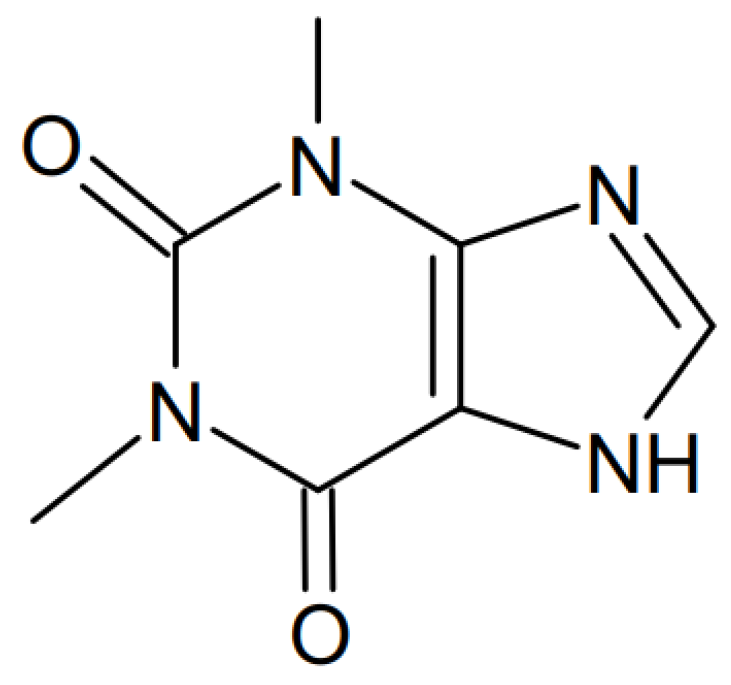
Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
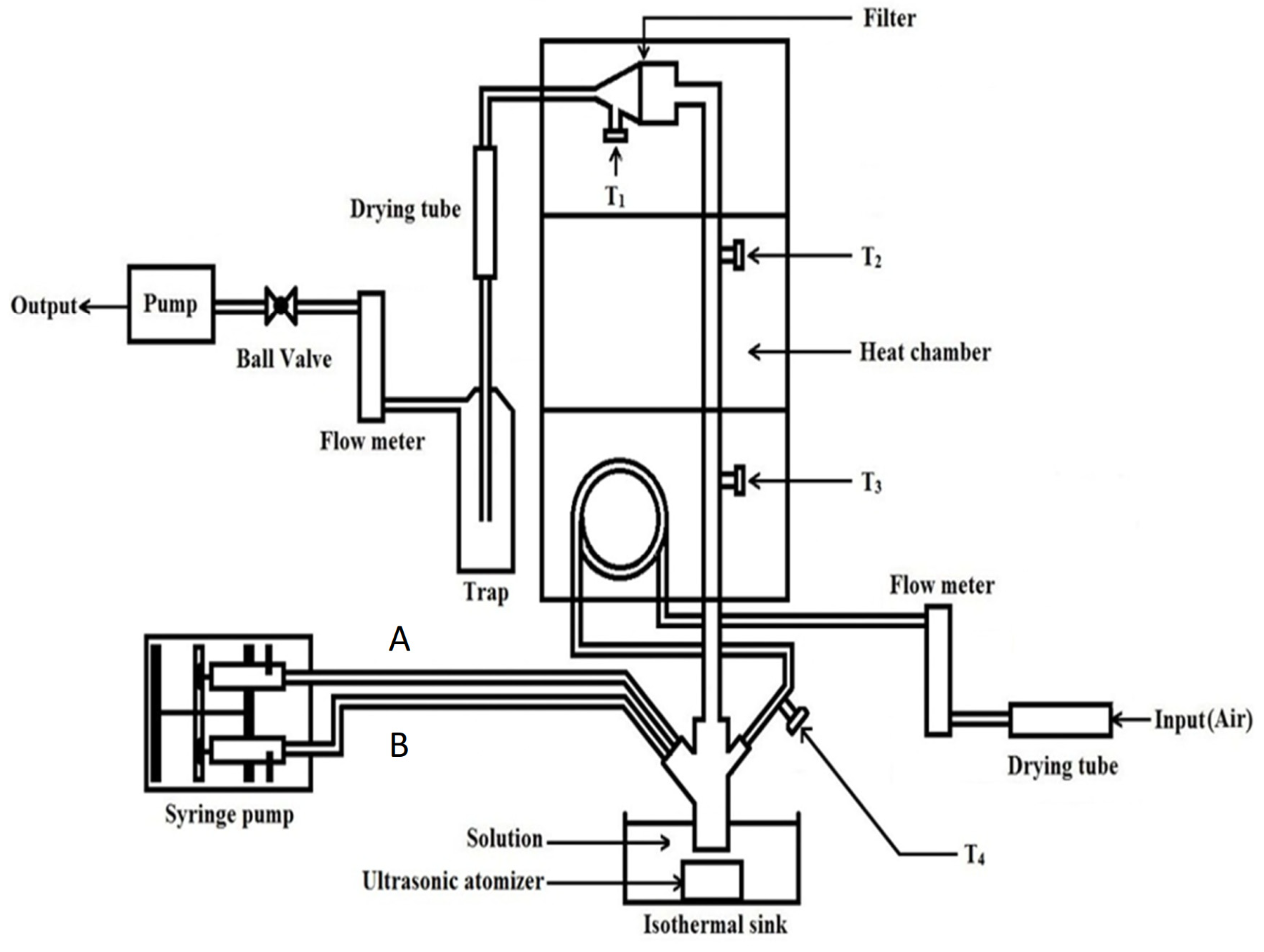
2.2. Preparation of Pectin/Chitosan Hydrochloride Microparticles
2.3. Characteristics of Pectin/Chitosan Hydrochloride Microparticles
2.4. The Release Profile of TH from the Prepared Pectin/Chitosan Hydrochloride Microparticles
3. Results and Discussions
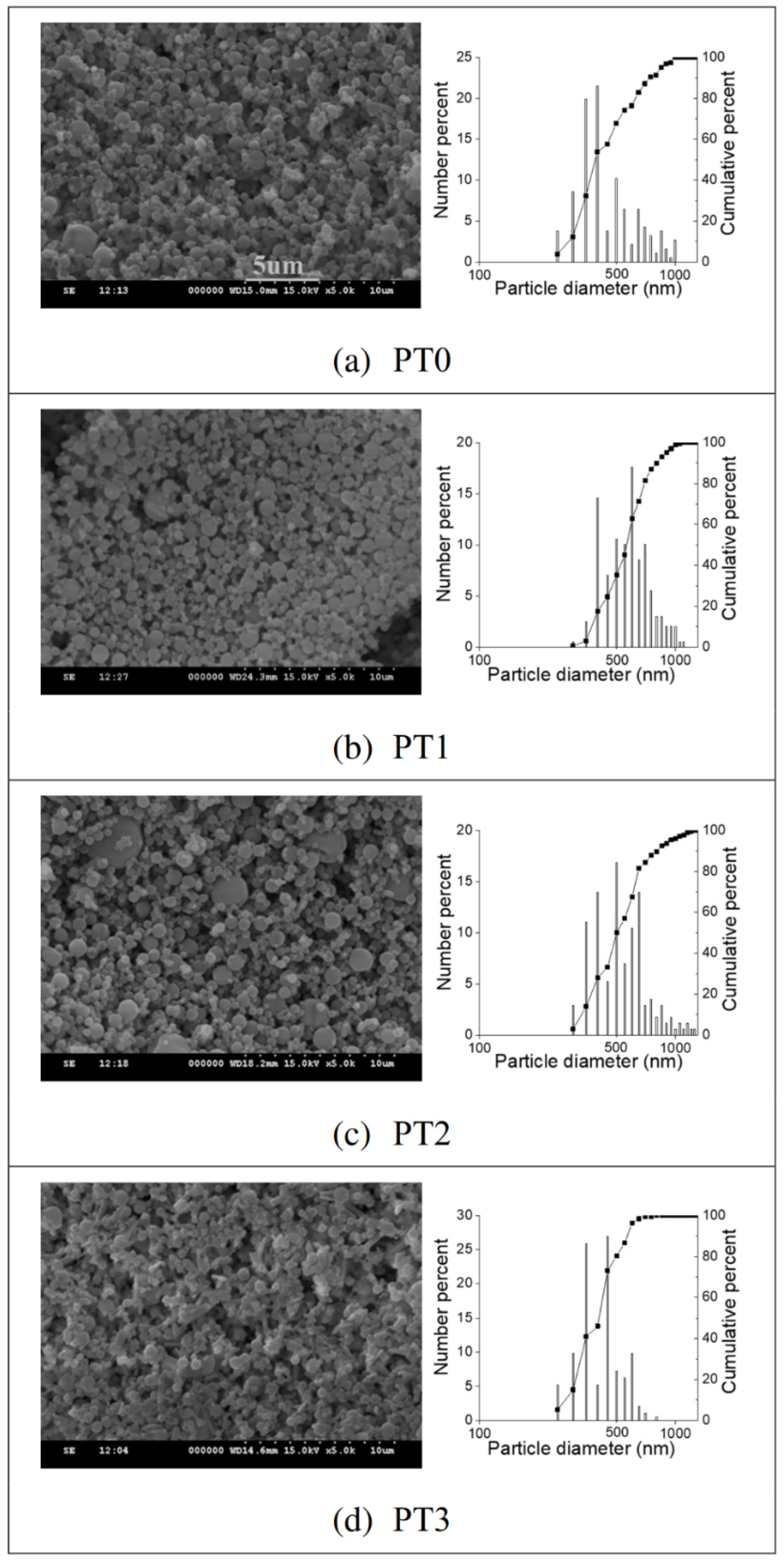
3.1. Characteristics of Pectin/Chitosan Hydrochloride Microparticles
3.2. Characteristics of Theophylline-Loaded Pectin Particles
3.3. Release of TH from the Pectin Particles in PBS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ahmed, O.; Abdel-Halim, M.; Farid, A.; Elamir, A. Taurine loaded chitosan-pectin nanoparticle shows curative effect against acetic acid-induced colitis in rats. Chem.-Biol. Int. 2022, 351, 109715. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.B.; Lin, J. Swelling behavior and the release of protein from chitosan–pectin composite particles. Carbohydr. Polym. 2000, 43, 163–169. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Assifaoui, A.; Lerbret, A.; Uyen, H.T.D.; Neiers, F.; Chambin, O.; Loupiac, C.; Cousin, F. Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca2+ and Zn2+): A process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter 2015, 11, 551–560. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Lu, Y.; Xu, H.; Chen, G.; Wu, W. Physicochemical characterization of a pectin/calcium matrix containing a large fraction of calcium chloride: Implications for sigmoidal release characteristics. J. Appl. Polym. Sci. 2009, 113, 2418–2428. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.N.; Mukhamedjanova, M.Y.; Voropaeva, N.L.; Vasilyeva, S.; Faizieva, R.; Ruban, I.N. Characteristics of interactions in the pectin–chitosan system. Chromatographia 2004, 59, 779–782. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Cerchiara, T.; Sorrenti, M.; Bettinetti, G.; Rodriguez, L.; Zecchi, V. Chitosan/pectin polyelectrolyte complexes: Selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur. J. Pharm. Sci. 2008, 35, 435–441. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Meshali, M.M.; Gabr, K.E. Effect of interpolymer complex formation of chitosan with pectin or acacia on the release behavior of chlorpromazine HCl. Int. J. Pharm. 1993, 89, 177–181. [Google Scholar] [CrossRef]
- Saladini, B.; Bigucci, F.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Microparticles based on chitosan/pectin polyelectrolyte complexes for nasal delivery of tacrine hydrochloride. Drug Deliv. Transl. Res. 2013, 3, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Bardi, G.; Gamucci, O.; Mazzolai, B. Targeted drug delivery across biological barriers using polymer nanoparticles. Future Sci. 2013, 96–109. [Google Scholar]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Wu, T.H.; Chou, C.W.; Shen, L.H. Study on the Characterization and controlled drug release carrier of thiolated chitosan-based nanoparticles. Adv. Mater. Res. 2012, 476, 1480–1483. [Google Scholar]
- Wu, T.H.; Shen, L.H.; Chou, C.W. Study on the Preparation, Property and drug release carrier of chitosan-based nanoparticles for cancer treatment. Adv. Mater. Res. 2011, 284, 1800–1803. [Google Scholar]
- Sarma, S.; Agarwal, S.; Bhuyan, P.; Hazarika, J.; Ganguly, M. Resveratrol-loaded chitosan–pectin core–shell nanoparticles as novel drug delivery vehicle for sustained release and improved antioxidant activities. R. Soc. Open Sci. 2022, 9, 210784. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.; Al-Bairmani, H.K.; Abbas, Z.S.; Rheima, A.M. Nanoscale synthesis of metal(II) theophylline complexes and assessment of their biological activity. Nano Biomed. Eng. 2020, 12, 139–147. [Google Scholar] [CrossRef]
