Synergistic Effect of Two Plasticizers on Thermal Stability, Transparency, and Migration Resistance of Zinc Arginine Stabilized PVC
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVC Sheets
2.3. Evaluation of PVC Sheets
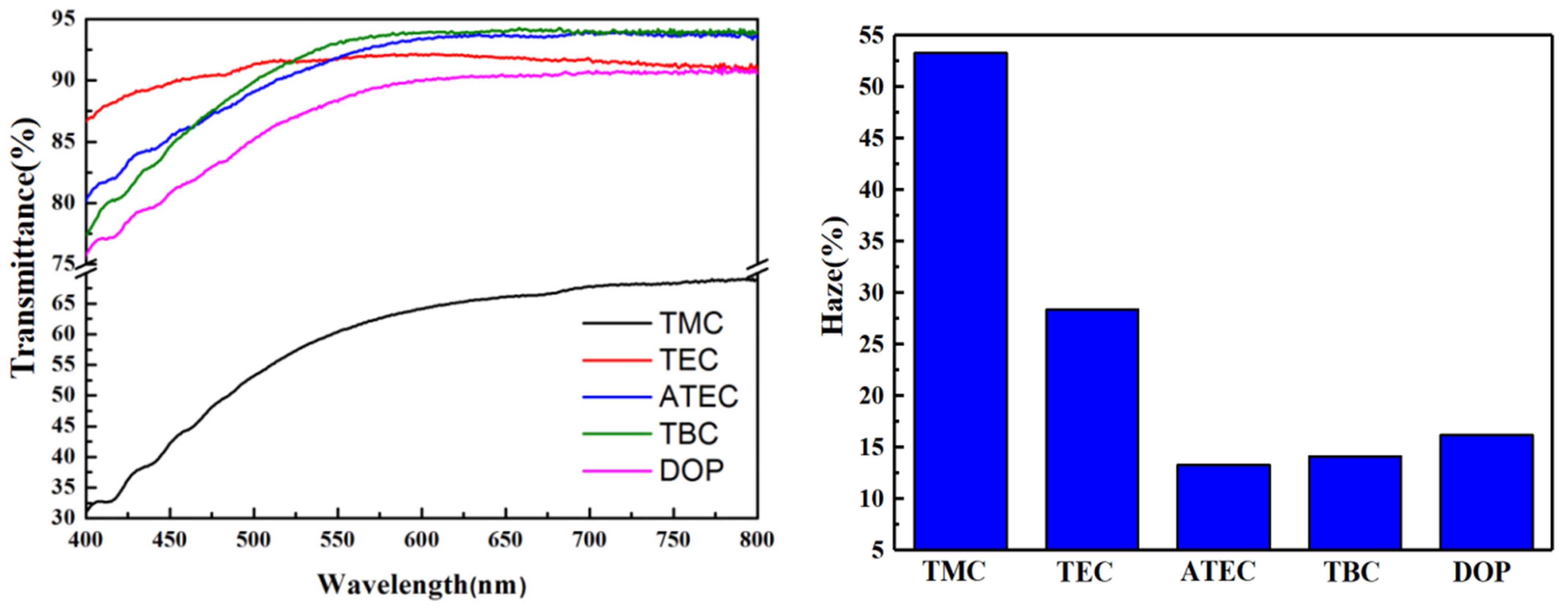
2.3.1. Transparency
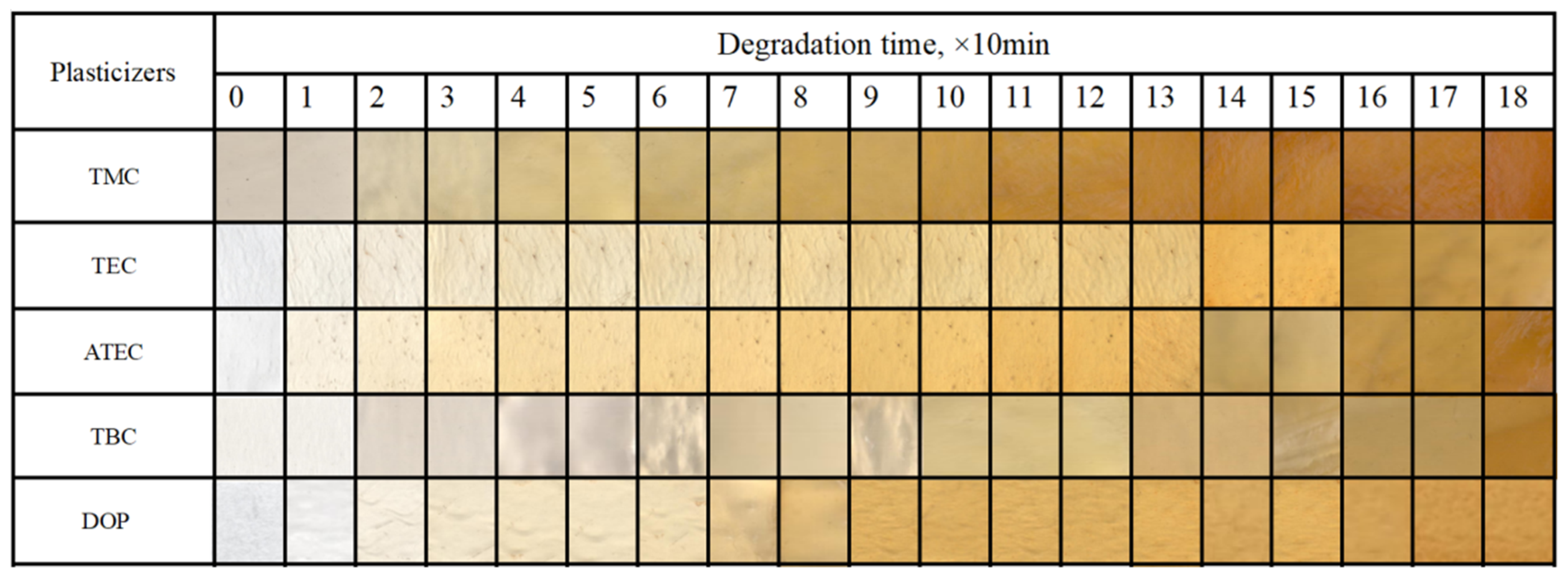
2.3.2. Static Thermal Stability
2.3.3. Mechanical Properties
2.3.4. Glass Transition Temperature
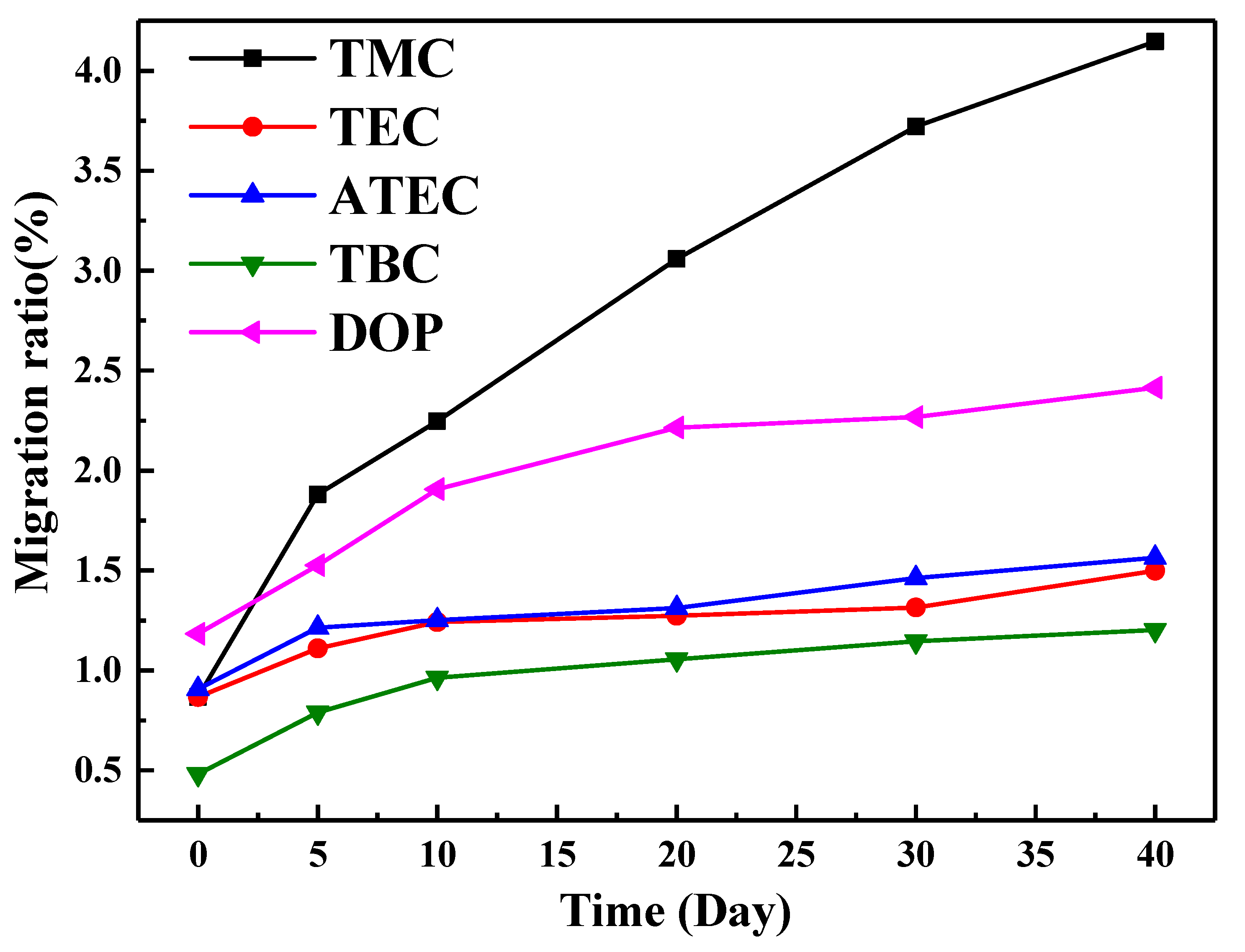
2.3.5. Migration Ratio of Zn(Arg)2
3. Results and Discussion
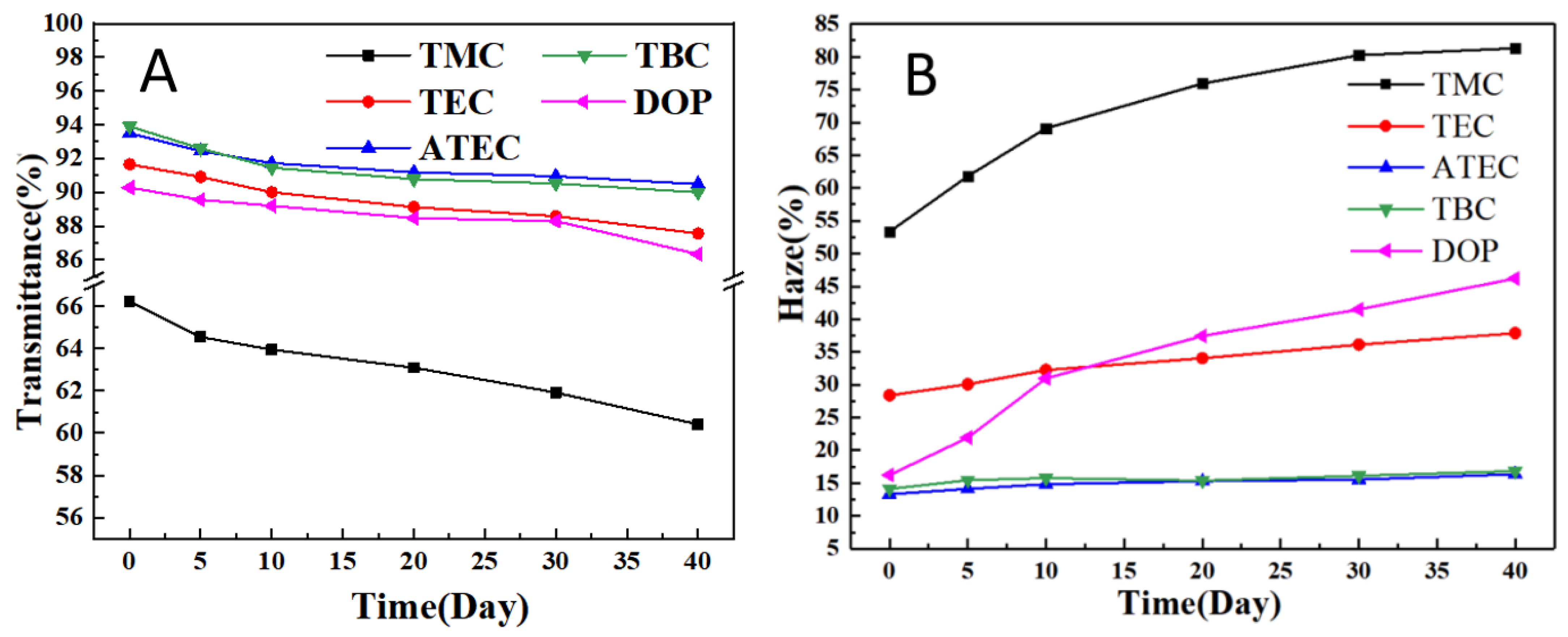
3.1. Effect of Citric Acid Esters on the Properties of PVC Stabilized by Zn(Arg)2
3.2. Calculation of Compatibility between Plasticizers and PVC
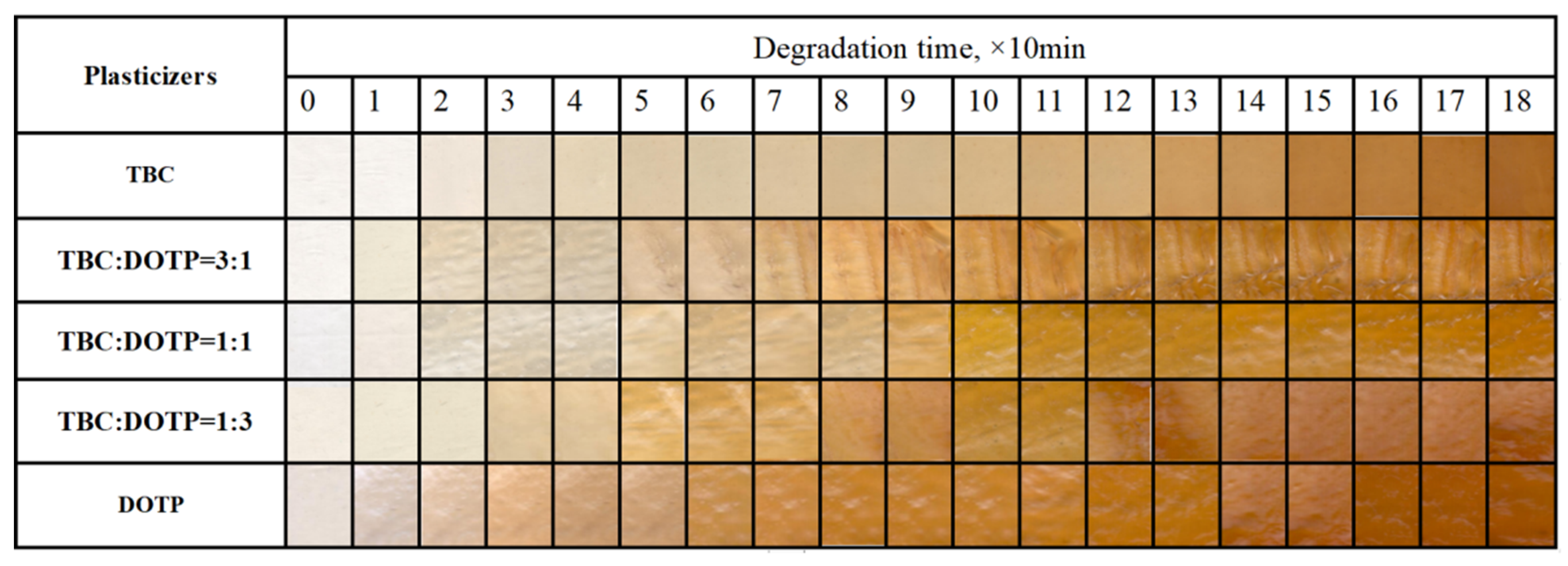
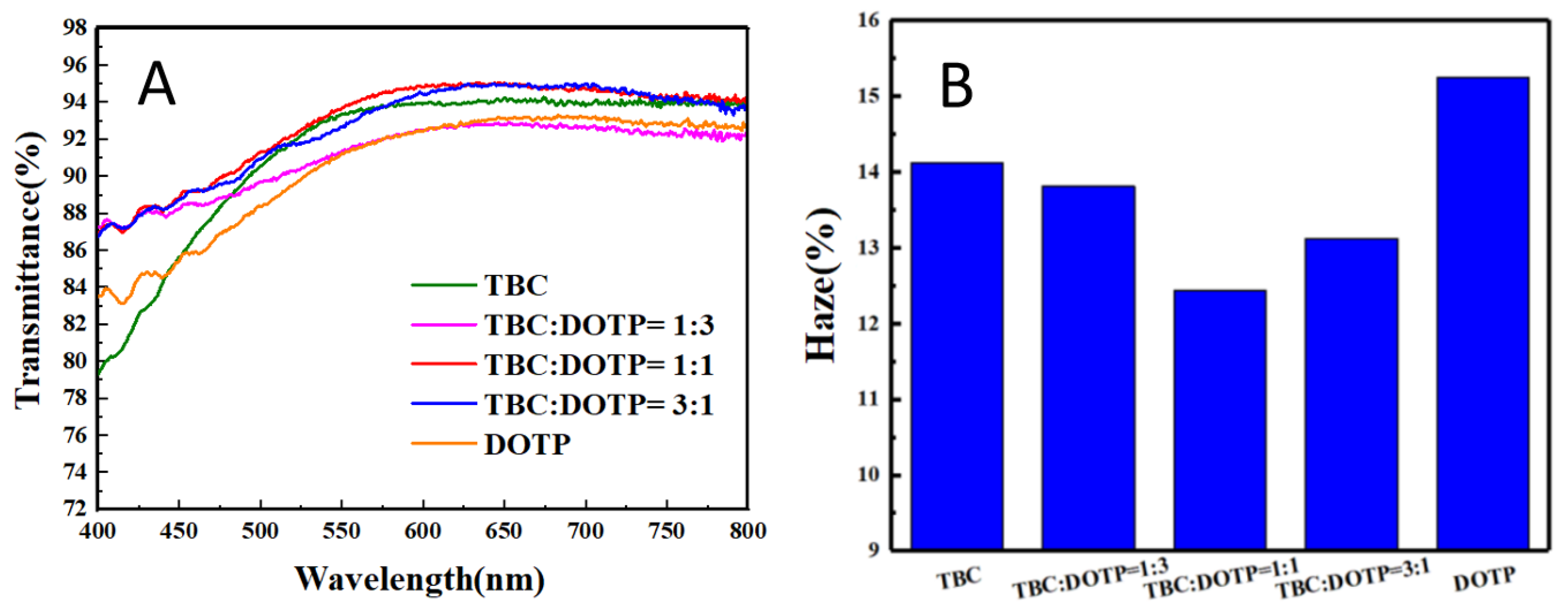
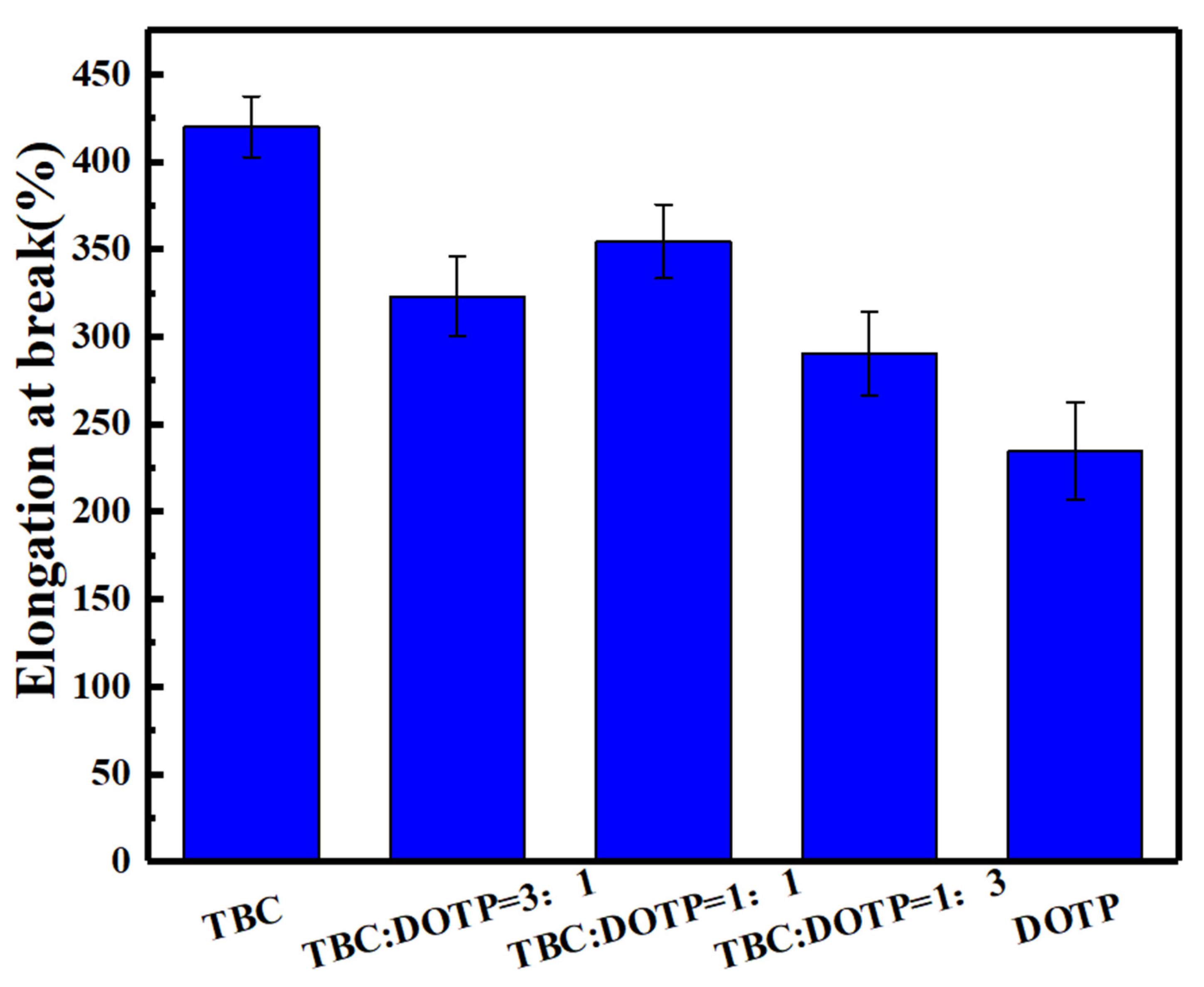
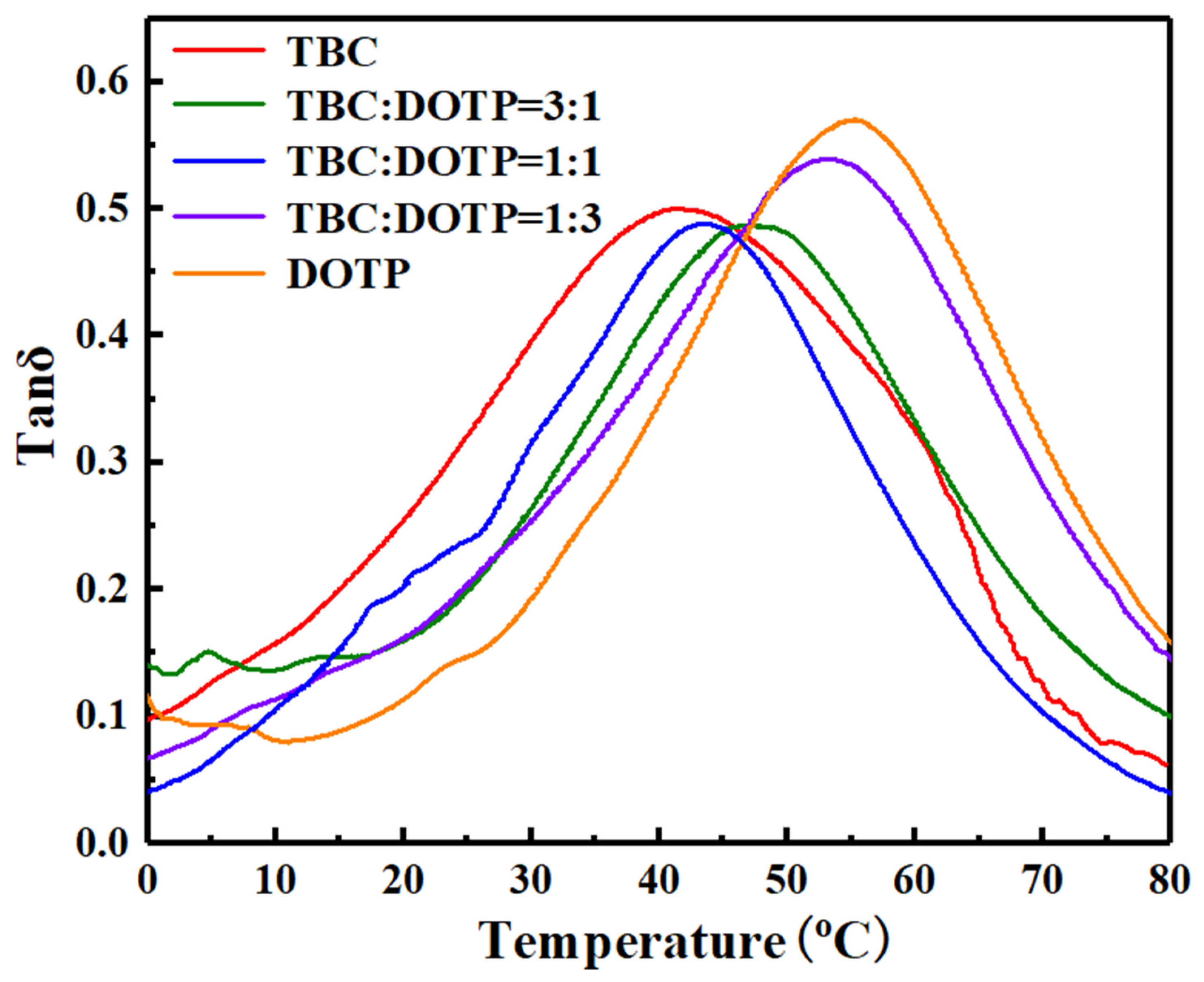
3.3. Synergistic Effect of Two Plasticizers for PVC Stabilized by Zn(Arg)2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chai, R.; Chen, S.; Zhang, J. Combined effect of hindered amine light stabilizer and ultraviolet absorbers on photodegradation of poly(vinyl chloride). J. Vinyl. Addit. Technol. 2012, 18, 17–25. [Google Scholar] [CrossRef]
- Moulay, S. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Guo, Y.; Leroux, F.; Tian, W.; Li, D.; Tang, P.; Feng, Y. Layered double hydroxides as thermal stabilizers for Poly(vinyl chloride): A review. Appl. Clay Sci. 2021, 211, 106198. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Zhang, K.; Ge, Z. Facile synthesis of exfoliated vermiculite nanosheets as a thermal stabilizer in polyvinyl chloride resin. RSC Adv. 2019, 9, 19675–19679. [Google Scholar] [CrossRef]
