoka, N.M.; Ren, J.; Langmi, H.W.; Mathe, M.; Bessarabov, D.; Petrik, L.F. Electrospun Zeolite-Templated Carbon Composite Fibres for Hydrogen Storage Applications. Res. Chem. Intermed. 2017, 43, 4095–4102. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Hurst, K.E.; Parilla, P.A.; Gennett, T.; Brown, C.M.; Zacharia, R.; Tylianakis, E.; Klontzas, E.; Froudakis, G.E.; et al. Outlook and Challenges for Hydrogen Storage in Nanoporous Materials. Appl. Phys. A 2016, 122, 151. [Google Scholar] [CrossRef] [Green Version]
- Rochat, S.; Polak-Kraśna, K.; Tian, M.; Holyfield, L.T.; Mays, T.J.; Bowen, C.R.; Burrows, A.D. Hydrogen Storage in Polymer-Based Processable Microporous Composites. J. Mater. Chem. A 2017, 5, 18752–18761. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Rochat, S.; Polak-Kraśna, K.; Holyfield, L.T.; Burrows, A.D.; Bowen, C.R.; Mays, T.J. Nanoporous Polymer-Based Composites for Enhanced Hydrogen Storage. Adsorption 2019, 25, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous Materials for Hydrogen Storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Kostoglou, N.; Koczwara, C.; Stock, S.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; Paris, O.; Rebholz, C.; Mitterer, C. Nanoporous Polymer-Derived Activated Carbon for Hydrogen Adsorption and Electrochemical Energy Storage. Chem. Eng. J. 2022, 427, 131730. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Rozière, J.; Jones, D.J. High-Purity Hydrogen Generation via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680. [Google Scholar] [CrossRef]
- Stark, K.; Emelyanenko, V.N.; Zhabina, A.A.; Varfolomeev, M.A.; Verevkin, S.P.; Müller, K.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Carbazole Partly and Fully Hydrogenated Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7953–7966. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Zhi, C.; Bando, Y.; Golberg, D. Boron Nitride Porous Microbelts for Hydrogen Storage. ACS Nano 2013, 7, 1558–1565. [Google Scholar] [CrossRef]
- He, T.; Wu, H.; Wu, G.; Wang, J.; Zhou, W.; Xiong, Z.; Chen, J.; Zhang, T.; Chen, P. Borohydride Hydrazinates: High Hydrogen Content Materials for Hydrogenstorage. Energy Environ. Sci. 2012, 5, 5686–5689. [Google Scholar] [CrossRef]
- Kato, R.; Yoshimasa, K.; Egashira, T.; Oya, T.; Oyaizu, K.; Nishide, H. A Ketone/Alcohol Polymer for Cycle of Electrolytic Hydrogen-Fixing with Water and Releasing under Mild Conditions. Nat. Commun. 2016, 7, 13032. [Google Scholar] [CrossRef]
- Kato, R.; Oya, T.; Shimazaki, Y.; Oyaizu, K.; Nishide, H. A Hydrogen-Storing Quinaldine Polymer: Nickel-Electrodeposition-Assisted Hydrogenation and Subsequent Hydrogen Evolution. Polym. Int. 2017, 66, 647–652. [Google Scholar] [CrossRef]
- Staudinger, H.; Geiger, E.; Huber, E. Über Hochpolymere Verbindungen, 15. Mitteilung: Über Die Reduktion Des Polystyrols. Ber. Der Dtsch. Chem. Ges. (A B Ser.) 1929, 62, 263–267. [Google Scholar] [CrossRef]
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2014, 136, 8564–8567. [Google Scholar] [CrossRef]
- Oka, K.; Kaiwa, Y.; Furukawa, S.; Nishide, H.; Oyaizu, K. Reversible Hydrogen Fixation and Release under Mild Conditions by Poly(Vinylquinoxaline). ACS Appl. Polym. Mater. 2020, 2, 2756–2760. [Google Scholar] [CrossRef]
- Kaiwa, Y.; Oka, K.; Nishide, H.; Oyaizu, K. Facile Reversible Hydrogenation of a Poly(6-Vinyl-2,3-Dimethyl-1,2,3,4-Tetrahydroquinoxaline) Gel-like Solid. Polym. Adv. Technol. 2021, 32, 1162–1167. [Google Scholar] [CrossRef]
- Oka, K.; Kataoka, M.; Nishide, H.; Oyaizu, K. Poly(Vinyl Diphenylquinoxaline) as a Hydrogen Storage Material toward Rapid Hydrogen Evolution. MRS Commun. 2022, 12, 213–216. [Google Scholar] [CrossRef]
