Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Fabricating TiO2-CuO Binary Composite
2.3. Synthesis of PANI@TiO2-CuO Ternary Composite
2.4. Measurements
2.5. Electrochemical Investigation
3. Results
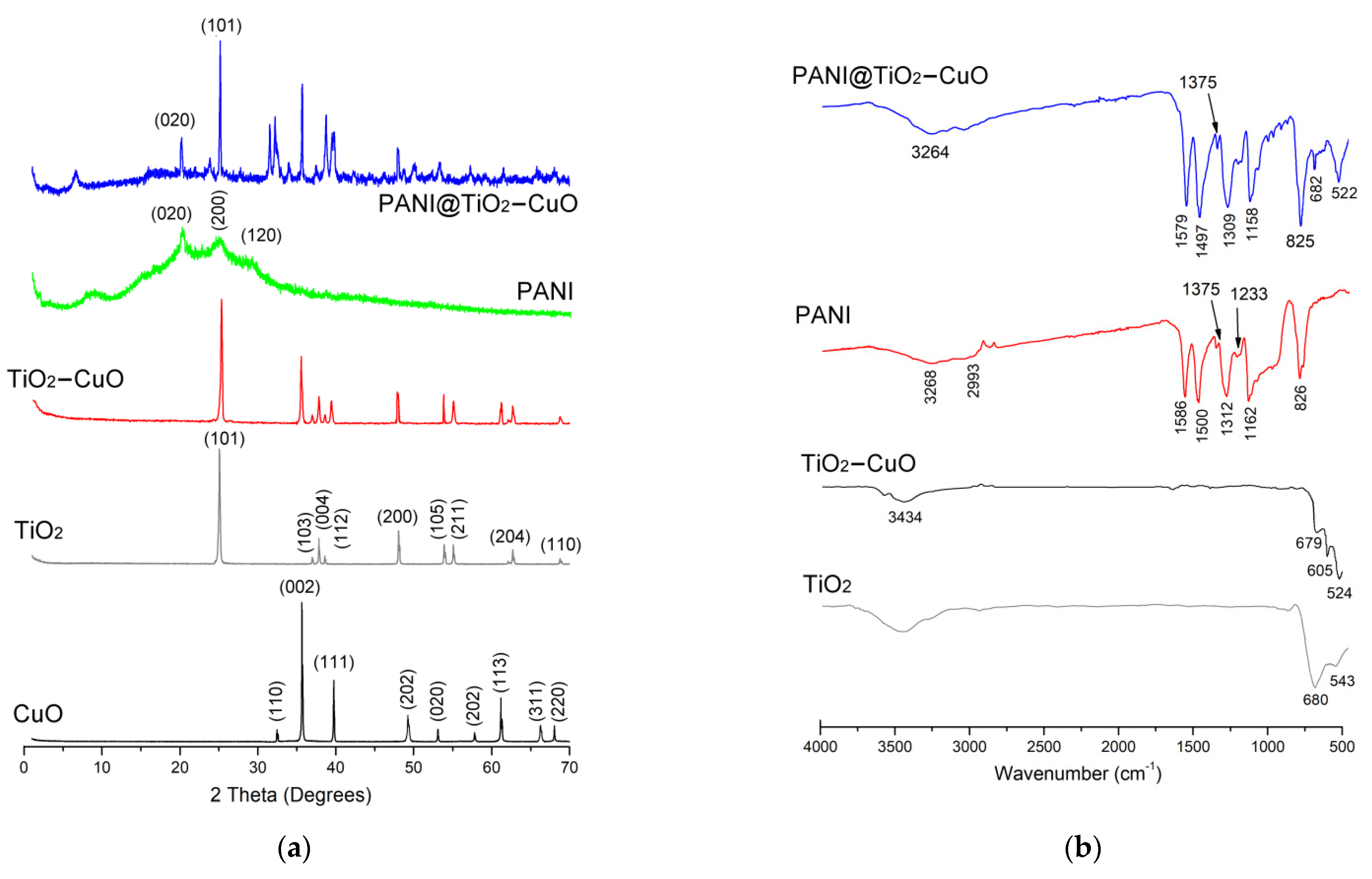
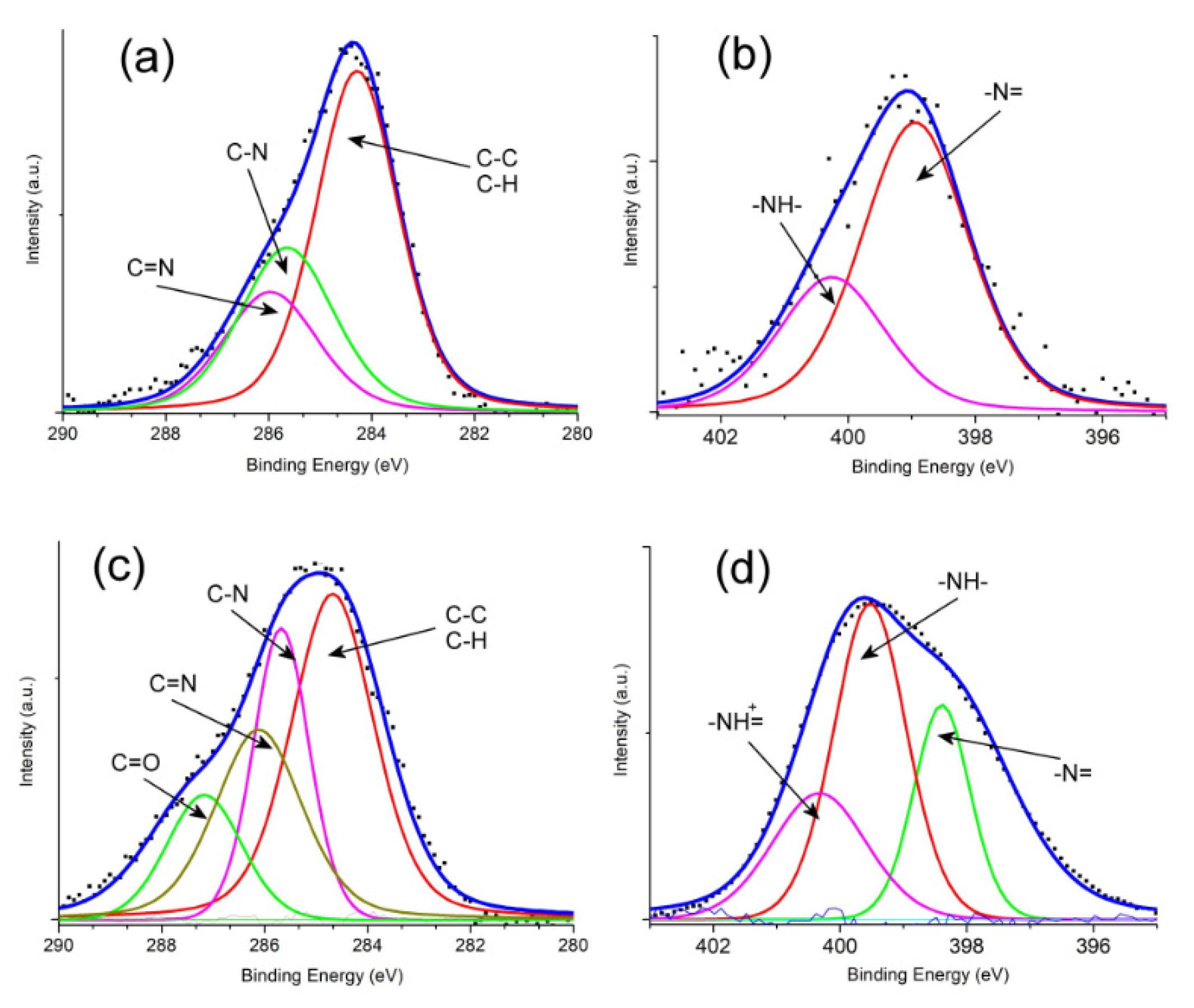
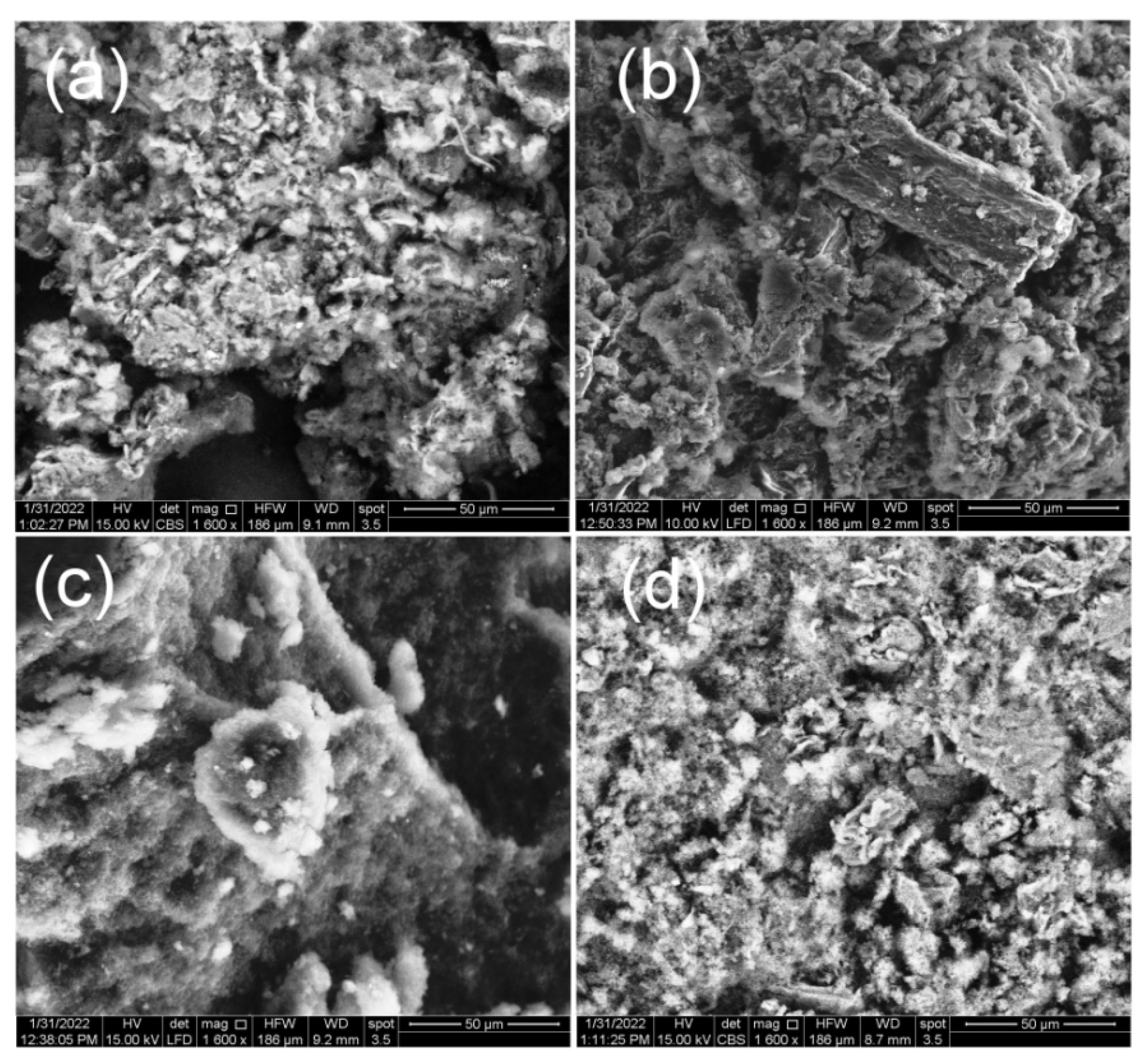
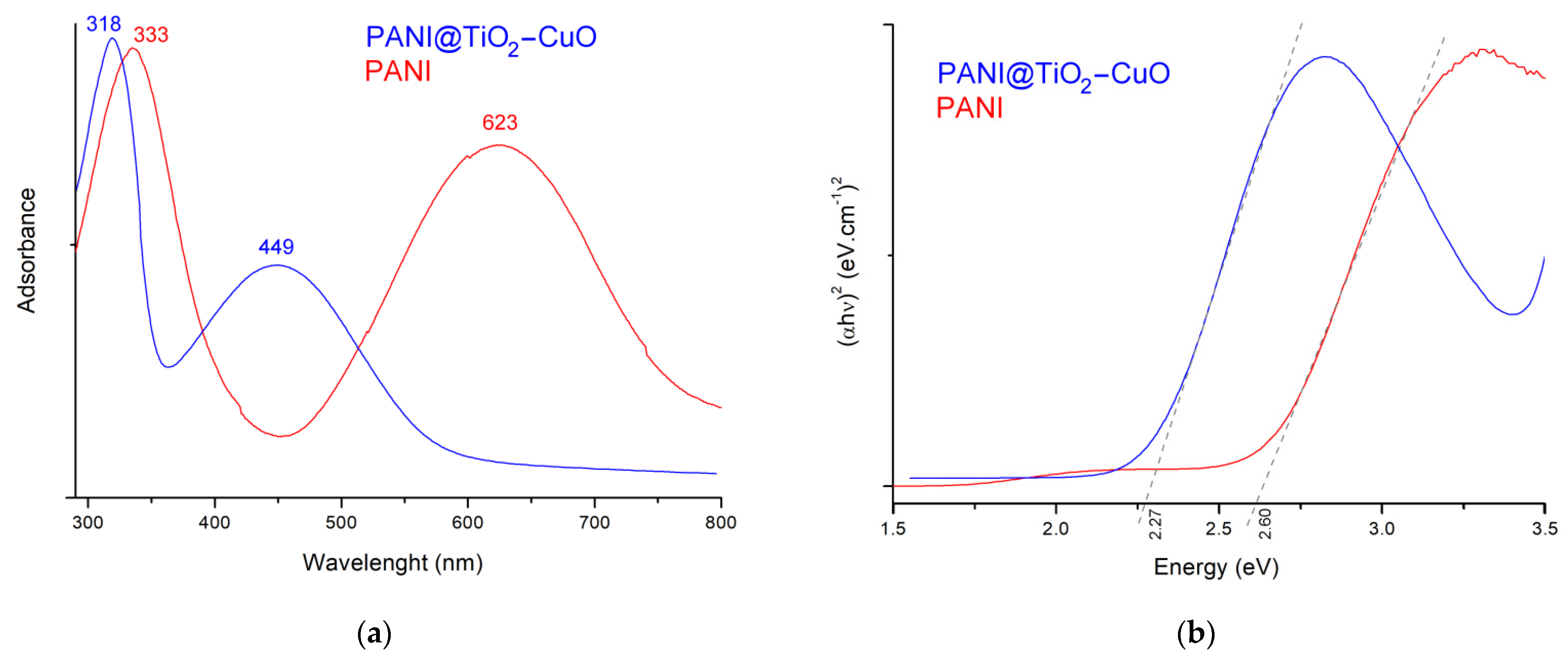
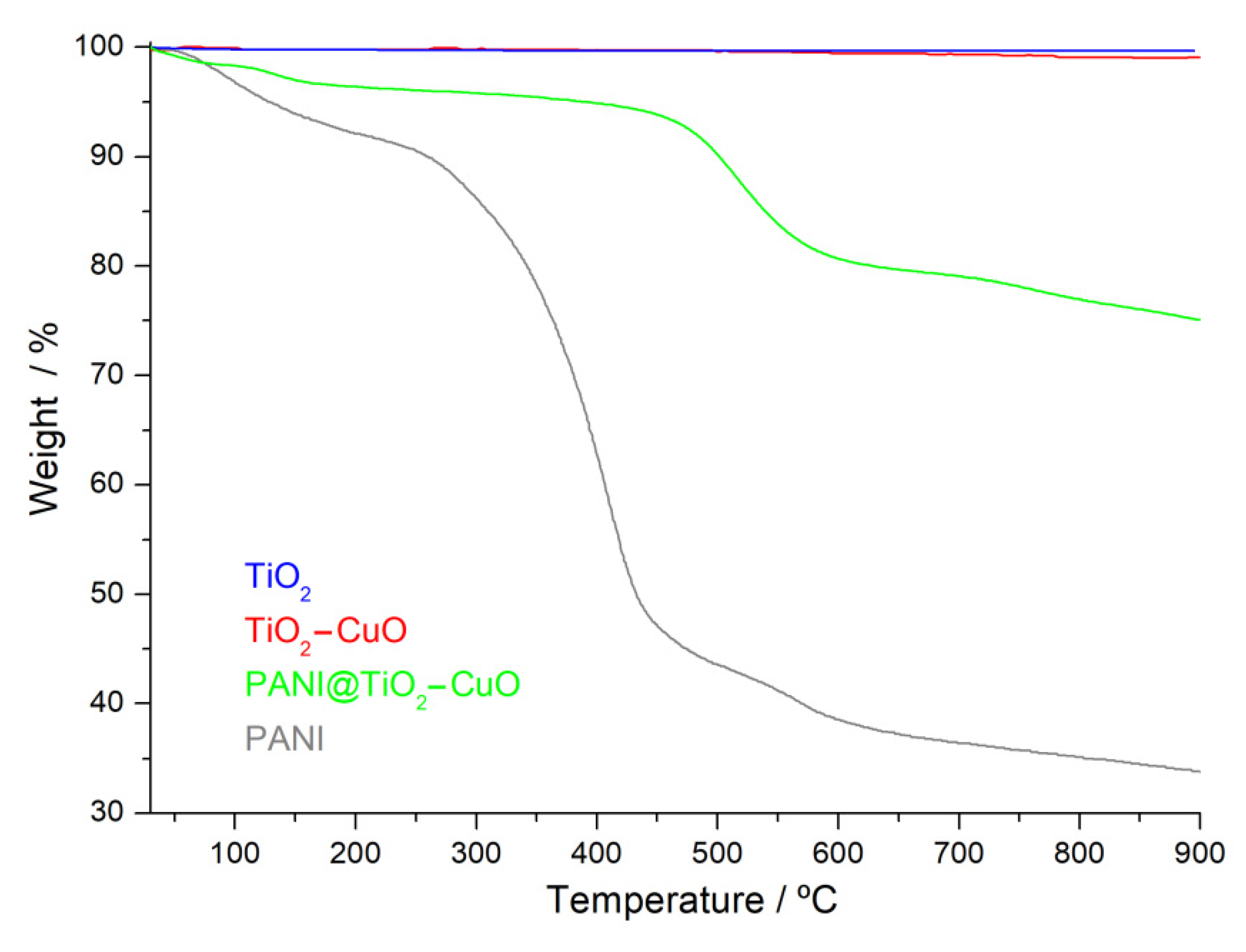
3.1. Structure and Morphology Analysis
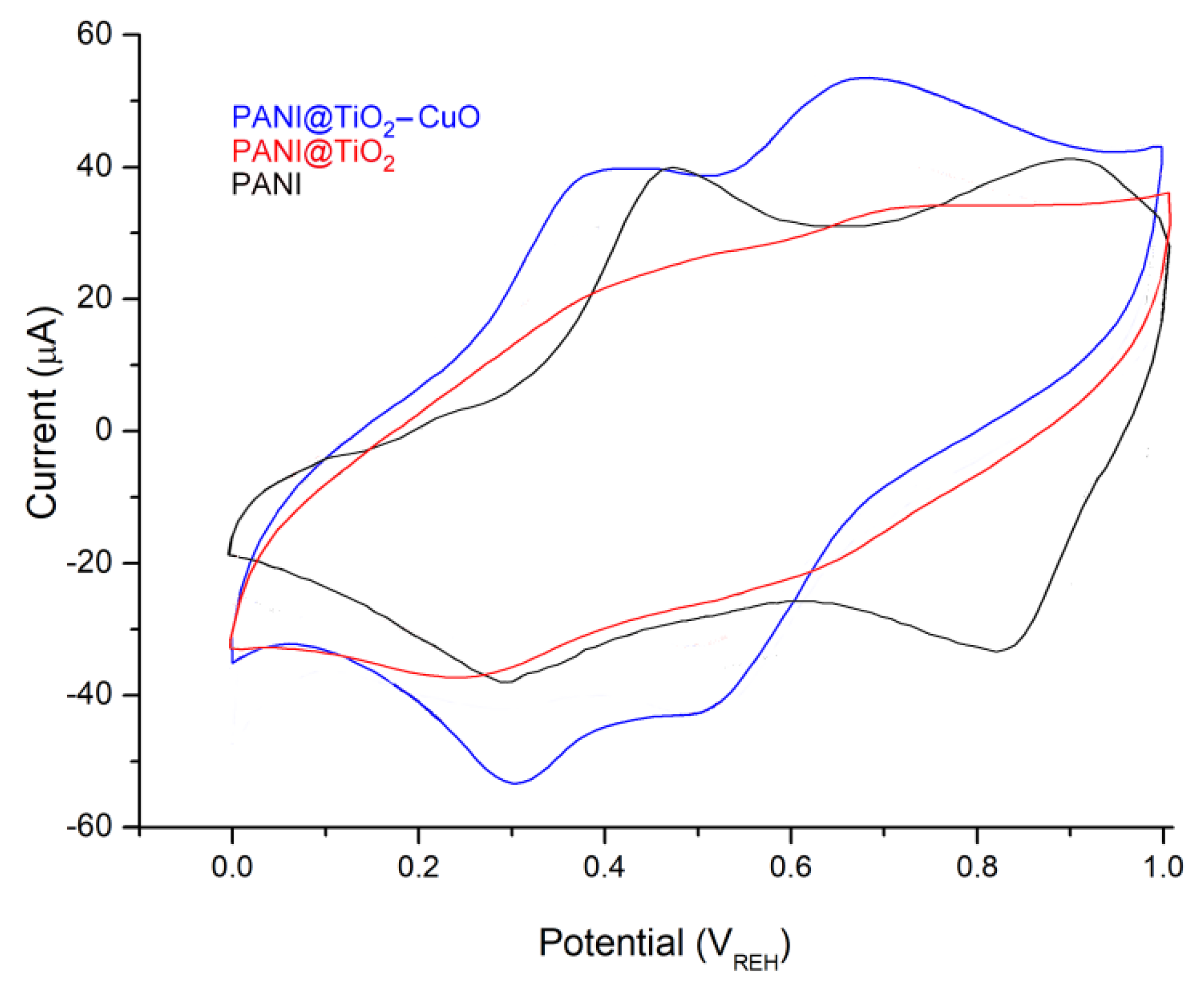
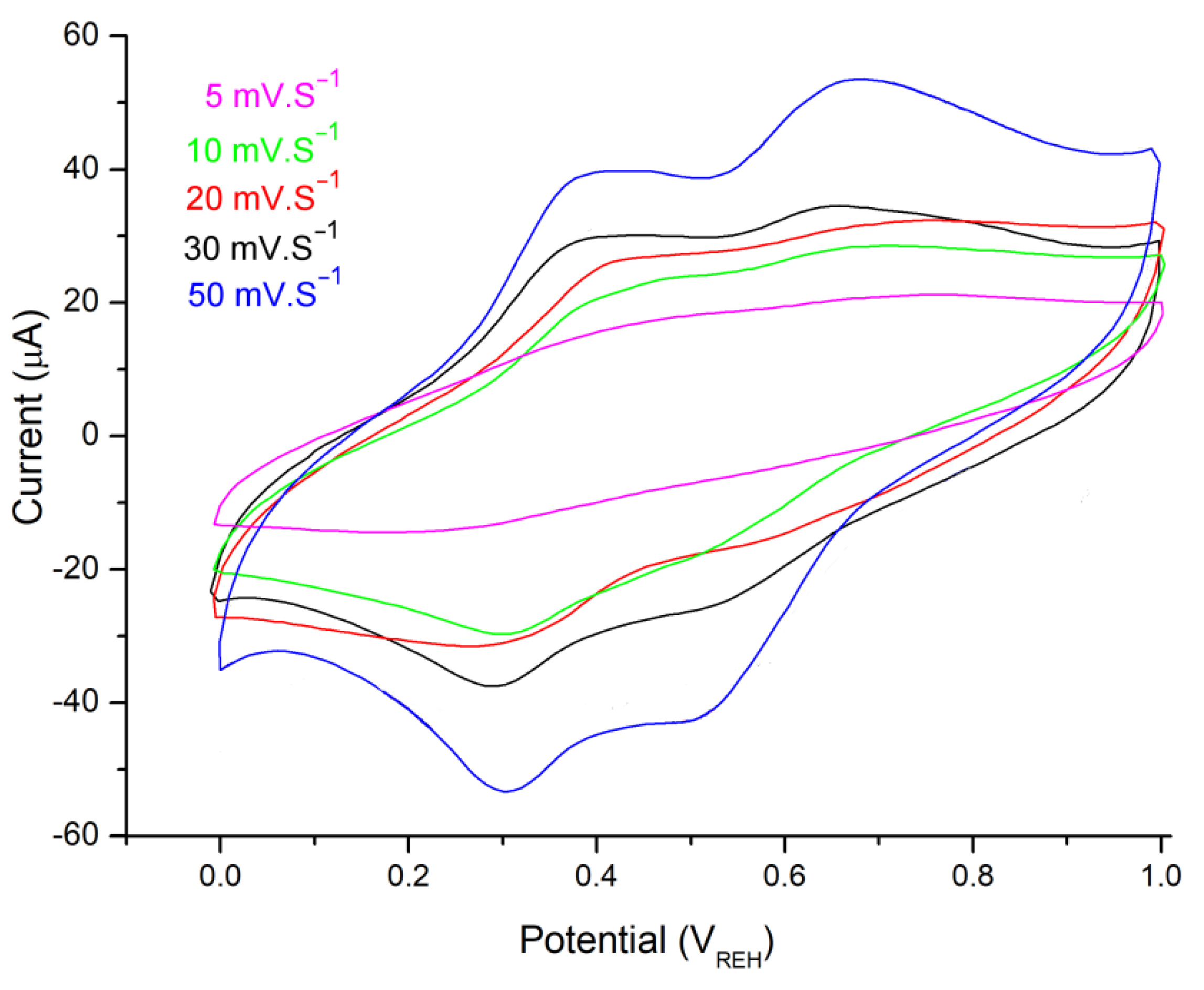
3.2. Electrochemical Properties
3.3. Galvanostatic Charge Discharge (GCD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Das, T.; Pandey, V.K.; Verma, B. Nanoarchitectonics of GO/PANI/CoFe2O4 (Graphene Oxide/polyaniline/Cobalt Ferrite) based hybrid composite and its use in fabricating symmetric supercapacitor devices. J. Mol. Struct. 2022, 1266, 133515. [Google Scholar] [CrossRef]