- Bobrovs, R.; Seton, L.; Dempster, N. The reluctant polymorph: Investigation into the effect of self-association on the solvent-mediated phase transformation and nucleation of theophylline. Cryst. Eng. Comm. 2015, 17, 5237–5251. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.H.; Cheng, K.C.; Song, Y.L. Investigations of the influences of processing conditions on the properties of spray dried chitosan-tripolyphosphate particles loaded with theophylline. Sci. Rep. 2020, 10, 1155. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Effect of different cation in situ cross-linking on the properties of pethe ctin-thymol active film. Food Hydrocoll. 2022, 128, 107594. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding behavior of calcium to polyuronates: Comparison of pectin with alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly (acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Dinu, M.V.; Okay, O. Preparation of macroporous poly(acrylamide) hydrogels in DMSO/water mixture at subzero temperatures. Polym. Bull. 2008, 60, 169–180. [Google Scholar] [CrossRef]
- Oh, K.S.; Oh, J.S.; Choi, H.S.; Bae, Y.C. Effect of cross-linking density on swelling behavior of NIPA gel particles. Macromolecules 1998, 31, 7328–7335. [Google Scholar] [CrossRef]


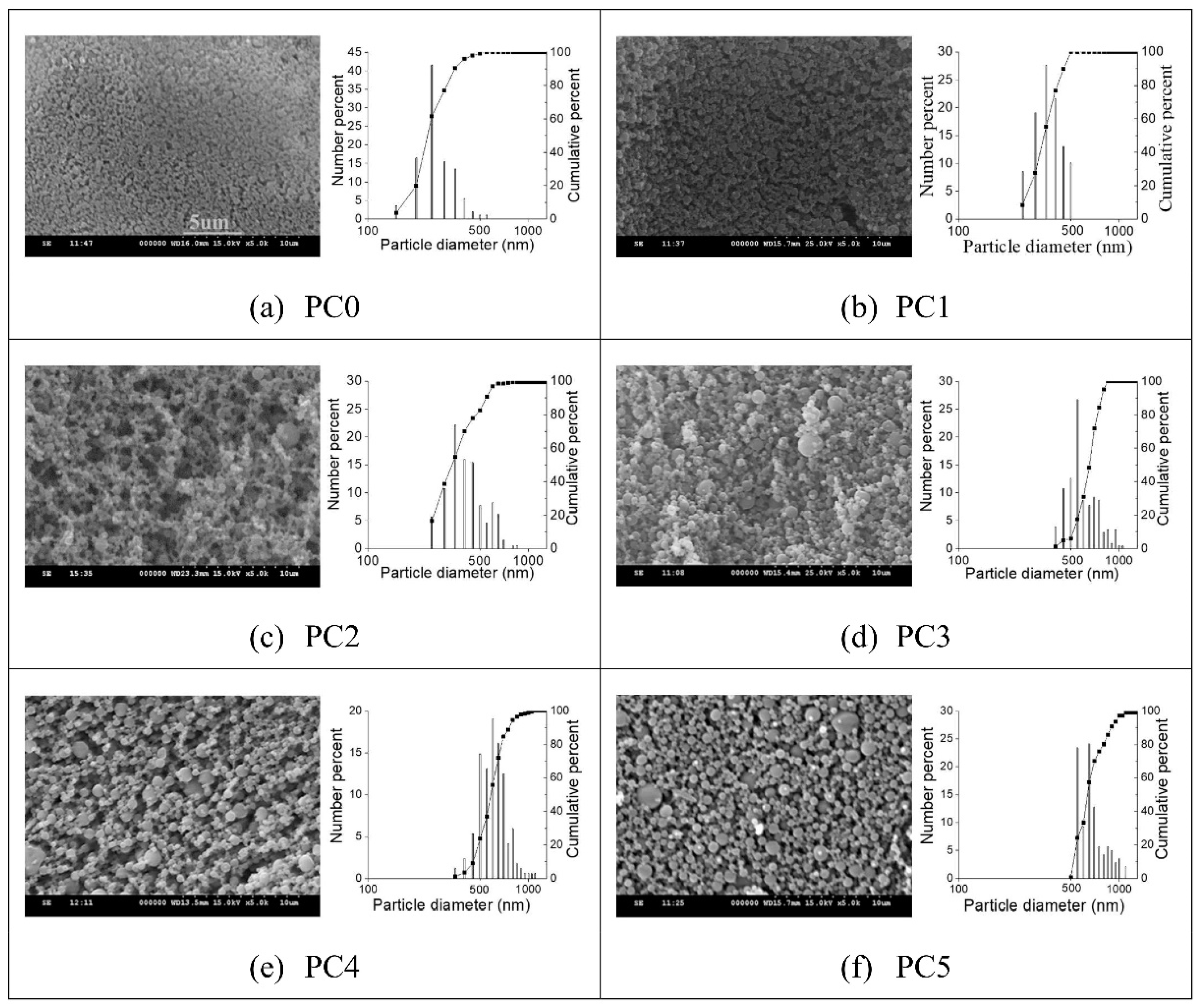
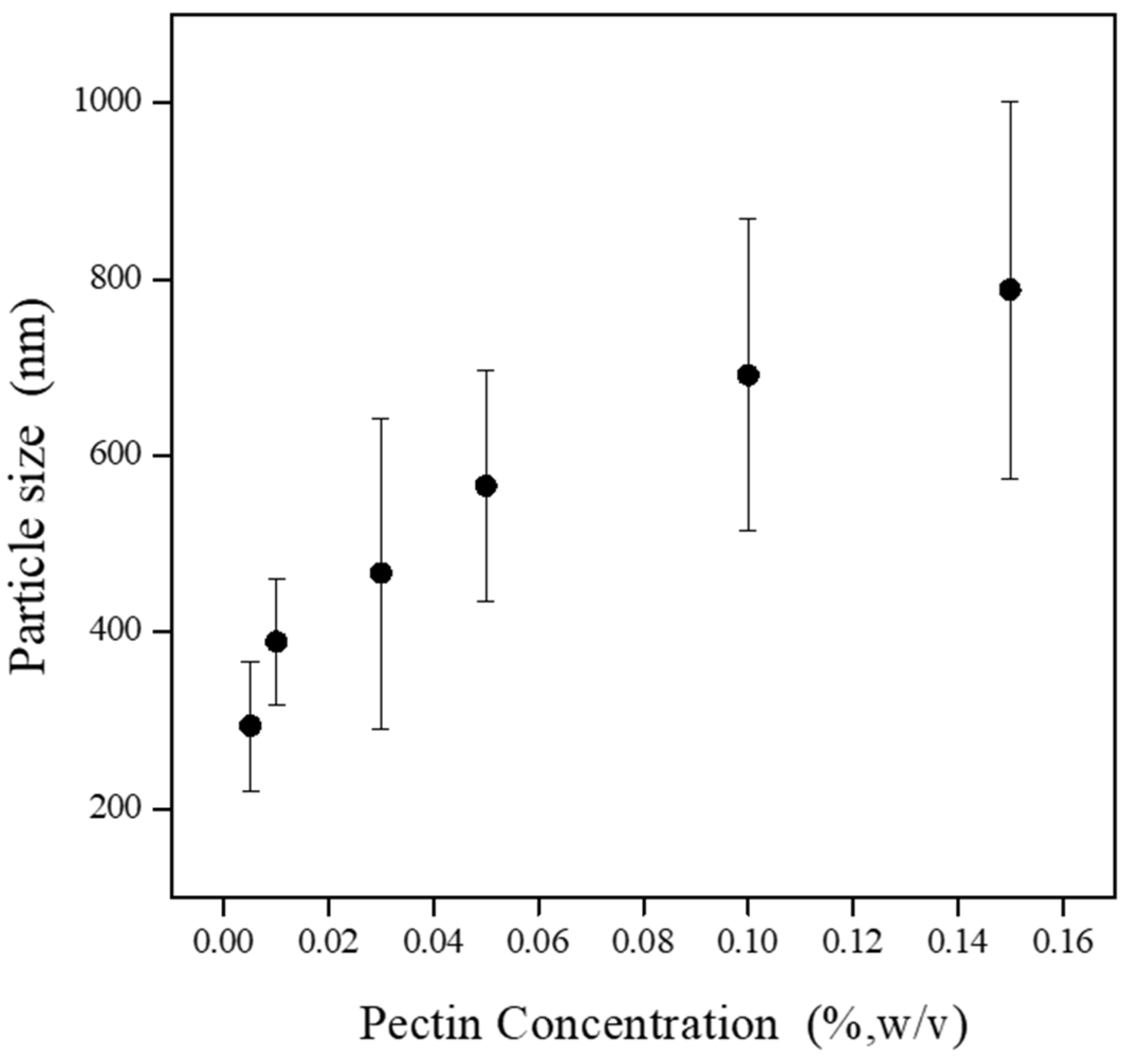


| Sample | Pectin (mg/mL) | CHC (mg/mL) | Dn (nm) | DVM (nm) | G.S.D. |
|---|---|---|---|---|---|
| PC0 | 0.05 | 0.01 | 294 (±73) | 296 (±95) | 1.38 |
| PC1 | 0.1 | 0.02 | 389 (±72) | 384 (±127) | 1.25 |
| PC2 | 0.3 | 0.06 | 467 (±176) | 485 (±144) | 1.52 |
| PC3 | 0.5 | 0.1 | 566 (±115) | 537 (±176) | 1.31 |
| PC4 | 1 | 0.2 | 691 (±177) | 645 (±191) | 1.19 |
| PC5 | 1.5 | 0.3 | 788 (±214) | 763 (±225) | 1.31 |
| Sample | Pectin (mg/mL) | CHC (mg/mL) | CaCl2 (mg/mL) | Dn (nm) | DVM (nm) | G.S.D. | Zeta Potential (mV) | LC (%) | EE (%) |
|---|---|---|---|---|---|---|---|---|---|
| PT0 | 0.5 | 0 | 0 | 553 (±248) | 558 (±161) | 1.70 | −16.3 | 23 | 82 |
| PT1 | 0.5 | 0.1 | 0 | 617 (±166) | 641 (±182) | 1.33 | −13.6 | 23 | 92 |
| PT2 | 0.5 | 0.2 | 0 | 648 (±269) | 633 (±174) | 1.39 | −8.40 | 19 | 86 |
| PT3 | 0.5 | 0 | 0.1 | 502 (±128) | 503 (±160) | 1.27 | −8.50 | 23 | 92 |