- Hazer, B.; Ashby, R.D. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2020, 344, 128644. [Google Scholar] [CrossRef]
- Hazer, B.; Ashby, R.D. Synthesis of poly vinyl chloride/chlorinated polypropylene-active natural substance derivatives for potential packaging materials application. Tannic acid, menthol and lipoic acid. Food Chem. 2022, 403, 134475. [Google Scholar] [CrossRef]
- Starnes, W.H. Overview and assessment of recent research on the structural defects in poly(vinyl chloride). Polym. Degrad. Stab. 2012, 97, 1815–1821. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Asawakosinchai, A.; Jubsilp, C.; Mora, P.; Rimdusit, S. Organic Heat Stabilizers for Polyvinyl Chloride (PVC): A Synergistic Behavior of Eugenol and Uracil Derivative. J. Mater. Eng. Perform. 2017, 26, 4781–4788. [Google Scholar] [CrossRef]
- Kalouskova, R.; Novotna, M.; Vymazal, Z. Investigation of thermal stabilization of poly(vinyl chloride) by lead stearate and its combination with synthetic hydrotalcite. Polym. Degrad. Stab. 2004, 85, 903–909. [Google Scholar] [CrossRef]
- George, A.S. A review of the migration of food-contact organotin stabilizers from poly(vinyl chloride). Polymer 1982, 9, 1385–1387. [Google Scholar]
- Balköse, D.; Gökçel, H.İ.; Göktepe, S.E. Synergism of Ca/Zn soaps in poly(vinyl chloride) thermal stability. Eur. Polym. J. 2001, 37, 1191–1197. [Google Scholar] [CrossRef]