- Navarro, N.C.R.; Kajiura, R.; Miura, A.; Tadanaga, K. Organic–Inorganic Hybrid Materials for Interface Design in All-Solid-State Batteries with a Garnet-Type Solid Electrolyte. ACS Appl. Energy Mater. 2020, 3, 11260–11268. [Google Scholar] [CrossRef]
- Yang, L.; Pan, L.; Xiang, H.; Fei, X.; Zhu, M. Organic–Inorganic Hybrid Conductive Network to Enhance the Electrical Conductivity of Graphene-Hybridized Polymeric Fibers. Chem. Mater. 2022, 34, 2049–2058. [Google Scholar] [CrossRef]
- Maity, N.; Ghosh, R.; Nandi, A.K. Optoelectronic Properties of Self-Assembled Nanostructures of Polymer Functionalized Polythiophene and Graphene. Langmuir 2018, 34, 7585–7597. [Google Scholar] [CrossRef]
- Miyashita, R.; Goto, H. Electro-Magneto-Optically Active Polyaniline/Hydroxypropyl Cellulose Composite. ACS Appl. Polym. Mater. 2022, 4, 796–805. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Luo, X.; Cao, Y.; Gong, X.; Xu, W. Molecular Engineering of Polyaniline with Ultrathin Polydopamine and Monolayer Graphene for All-Solid-State Flexible Microsupercapacitors. ACS Appl. Energy Mater. 2021, 4, 10069–10080. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Firda, P.B.D.; Malik, Y.T.; Oh, J.K.; Wujcik, E.K.; Jeon, J.W. Enhanced Chemical and Electrochemical Stability of Polyaniline-Based Layer-by-Layer Films. Polymers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Rajkumar, S.; Ezhilarasi, J.C.; Saranya, P.; Merlin, J.P. Fabrication of CoWO4/PANI composite as electrode material for energy storage applications. J. Phys. Chem. Solids 2022, 162, 110500. [Google Scholar] [CrossRef]