| Sample | RTH (30 min) | RTH (180 min) | t0.6 (mins) | k | n | R2 |
|---|---|---|---|---|---|---|
| PT0 | 0.61 | 0.73 | 28.5 | 0.0615 | 0.706 | 0.9804 |
| PT1 | 0.44 | 0.71 | 48.5 | 0.0438 | 0.681 | 0.9852 |
| PT2 | 0.36 | 0.72 | 55.0 | 0.0068 | 1.141 | 0.9933 |
| PT3 | 0.38 | 0.66 | 66.5 | 0.0304 | 0.732 | 0.9887 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-C.; Hu, C.-C.; Li, C.-Y.; Li, S.-C.; Cai, Z.-W.; Wei, Y.; Don, T.-M. Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer. Polymers 2022, 14, 4538. https://doi.org/10.3390/polym14214538
Cheng K-C, Hu C-C, Li C-Y, Li S-C, Cai Z-W, Wei Y, Don T-M. Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer. Polymers. 2022; 14(21):4538. https://doi.org/10.3390/polym14214538
Chicago/Turabian StyleCheng, Kuo-Chung, Chia-Chien Hu, Chih-Ying Li, Shih-Chi Li, Zhi-Wei Cai, Yang Wei, and Trong-Ming Don. 2022. "Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer" Polymers 14, no. 21: 4538. https://doi.org/10.3390/polym14214538
APA StyleCheng, K.-C., Hu, C.-C., Li, C.-Y., Li, S.-C., Cai, Z.-W., Wei, Y., & Don, T.-M. (2022). Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer. Polymers, 14(21), 4538. https://doi.org/10.3390/polym14214538