- Vrandečić, N.S.; Klarić, I.; Roje, U. Effect of Ca/Zn stabiliser on thermal degradation of poly(vinyl chloride)/chlorinated polyeth—Ylene blends. Polym. Degrad. Stab. 2001, 74, 203–212. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, L.; Fang, Y.; Zhang, H.; Chen, D.; Xu, K.; Chen, M. Hydroxylbenzylthioethers as novel organic thermal stabi—Lizers for rigid PVC. Polym. Degrad. Stab. 2007, 92, 503–508. [Google Scholar] [CrossRef]
- Santamaria, E. New Insights into the Degradation Mechanism of Poly(vinyl chloride), Part II. Limited Polyene Sequence Length during Degradation and Synergism between Costabilizers. J. Appl. Polym. Sci. 2004, 93, 2744–2763. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, Z.; Rong, Y.; Deng, L.; Wu, J. A zinc Schiff base complex as high—Efficiency stabilizer for flexible poly(vinyl chloride) against thermal degradation. J. Vinyl. Addit. Technol. 2021, 27, 367–375. [Google Scholar] [CrossRef]
- Han, W.; Zhang, M.; Kong, Y.; Li, D.; Liu, L.; Tang, S.; Ding, J.; Liu, S. Pentaerythritol stearate ester-based tin (II) metal alkoxides: A tri-functional organotin as poly (vinyl chloride) thermal stabilizers. Polym. Degrad. Stab. 2020, 175, 109129. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.; Xia, J.; Li, S.; Li, M. Phosphate ester groups-containing ricinoleic acid-based Ca/Zn: Preparation and application as novel thermal stabilizer for PVC. J. Appl. Polym. Sci. 2018, 135, 45940. [Google Scholar] [CrossRef]
- Ureta, E.; Cantum, E. Zinc Maleate and Zinc Anthranilate as Thermal Stabilizers for PVC. J. Appl. Polym. Sci. 2000, 77, 2603–2605. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A.; Fahmy, M.M.; Ahmed, M.H. Thermally stable antimicrobial PVC/maleimido phenyl urea composites. Polym. Bull. 2014, 71, 2833–2849. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Yu, X.; Zhang, Y.; Yu, Y.; Zhou, M.; Tang, S. Study on pentaerythritol-zinc as a novel thermal stabilizer for rigid poly(vinyl chloride). J. Appl. Polym. Sci. 2012, 126, 569–574. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Y.; Shentu, B.; Weng, Z. Preparation and characterization of zinc-mannitol complexes as PVC thermal stabilizers with high efficiency. Polym. Degrad. Stab. 2016, 133, 399–403. [Google Scholar] [CrossRef]
- Ye, F.; Ye, Q.; Zhan, H.; Ge, Y.; Ma, X.; Wang, X. Synthesis and Study of Zinc Orotate and its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride). Polymers 2019, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, S.; Tang, W.; Qu, Y.; Wang, X. Synthesis and application of uracil derivatives as novel thermal stabilizers for rigid poly(vinyl chloride). Polym. Degrad. Stab. 2013, 98, 659–665. [Google Scholar] [CrossRef]
- Chen, S.; Xu, X.; Zhang, J.; Tang, W.; Qu, Y.; Wang, X. Efficiency and mechanism for the stabilizing action of N,N’-bis(phe nylcarbamoyl)alkyldiamines as thermal stabilizers and co-stabilizers for poly(vinyl chloride). Polym. Degrad. Stab. 2014, 105, 178–184. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Shi, Y.; Wu, B.; Ma, M.; Wang, X. Novel organic antibacterial thermal stabilizers for transparent poly(vinyl chloride). J. Therm. Anal. Calorim. 2015, 122, 1435–1444. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.; Chen, S.; Wang, M.; Ma, M.; Shi, Y.; Wang, X. Bis-uracil based high efficient heat stabilizers used in super transparent soft poly(vinyl chloride). Polym. Degrad. Stab. 2018, 149, 143–151. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, S.; Ma, B.; Chen, S.; Ma, M.; He, H.; Wang, X. A new method for designing bis-uracil derivatives as highly efficient and transparent PVC thermal stabilizer with excellent migration resistance. Polym. Degrad. Stab. 2021, 186, 109504. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Lu, S.; He, H.; Ma, M.; Shi, Y.; Chen, S. A novel double agent of triazole-based zinc-containing complex which constituted Zn/Zn stabilizer system with zinc stearate as thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2019, 168, 108953. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Wang, Y.; Lu, S.; Ma, M.; Shi, Y.; Chen, S. A new theory of “two-step stabilization mechanism” for triazole-based zinc-containing complex as thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2019, 167, 86–93. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, S.; Ma, B.; Ma, M.; He, H.; Chen, S.; Wang, X. A “one stop” thermal stabilizer, zinc arginine complex, with excellent comprehensive thermal stability effect on poly(vinyl chloride). Polym. Degrad. Stab. 2019, 167, 58–66. [Google Scholar] [CrossRef]
- Pyeon, H.B.; Park, J.E.; Suh, D.H. Non-phthalate plasticizer from camphor for flexible PVC with a wide range of available temperature. Polym. Test. 2017, 63, 375–381. [Google Scholar] [CrossRef]
- Coltro, L.; Pitta, J.B.; Madaleno, E. Performance evaluation of new plasticizers for stretch PVC films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Wang, X.; Tam, K.C. UV-Absorbing Cellulose Nanocrystals as Functional Reinforcing Fillers in Poly(vinylchloride) Films. ACS Appl. Nano Mater. 2018, 1, 632–641. [Google Scholar] [CrossRef]