- Kato, R.; Oka, K.; Yoshimasa, K.; Nakajima, M.; Nishide, H.; Oyaizu, K. Reversible Hydrogen Releasing and Fixing with Poly(Vinylfluorenol) through a Mild Ir-Catalyzed Dehydrogenation and Electrochemical Hydrogenation. Macromol. Rapid Commun. 2019, 40, 1900139. [Google Scholar] [CrossRef]
- Miyake, J.; Ogawa, Y.; Tanaka, T.; Ahn, J.; Oka, K.; Oyaizu, K.; Miyatake, K. Rechargeable Proton Exchange Membrane Fuel Cell Containing an Intrinsic Hydrogen Storage Polymer. Commun. Chem. 2020, 3, 3–4. [Google Scholar] [CrossRef]
- Oka, K.; Tobita, Y.; Kataoka, M.; Murao, S.; Kobayashi, K.; Furukawa, S.; Nishide, H.; Oyaizu, K. Synthesis of Vinyl Polymers Substituted with 2-Propanol and Acetone and Investigation of Their Reversible Hydrogen Storage Capabilities. Polym. J. 2021, 53, 799–804. [Google Scholar] [CrossRef]
- Oka, K.; Kaiwa, Y.; Kataoka, M.; Fujita, K.; Oyaizu, K. A Polymer Sheet-Based Hydrogen Carrier. Eur. J. Org. Chem. 2020, 2020, 5876–5879. [Google Scholar] [CrossRef]
- Oka, K.; Tobita, Y.; Kataoka, M.; Kobayashi, K.; Kaiwa, Y.; Nishide, H.; Oyaizu, K. Hydrophilic Isopropanol/Acetone-substituted Polymers for Safe Hydrogen Storage. Polym. Int. 2022, 71, 348–351. [Google Scholar] [CrossRef]
- Crabtree, R.H. Homogeneous Transition Metal Catalysis of Acceptorless Dehydrogenative Alcohol Oxidation: Applications in Hydrogen Storage and to Heterocycle Synthesis. Chem. Rev. 2017, 117, 9228–9246. [Google Scholar] [CrossRef] [PubMed]
- Hodoshima, S.; Takaiwa, S.; Shono, A.; Satoh, K.; Saito, Y. Hydrogen Storage by Decalin/Naphthalene Pair and Hydrogen Supply to Fuel Cells by Use of Superheated Liquid-Film-Type Catalysis. Appl. Catal. A Gen. 2005, 283, 235–242. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, L.; Xu, L.; Wan, C.; An, Y.; Ye, M. Recent Developments of Effective Catalysts for Hydrogen Storage Technology Using N-Ethylcarbazole. Catalysts 2020, 10, 648. [Google Scholar] [CrossRef]
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of Hydrogenation and Dehydrogenation Based on Dibenzyltoluene as Liquid Organic Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [Green Version]
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar] [CrossRef]
- Cho, S.J.; Choo, K.; Kim, D.P.; Kim, J.W. H2 Sorption in HCl-Treated Polyaniline and Polypyrrole. Catal. Today 2007, 120, 336–340. [Google Scholar] [CrossRef]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Room Temperature Reversible Hydrogen Storage in Polyaniline (PANI) Nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 4561–4565. [Google Scholar] [CrossRef]
- Attia, N.F.; Geckeler, K.E. Polyaniline as a Material for Hydrogen Storage Applications. Macromol. Rapid Commun. 2013, 34, 1043–1055. [Google Scholar] [CrossRef]
- Ebrahim, R.; Zomorrodian, A.; Attia, N.; Geckeler, K.; Ignatiev, A. Cyclical Hydrogen Storage and Retention in Polyaniline. Energy Sci. Eng. 2016, 4, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhu, H.; Liu, Y. Preparation of Activated Rectangular Polyaniline-Based Carbon Tubes and Their Application in Hydrogen Adsorption. Int. J. Hydrogen Energy 2011, 36, 11738–11745. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. Preparation and Hydrogen Storage Capacity of Highly Porous Activated Carbon Materials Derived from Polythiophene. Int. J. Hydrogen Energy 2011, 36, 15658–15663. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, D.; Liu, W.; Liu, H.; Zhao, J.; Chen, P.; Wang, Q.; Wang, X.; Zou, Y. Fabrication of Porous Polyaniline/MWCNTs Coated Co9S8 Composite for Electrochemical Hydrogen Storage Application. J. Phys. Chem. Solids 2021, 157, 110235. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Liu, W.; Liu, H.; Chen, P.; Zhao, J.; Cheng, Y.; Wang, Q.; Wang, X. Preparation of Porous Polyaniline/RGO Composite and Its Application in Improving the Electrochemical Properties of Co9S8 Hydrogen Storage Alloy. Int. J. Hydrogen Energy 2021, 46, 40239–40250. [Google Scholar] [CrossRef]