- Lahreche, S.; Moulefera, I.; El Kebir, A.; Sabantina, L.; Kaid, M.; Benyoucef, A. Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers 2022, 10, 7. [Google Scholar] [CrossRef]
- Daikh, S.; Ouis, D.; Benyoucef, A.; Mouffok, B. Equilibrium, kinetic and thermodynamic studies for evaluation of adsorption capacity of a new potential hybrid adsorbent based on polyaniline and chitosan for Acetaminophen. Chem. Phys. Lett. 2022, 17, 139565. [Google Scholar] [CrossRef]
- Song, Y.; Su, Z.; Zhao, Z.; Lin, S.; Wang, D. A new As3Mo8V4/PANi/rGO composite for high performance supercapacitor electrode. Ceram. Int. 2021, 47, 21367–21372. [Google Scholar] [CrossRef]
- Maaza, L.; Djafri, F.; Belmokhtar, A.; Benyoucef, A. Evaluation of the influence of Al2O3 nanoparticles on the thermal stability and optical and electrochemical properties of PANI-derived matrix reinforced conducting polymer composites. J. Phys. Chem. Solids 2021, 152, 109970. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers 2021, 13, 1360. [Google Scholar] [CrossRef]
- Xiao, T.; Zhao, J.; Wang, X.; Li, Z.; Song, M.; Li, H.; Wang, X. Preparation and performance of PANI-TiO2 nanotube arrays composite electrode by in-situ microcavity polymerization. Mater. Chem. Phys. 2020, 240, 122179. [Google Scholar] [CrossRef]
- Shao, Z.; Li, H.; Li, M.; Li, C.; Qu, C.; Yang, B. Fabrication of polyaniline nanowire/TiO2 nanotube array electrode for supercapacitors. Energy 2015, 87, 578–585. [Google Scholar] [CrossRef]
- Belhadj, H.