- Gama, N.V.; Santos, R.; Godinho, B.; Silva, R.; Ferreira, A. Methyl Acetyl Ricinoleate as Polyvinyl Chloride Plasticizer. J. Polym. Environ. 2019, 27, 703–709. [Google Scholar] [CrossRef]
- Park, C.K.; Jung, J.H.; Kim, S.H. Biopolyurethane/Diethylhexyl Phthalate Hybrid Plasticizer for Flexible Polyvinyl Chlo ride. Fiber. Polym. 2020, 21, 1180–1186. [Google Scholar] [CrossRef]
- Chu, H.Y.; Li, H.B.; Sun, X.Y.; Zhang, Y.W. Synthesis of biomass—Based plasticizer with excellent durability for high transparent polyvinyl chloride film. J. Vinyl. Addit. Technol. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Xia, J.; Ding, C.; Wang, M.; Xu, L.; Yang, X.; Huang, K. Tung oil based plasticizer and auxiliary stabilizer for poly(vinyl chloride). Mater. Des. 2017, 122, 366–375. [Google Scholar] [CrossRef]
- Mehta, B.; Kathalewar, M.; Sabnis, A. Diester Based on Castor Oil Fatty Acid as Plasticizer for Poly(vinyl chloride). J. Appl. Polym. Sci. 2014, 131, 2928–2935. [Google Scholar] [CrossRef]
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. Analytical methods for the deter mination of DEHP plasticizer alternatives present in medical devices: A review. Talanta 2014, 129, 39–54. [Google Scholar] [CrossRef]
- Venkatram, C.S. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters A Users Handbook, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 0849372488(1). [Google Scholar]
- Wieneke, J.U.; Kommoss, B.; Gaer, O.; Prykhodko, I.; Ulbricht, M. Systematic Investigation of Dispersions of Unmodified Inorganic Nanoparticles in Organic Solvents with Focus on the Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2012, 51, 327–334. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamamoto, H. Evaluation of adsorption of organic solvents to modified hydrophobic silica adsorbents based on Hansen solubility parameter. Sep. Purif. Technol. 2019, 210, 907–912. [Google Scholar] [CrossRef]