- Li, M.M.; Wang, C.C.; Zhou, Y.T.; Yang, C.C. Clarifying the Polyaniline Effect on Superior Electrochemical Performances of Hydrogen Storage Alloys. Electrochim. Acta 2021, 365, 137336. [Google Scholar] [CrossRef]
- Lochan, R.C.; Head-Gordon, M. Computational Studies of Molecular Hydrogen Binding Affinities: The Role of Dispersion Forces, Electrostatics, and Orbital Interactions. Phys. Chem. Chem. Phys. 2006, 8, 1357. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Chaine, R.; Bose, T. Low-Pressure Adsorption Storage of Hydrogen. Int. J. Hydrogen Energy 1994, 19, 161–164. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Ilinitch, O.M.; Fenelonov, V.B.; Lapkin, A.A.; Okkel, L.G.; Terskikh, V.V.; Zamaraev, K.I. Intrinsic Microporosity and Gas Transport in Polyphenylene Oxide Polymers. Microporous Mesoporous Mater. 1999, 31, 97–110. [Google Scholar] [CrossRef]
- Yoshida, A.; Okuyama, T.; Mori, Y.; Saito, N.; Naito, S. Hydrogen Storage Material Composed of Polyacetylene and LiH and Investigation of Its Mechanisms. Chem. Mater. 2014, 26, 4076–4081. [Google Scholar] [CrossRef]
- Chau, J.; Basak, P.; Sirkar, K.K. Reverse Osmosis Separation of Particular Organic Solvent Mixtures by a Perfluorodioxole Copolymer Membrane. J. Memb. Sci. 2018, 563, 541–551. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Mustain, W.E.; Kohl, P.A. Poly(Norbornene) Anion Conductive Membranes: Homopolymer, Block Copolymer and Random Copolymer Properties and Performance. J. Mater. Chem. A 2020, 8, 17568–17578. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Wakimoto, K.; Wang, Z.; Isfahani, A.P.; Suma, T.; Sivaniah, E.; Ghalei, B. A Facile Synthesis of Contorted Spirobisindane-Diamine and Its Microporous Polyimides for Gas Separation. RSC Adv. 2018, 8, 6326–6330. [Google Scholar] [CrossRef] [Green Version]
- Ramimoghadam, D.; Gray, E.M.; Webb, C.J. Review of Polymers of Intrinsic Microporosity for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Boyd, S.E.; Brown, C.L.; Gray, E.M.A.; Webb, C.J. The Effect of Thermal Treatment on the Hydrogen-Storage Properties of PIM-1. ChemPhysChem 2019, 20, 1613–1623. [Google Scholar] [CrossRef]
- Bambalaza, S.E.; Langmi, H.W.; Mokaya, R.; Musyoka, N.M.; Khotseng, L.E. Co-Pelletization of a Zirconium-Based Metal-Organic Framework (UiO-66) with Polymer Nanofibers for Improved Useable Capacity in Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 8607–8620. [Google Scholar] [CrossRef]
- Davy, H.I. The Bakerian Lecture. On Some of the Combinations of Oxymuriatic Gas and Oxygene, and on the Chemical Relations of These Principles, to Inflammable Bodies. Philos. Trans. R. Soc. Lond. 1811, 101, 1–35. [Google Scholar] [CrossRef]
- Franks, F. Water: A Comprehensive Treatise; Franks, F., Ed.; Springer: Boston, MA, US, 1979; ISBN 978-1-4684-8020-7. [Google Scholar]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrogen Storage in Clathrate Hydrates: Current State of the Art and Future Directions. Appl. Energy 2014, 122, 112–132. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Montes-Campos, H.; Pérez-Rodríguez, M.; Piñeiro, M.M. Evaluation of Hydrogen Storage Ability of Hydroquinone Clathrates Using Molecular Simulations. J. Mol. Liq. 2022, 360, 119487. [Google Scholar] [CrossRef]
- Su, F.; Bray, C.L.; Tan, B.; Cooper, A.I. Rapid and Reversible Hydrogen Storage in Clathrate Hydrates Using Emulsion-Templated Polymers. Adv. Mater. 2008, 20, 2663–2666. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, H.; Yang, G.; Wang, Y.; Li, G.; Lang, X. Reduction Clathrate Hydrates Growth Rates and Adhesion Forces on Surfaces of Inorganic or Polymer Coatings. Energy Fuels 2020, 34, 13566–13579. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, W.; Ahn, Y.-H.; Lee, J.W. Confined Tetrahydrofuran in a Superabsorbent Polymer for Sustainable Methane Storage in Clathrate Hydrates. Chem. Eng. J. 2021, 411, 128512. [Google Scholar] [CrossRef]
- Strobel, T.A.; Koh, C.A.; Sloan, E.D. Hydrogen Storage Properties of Clathrate Hydrate Materials. Fluid Phase Equilib. 2007, 261, 382–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Kumar, R.; Linga, P. Solidified Hydrogen Storage (Solid-HyStore) via Clathrate Hydrates. Chem. Eng. J. 2022, 431, 133702. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The Atom, the Molecule, and the Covalent Organic Framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Wang, W. Covalent Organic Frameworks (COFs): From Design to Applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Sharma, V.; Kumar, D. Improved Designs of Multifunctional Covalent-Organic Frameworks: Hydrogen Storage, Methane Storage, and Water Harvesting. Mini Rev. Org. Chem. 2021, 18, 1026–1036. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Xu, Y.; Pang, H. Two-Dimensional MOF and COF Nanosheets: Synthesis and Applications in Electrochemistry. Chem.—A Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, E.; Klontzas, E.; Froudakis, G.E. Multi-Scale Theoretical Investigation of Hydrogen Storage in Covalent Organic Frameworks. Nanoscale 2011, 3, 856. [Google Scholar] [CrossRef]
- Shangguan, W.; Zhao, H.; Dai, J.-Q.; Cai, J.; Yan, C. First-Principles Study of Hydrogen Storage of Sc-Modified Semiconductor Covalent Organic Framework-1. ACS Omega 2021, 6, 21985–21993. [Google Scholar] [CrossRef]
- Tong, M.; Zhu, W.; Li, J.; Long, Z.; Zhao, S.; Chen, G.; Lan, Y. An Easy Way to Identify High Performing Covalent Organic Frameworks for Hydrogen Storage. Chem. Commun. 2020, 56, 6376–6379. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Yusenko, K.; Fischer, R.A. Metallocenes@COF-102: Organometallic Host–Guest Chemistry of Porous Crystalline Organic Frameworks. Chem. Commun. 2011, 47, 8506. [Google Scholar] [CrossRef]
- Han, S.S.; Furukawa, H.; Yaghi, O.M.; Goddard, W.A. Covalent Organic Frameworks as Exceptional Hydrogen Storage Materials. J. Am. Chem. Soc. 2008, 130, 11580–11581. [Google Scholar] [CrossRef] [Green Version]
- Klontzas, E.; Tylianakis, E.; Froudakis, G.E. Designing 3D COFs with Enhanced Hydrogen Storage Capacity. Nano Lett. 2010, 10, 452–454. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Bian, L.-Y.; Li, X.-D.; Huang, X.-Y.; Yang, P.; Wang, Y.-D.; Liu, X.-Y.; Chen, Z. Molecular Simulation on Hydrogen Storage Properties of Five Novel Covalent Organic Frameworks with the Higher Valency. Int. J. Hydrogen Energy 2022, 47, 29390–29398. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Park, H.J.; Lim, D.W.; Yang, W.S.; Oh, T.R.; Suh, M.P. A Highly Porous Metal-Organic Framework: Structural Transformations of a Guest-Free MOF Depending on Activation Method and Temperature. Chem.—A Eur. J. 2011, 17, 7251–7260. [Google Scholar] [CrossRef]
- Tedds, S.; Walton, A.; Broom, D.P.; Book, D. Characterisation of Porous Hydrogen Storage Materials: Carbons, Zeolites, MOFs and PIMs. Faraday Discuss. 2011, 151, 75. [Google Scholar] [CrossRef]
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent Advancements in MOFs Synthesis and Their Green Applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593. [Google Scholar] [CrossRef]