- Raynal, M.; Bouteiller, L. Organogel formation rationalized by Hansen solubility parameters. Chem. Commun. 2011, 47, 8271. [Google Scholar] [CrossRef] [PubMed]
- Takashi, S.; Sadao, A. Comparison of Hansen solubility parameter of asphaltenes extracted from bitumen produced in different geographical regions. Energ. Fuels 2014, 28, 891–897. [Google Scholar]
- Zhao, L.; Wang, Q.; Ma, K. Solubility Parameter of Ionic Liquids: A Comparative Study of Inverse Gas Chromatography and Hansen Solubility Sphere. ACS Sustain. Chem. Eng. 2019, 7, 10544–10551. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Ma, K. Determination of 3D Solubility Parameters of Raw Coal by Inverse Gas Chromatography and Hansen Solubility Parameter Software. Chin. J. Chem. Eng. 2017, 68, 4494–4499. [Google Scholar]
- Weng, M. Effect of optimization methods on Hansen solubility ellipsoids. J. Appl. Polym. Sci. 2017, 134, 44621. [Google Scholar] [CrossRef]
- Agata, Y.; Yamamoto, H. Determination of Hansen solubility parameters of ionic liquids using double-sphere type of Hansen solubility sphere method. Chem. Phys. 2018, 513, 165–173. [Google Scholar] [CrossRef]


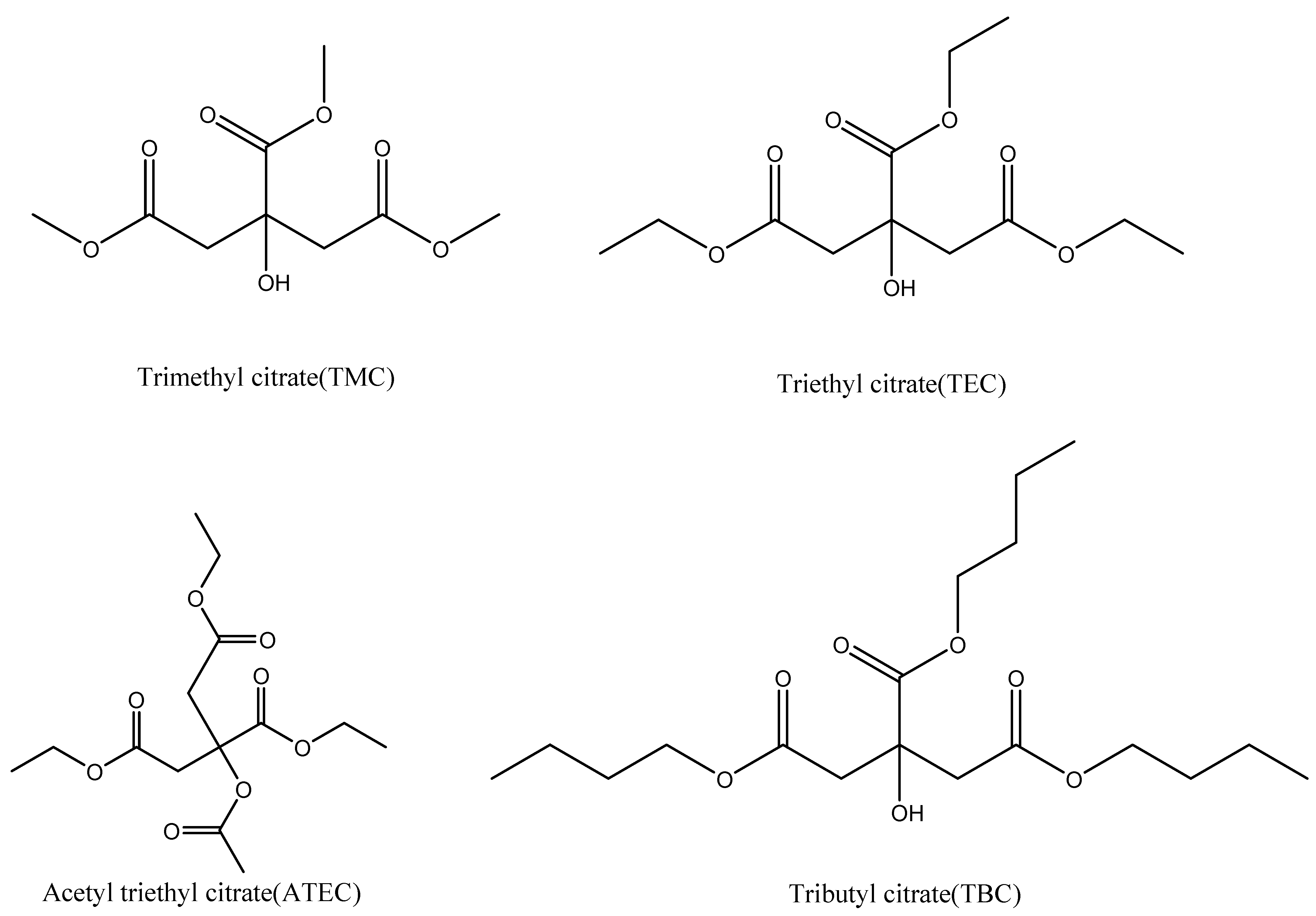





| Fdi | Fpi | Ehi | V | N | |
|---|---|---|---|---|---|
| J-CH3 | 420 | 0 | 0 | 33.5 | 3 |
| J-CH2- | 270 | 0 | 0 | 16.1 | 2 + 3n |
| J-C=O- | 290 | 770 | 2000 | 10.8 | 3 or 4 |
| J-O- | 100 | 400 | 3000 | 3.8 | 3 |
| J-OH | 210 | 500 | 20,000 | 13 | 1 |