- Kudiiarov, V.; Lyu, J.; Semyonov, O.; Lider, A.; Chaemchuen, S.; Verpoort, F. Prospects of Hybrid Materials Composed of MOFs and Hydride-Forming Metal Nanoparticles for Light-Duty Vehicle Hydrogen Storage. Appl. Mater. Today 2021, 25, 101208. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Balderas-Xicohténcatl, R.; Schlichtenmayer, M.; Hirscher, M. Volumetric Hydrogen Storage Capacity in Metal-Organic Frameworks. Energy Technol. 2018, 6, 578–582. [Google Scholar] [CrossRef]
- Grünker, R.; Bon, V.; Müller, P.; Stoeck, U.; Krause, S.; Mueller, U.; Senkovska, I.; Kaskel, S. A New Metal–Organic Framework with Ultra-High Surface Area. Chem. Commun. 2014, 50, 3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biserčić, M.S.; Marjanović, B.; Zasońska, B.A.; Stojadinović, S.; Ćirić-Marjanović, G. Novel Microporous Composites of MOF-5 and Polyaniline with High Specific Surface Area. Synth. Met. 2020, 262, 116348. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Du, H.; Yuan, Y.; Qu, H.; Zou, M. First-Principles Investigation on Hydrogen Storage Performance of Li, Na and K Decorated Borophene. Appl. Surf. Sci. 2018, 427, 1030–1037. [Google Scholar] [CrossRef]
- Orcajo, G.; Montes-Andrés, H.; Villajos, J.A.; Martos, C.; Botas, J.A.; Calleja, G. Li-Crown Ether Complex Inclusion in MOF Materials for Enhanced H2 Volumetric Storage Capacity at Room Temperature. Int. J. Hydrogen Energy 2019, 44, 19285–19293. [Google Scholar] [CrossRef]
- Montes-Andrés, H.; Orcajo, G.; Mellot-Draznieks, C.; Martos, C.; Botas, J.A.; Calleja, G. Novel Ni-IRMOF-74 Postsynthetically Functionalized for H 2 Storage Applications. J. Phys. Chem. C 2018, 122, 28123–28132. [Google Scholar] [CrossRef]
- El Kassaoui, M.; Lakhal, M.; Abdellaoui, M.; Benyoussef, A.; El Kenz, A.; Loulidi, M. Modeling Hydrogen Adsorption in the Metal Organic Framework (MOF-5, Connector): Zn4O(C8H4O4)3. Int. J. Hydrogen Energy 2020, 45, 33663–33674. [Google Scholar] [CrossRef]
- Yan, Y.; da Silva, I.; Blake, A.J.; Dailly, A.; Manuel, P.; Yang, S.; Schröder, M. High Volumetric Hydrogen Adsorption in a Porous Anthracene-Decorated Metal–Organic Framework. Inorg. Chem. 2018, 57, 12050–12055. [Google Scholar] [CrossRef] [Green Version]
- Burress, J.; Kraus, M.; Beckner, M.; Cepel, R.; Suppes, G.; Wexler, C.; Pfeifer, P. Hydrogen Storage in Engineered Carbon Nanospaces. Nanotechnology 2009, 20, 204026. [Google Scholar] [CrossRef] [Green Version]
- Geloso, B. Hydrogen Storage on Chemically Activated Carbons and Carbon Materials at High Temperatures. Ann. Chem. 1926, 18, 413–426. [Google Scholar]
- Rimza, T.; Saha, S.; Dhand, C.; Dwivedi, N.; Patel, S.S.; Singh, S.; Kumar, P. Carbon-Based Sorbents for Hydrogen Storage: Challenges and Sustainability at Operating Conditions for Renewable Energy. ChemSusChem 2022, 15, e202200281. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen Adsorption in Different Carbon Nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Zhao, X.B.; Xiao, B.; Fletcher, A.J.; Thomas, K.M. Hydrogen Adsorption on Functionalized Nanoporous Activated Carbons. J. Phys. Chem. B 2005, 109, 8880–8888. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High Density Hydrogen Storage in Superactivated Carbons from Hydrothermally Carbonized Renewable Organic Materials. Energy Environ. Sci. 2011, 4, 1400. [Google Scholar] [CrossRef] [Green Version]
- Kuchta, B.; Firlej, L.; Roszak, S.; Pfeifer, P. A Review of Boron Enhanced Nanoporous Carbons for Hydrogen Adsorption: Numerical Perspective. Adsorption 2010, 16, 413–421. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Hydrogen Storage in High Surface Area Carbons with Identical Surface Areas but Different Pore Sizes: Direct Demonstration of the Effects of Pore Size. J. Phys. Chem. C 2012, 116, 25734–25740. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Fernández-Huerta, N.; Izquierdo, M.T.; Celzard, A. Impact of Synthesis Conditions of KOH Activated Carbons on Their Hydrogen Storage Capacities. Int. J. Hydrogen Energy 2012, 37, 14278–14284. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Effect of Platinum Doping of Activated Carbon on Hydrogen Storage Behaviors of Metal-Organic Frameworks-5. Int. J. Hydrogen Energy 2011, 36, 8381–8387. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Zlotea, C.; Izquierdo, M.T.; Chevalier-César, C.; Latroche, M.; Celzard, A. Activated Carbons Doped with Pd Nanoparticles for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 5072–5080. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A Review on the Mechanical and Electrical Properties of Graphite and Modified Graphite Reinforced Polymer Composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Watanabe, K.; Soma, M.; Onishi, T.; Tamaru, K. Sorption of Molecular Hydrogen by Potassium Graphite. Nat. Phys. Sci. 1971, 233, 160–161. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.-K.; Hua, T.Q. Sorbent Material Property Requirements for On-Board Hydrogen Storage for Automotive Fuel Cell Systems. Int. J. Hydrogen Energy 2015, 40, 6373–6390. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lee, K.; Sun, Y.-Y.; Lucking, M.; Chen, Z.; Zhao, J.J.; Zhang, S.B. Graphene Oxide as an Ideal Substrate for Hydrogen Storage. ACS Nano 2009, 3, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Darkrim, F.L.; Malbrunot, P.; Tartaglia, G.P. Review of Hydrogen Storage by Adsorption in Carbon Nanotubes. Int. J. Hydrogen Energy 2002, 27, 193–202. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Ci, L.; Xu, C.; Mao, Z.; Wei, B.; Liang, J.; Wu, D. Hydrogen Uptake by Graphitized Multi-Walled Carbon Nanotubes under Moderate Pressure and at Room Temperature. Carbon 2001, 39, 2077–2079. [Google Scholar] [CrossRef]
- Ding, R.G.; Lu, G.Q.; Yan, Z.F.; Wilson, M.A. Recent Advances in the Preparation and Utilization of Carbon Nanotubes for Hydrogen Storage. J. Nanosci. Nanotechnol. 2001, 1, 7–29. [Google Scholar] [CrossRef]
- Rather, S.U. Preparation, Characterization and Hydrogen Storage Studies of Carbon Nanotubes and Their Composites: A Review. Int. J. Hydrogen Energy 2020, 45, 4653–4672. [Google Scholar] [CrossRef]
- Rather, S.; Nahm, K.S. Hydrogen Uptake of High-Energy Ball Milled Nickel-Multiwalled Carbon Nanotube Composites. Mater. Res. Bull. 2014, 49, 525–530. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Wu, C.-Z.; Xu, S.-T.; Cheng, H.-M. Hydrogen Storage in Carbon Nanotubes Revisited. Carbon 2010, 48, 452–455. [Google Scholar] [CrossRef]
- Chen, P.; Wu, X.; Lin, J.; Tan, K.L. High H 2 Uptake by Alkali-Doped Carbon Nanotubes Under Ambient Pressure and Moderate Temperatures. Science 1999, 285, 91–93. [Google Scholar] [CrossRef]
- Yang, R.T. Hydrogen Storage by Alkali-Doped Carbon Nanotubes–Revisited. Carbon 2000, 38, 623–626. [Google Scholar] [CrossRef]
- Lachawiec, A.J.; Qi, G.; Yang, R.T. Hydrogen Storage in Nanostructured Carbons by Spillover: Bridge-Building Enhancement. Langmuir 2005, 21, 11418–11424. [Google Scholar] [CrossRef]
- Broom, D.P.; Hirscher, M. Irreproducibility in Hydrogen Storage Material Research. Energy Environ. Sci. 2016, 9, 3368–3380. [Google Scholar] [CrossRef] [Green Version]