| J-C- | −70 | 0 | 0 | −19.2 | 1 |
| δd | δp | δh | δ | Ra | Ra/Ro | |
|---|---|---|---|---|---|---|
| TMC | 18.26 | 9.30 | 14.34 | 25.01 | 11.12 | 1.36 |
| TEC | 17.93 | 7.25 | 12.65 | 23.11 | 9.29 | 1.13 |
| TBC | 17.58 | 5.03 | 10.54 | 21.10 | 7.66 | 0.93 |
| ATEC | 17.82 | 6.84 | 8.88 | 21.05 | 5.58 | 0.68 |
| DOP | 18.57 | 2.17 | 5.94 | 19.62 | 6.47 | 0.79 |
| PVC | 17.61 | 7.79 | 3.42 | 19.56 | 8.20 | — |
| Plasticizers | DOP | TBC |
|---|---|---|
| Solubility (g) | 0.40 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Yao, Y.; Lu, S.; Chen, L.; Chen, S.; He, H.; Ma, M.; Wang, X. Synergistic Effect of Two Plasticizers on Thermal Stability, Transparency, and Migration Resistance of Zinc Arginine Stabilized PVC. Polymers 2022, 14, 4560. https://doi.org/10.3390/polym14214560
Shi Y, Yao Y, Lu S, Chen L, Chen S, He H, Ma M, Wang X. Synergistic Effect of Two Plasticizers on Thermal Stability, Transparency, and Migration Resistance of Zinc Arginine Stabilized PVC. Polymers. 2022; 14(21):4560. https://doi.org/10.3390/polym14214560
Chicago/Turabian StyleShi, Yanqin, Yuchen Yao, Songyan Lu, Lukai Chen, Si Chen, Huiwen He, Meng Ma, and Xu Wang. 2022. "Synergistic Effect of Two Plasticizers on Thermal Stability, Transparency, and Migration Resistance of Zinc Arginine Stabilized PVC" Polymers 14, no. 21: 4560. https://doi.org/10.3390/polym14214560
APA StyleShi, Y., Yao, Y., Lu, S., Chen, L., Chen, S., He, H., Ma, M., & Wang, X. (2022). Synergistic Effect of Two Plasticizers on Thermal Stability, Transparency, and Migration Resistance of Zinc Arginine Stabilized PVC. Polymers, 14(21), 4560. https://doi.org/10.3390/polym14214560