- Tsivion, E.; Veccham, S.P.; Head-Gordon, M. High-Temperature Hydrogen Storage of Multiple Molecules: Theoretical Insights from Metalated Catechols. ChemPhysChem 2017, 18, 184–188. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Rather, S.; Naik, M.; Soo, C.S.; Nahm, K.-S. Hydrogen Uptake of Multiwalled Carbon Nanotubes Decorated with Pt–Pd Alloy Using Thermal Vapour Deposition Method. J. Alloys Compd. 2009, 480, L20–L24. [Google Scholar] [CrossRef]
- Mehrabi, M.; Reyhani, A.; Parvin, P.; Mortazavi, S.Z. Surface Structural Alteration of Multi-Walled Carbon Nanotubes Decorated by Nickel Nanoparticles Based on Laser Ablation/Chemical Reduction Methods to Enhance Hydrogen Storage Properties. Int. J. Hydrogen Energy 2019, 44, 3812–3823. [Google Scholar] [CrossRef]
- Vellingiri, L.; Annamalai, K.; Kandasamy, R.; Kombiah, I. Characterization and Hydrogen Storage Properties of SnO2 Functionalized MWCNT Nanocomposites. Int. J. Hydrogen Energy 2018, 43, 10396–10409. [Google Scholar] [CrossRef]
- Rather, S.U. Hydrogen Uptake of Manganese Oxide-Multiwalled Carbon Nanotube Composites. Int. J. Hydrogen Energy 2019, 44, 325–331. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Ghaedi, M.; Nasiri Kokhdan, S.; Vashaee, D. Remarkably Improved Electrochemical Hydrogen Storage by Multi-Walled Carbon Nanotubes Decorated with Nanoporous Bimetallic Fe–Ag/TiO2 Nanoparticles. Dalton Trans. 2019, 48, 898–907. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Thaweelap, N.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Chanlek, N.; Utke, O.; Pangon, A.; Utke, R. Hydrogen Adsorption of O/N-Rich Hierarchical Carbon Scaffold Decorated with Ni Nanoparticles: Experimental and Computational Studies. Int. J. Hydrogen Energy 2021, 46, 5427–5440. [Google Scholar] [CrossRef]
- Chambers, A.; Park, C.; Baker, R.T.K.; Rodriguez, N.M. Hydrogen Storage in Graphite Nanofibers. J. Phys. Chem. B 1998, 102, 4253–4256. [Google Scholar] [CrossRef]
- Poirier, E. Hydrogen Adsorption in Carbon Nanostructures. Int. J. Hydrogen Energy 2001, 26, 831–835. [Google Scholar] [CrossRef]
- Browning, D.J.; Gerrard, M.L.; Lakeman, J.B.; Mellor, I.M.; Mortimer, R.J.; Turpin, M.C. Studies into the Storage of Hydrogen in Carbon Nanofibers: Proposal of a Possible Reaction Mechanism. Nano Lett. 2002, 2, 201–205. [Google Scholar] [CrossRef]
- Hanada, K.; Shiono, H.; Matsuzaki, K. Hydrogen Uptake of Carbon Nanofiber under Moderate Temperature and Low Pressure. Diam. Relat. Mater. 2003, 12, 874–877. [Google Scholar] [CrossRef]
- Thiangviriya, S.; Utke, R. LiBH4 Nanoconfined in Activated Carbon Nanofiber for Reversible Hydrogen Storage. Int. J. Hydrogen Energy 2015, 40, 4167–4174. [Google Scholar] [CrossRef]
- Yadav, A.; Faisal, M.; Subramaniam, A.; Verma, N. Nickel Nanoparticle-Doped and Steam-Modified Multiscale Structure of Carbon Micro-Nanofibers for Hydrogen Storage: Effects of Metal, Surface Texture and Operating Conditions. Int. J. Hydrogen Energy 2017, 42, 6104–6117. [Google Scholar] [CrossRef]
- Chen, X.; Xue, Z.; Niu, K.; Liu, X.; Wei, L.; Zhang, B.; Li, Z.; Zeng, H.; Ren, Y.; Wu, Y.; et al. Li–Fluorine Codoped Electrospun Carbon Nanofibers for Enhanced Hydrogen Storage. RSC Adv. 2021, 11, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Kim, Y.K.; Seo, H.-J.; Jeong, S.M.; Kim, J.; Lim, S.K. The Enhanced Hydrogen Storage Capacity of Carbon Fibers: The Effect of Hollow Porous Structure and Surface Modification. Nanomaterials 2021, 11, 1830. [Google Scholar] [CrossRef]
- Xu, W.; Takahashi, K.; Matsuo, Y.; Hattori, Y.; Kumagai, M.; Ishiyama, S.; Kaneko, K.; Iijima, S. Investigation of Hydrogen Storage Capacity of Various Carbon Materials. Int. J. Hydrogen Energy 2007, 32, 2504–2512. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Balahmar, N.; Mokaya, R. Oxygen-Rich Microporous Carbons with Exceptional Hydrogen Storage Capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef]
- Kadri, A.; Jia, Y.; Chen, Z.; Yao, X. Catalytically Enhanced Hydrogen Sorption in Mg-MgH2 by Coupling Vanadium-Based Catalyst and Carbon Nanotubes. Materials 2015, 8, 3491–3507. [Google Scholar] [CrossRef] [Green Version]
- Chena, C.-H.; Huang, C.-C. Hydrogen Storage by KOH-Modified Multi-Walled Carbon Nanotubes. Int. J. Hydrogen Energy 2007, 32, 237–246. [Google Scholar] [CrossRef]



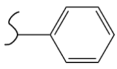
| Polymer | Hydrogenated Form | Molecule Weight of Monomer (g.mol−1) | Hydrogen Capacity (%wt) | Hydrogenation Condition | Ref. |
|---|---|---|---|---|---|
 | 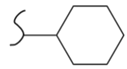 | 104 | 4.80 | 473 K, Ni catalyst | [39] |
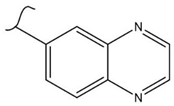 | 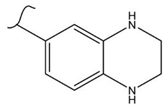 | 160 | 2.5 | 333 K, Ir catalyst 5 h | [37] |
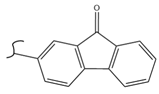 | 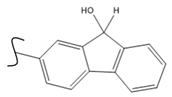 | 208 | 0.96 | 373 K, Ir catalyst 5 h | [44] |
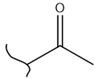 |  | 72 | 2.77 | 453 K, Ir catalyst 1.5 h | [42] |
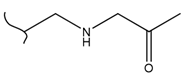 | 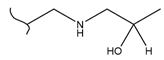 | 115 | 1.73 | 368 K, Ir catalyst 8 h | [48] |
 | 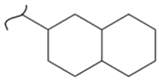 | 164 | 6.1 | 393 K, Ni catalyst 20 bar | [50] |
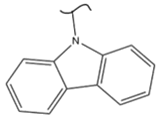 | 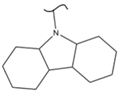 | 205 | 5.85 | 453 K, Pt or Pd catalyst 20–100 bar | [51] |
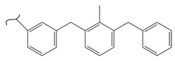 | 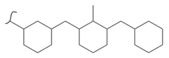 | 319 | 5.64 | 593 K, Pt catalyst >10 bar | [52] |
| Materials Group | H2 Gravimetric Capacity (%wt) | Process Condition | Carrier Properties | Ref. |
|---|---|---|---|---|
| AC | 0.70 | 303 K, 10 MPa | SSA: 3306 m2 g−1 Pore volume: 1.2 cm3 g−1 | [156] |
| AC (KOH-treated)-Oxygen-rich | 8.70 | 77 K, 30 MPa | SSA: 3800 m2 g−1 Pore volume: 1.8 cm3 g−1 | [157] |
| SWNH | 0.50 | 303 K, 10 MPa | SSA: 910 m2 g−1 Pore volume: 0.31 cm3 g−1 | [156] |
| AC (KOH-treated) | 6.60 | 77 K, 8 MPa | SSA: 3451 m2 g−1 Pore volume: 1.05 cm3 g−1 | [123] |
| Platinum-doped AC/MOF5 | 2.30 | 298 K, 100 MPa | SSA: 730 m2 g−1 Pore volume: 0.303 cm3 g−1 | [125] |
| CNT (Li-doped) | 20.00 | 678 K, 10 MPa | Energy density: 0.133 KWh/Kg | [137] |
| GNF | 58.37 | 298 K, 12 MPa | [148] | |
| SWCNT | 4.20 | 298 K, 10 MPa | [158] | |
| SWCNTs (50% purity) | 1.70 | 290 K, 12.04 MPa | SSA: 229 m2 g−1 | [136] |
| Pt-alloy CNT | 2.90 | 298 K, 3.2 MPa | Diameter: 10–40 nm | [142] |
| Ni-alloy CNT | 2.27 | 298 K, 3.2 MPa | Diameter: 10–40 nm | [142] |
| Ag-alloy CNT | 0.86 | 298 K, 3.2 MPa | Diameter: 10–40 nm | [142] |
| TiO2/Fe–Ag/MWCNT | 10.94 | 298 K, 0.1 MPa | [146] | |
| MgH2-CNT | 6.50 | 473 K, 2 MPa | [159] | |
| KOH-MWCNT | 4.47 | 823 K | [160] | |
| Treated-CNF | 6.50 | 273 K, 12 MPa | SSA: 51 m2 g−1 Fiber Diameter: 50–260 nm | [150] |
| KNO3-doped CNF | 5.10 | 373 K, 0.1 MPa | Diameter: 200–500 nm | [151] |
| Pd-doped Activated CNF | 5.43 | 77 K, 10 MPa | SSA: 3058 m2 g−1 | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifian, M.; Kern, W.; Riess, G. A Bird’s-Eye View on Polymer-Based Hydrogen Carriers for Mobile Applications. Polymers 2022, 14, 4512. https://doi.org/10.3390/polym14214512
Sharifian M, Kern W, Riess G. A Bird’s-Eye View on Polymer-Based Hydrogen Carriers for Mobile Applications. Polymers. 2022; 14(21):4512. https://doi.org/10.3390/polym14214512
Chicago/Turabian StyleSharifian, Mohammadhossein, Wolfgang Kern, and Gisbert Riess. 2022. "A Bird’s-Eye View on Polymer-Based Hydrogen Carriers for Mobile Applications" Polymers 14, no. 21: 4512. https://doi.org/10.3390/polym14214512