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Effects of Incorporating Titanium Dioxide with Titanium Carbide on Hybrid Materials Reinforced with Polyaniline: Synthesis, Characterization, Electrochemical and Supercapacitive Properties. Fibers 2022, 10, 46. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Patil, S.V.; Bulakhe, R.N.; Pusawale, S.N.; Shim, J.J.; Lokhande, C.D. Chemical synthesis of PANI–TiO2 composite thin film for supercapacitor application. RSC Adv. 2015, 5, 68939–68946. [Google Scholar] [CrossRef]
- Gottam, R.; Srinivasan, P.; Keloth, B. Improving the Performance of PANI-SA⋅TiO2 Supercapacitor Active Electrode Material via Emulsion Polymerization of Aniline with MWCNT. ChemistrySelect 2020, 5, 10098–10105. [Google Scholar] [CrossRef]
- Yoruk, O.; Bayrak, Y.; Ates, M. Design and assembly of supercapacitor based on reduced graphene oxide/TiO2/polyaniline ternary nanocomposite and its application in electrical circuit. Polym. Bull. 2022, 79, 2969–2993. [Google Scholar] [CrossRef]
- Kumar, N.; Joshi, N.C. Adsorption applications of synthetically prepared PANI-CuO based nanocomposite material. J. Indian Chem. Soc. 2022, 99, 100551. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of hollow CuO/PANI hybrid nanofibers for non-enzymatic electrochemical detection of H2O2 and glucose. Sens. Actuators B Chem. 2019, 286, 370–376. [Google Scholar] [CrossRef]
- Nekooie, R.; Shamspur, T.; Mostafavi, A. Novel CuO/TiO2/PANI nanocomposite: Preparation and photocatalytic investigation for chlorpyrifos degradation in water under visible light irradiation. J. Photochem. Photobiol. A Chem. 2021, 407, 113038. [Google Scholar] [CrossRef]
- Pang, Z.; Nie, Q.; Lv, P.; Yu, J.; Huang, F.; Wei, Q. Design of flexible PANI-coated CuO-TiO2-SiO2 heterostructure nanofibers with high ammonia sensing response values. Nanotechnology 2017, 28, 225501. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.L.; Ung, T.D.T.; Nguyen, Q.L. Synthesis and characterization of nano-CuO and CuO/TiO2 photocatalysts. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 025002. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers 2021, 13, 3344. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Z.; Wang, Z.; Liu, Y.; Wang, X.; Qiu, J. Dually Fixed SnO2 Nanoparticles on Graphene Nanosheets by Polyaniline Coating for Superior Lithium Storage. ACS Appl. Mater. Interfaces 2015, 7, 2444–2451. [Google Scholar] [CrossRef]
- Rathore, B.S.; Chauhan, N.P.S.; Jadoun, S.; Ameta, S.C.; Ameta, R. Synthesis and characterization of chitosan-polyaniline-nickel(II) oxide nanocomposite. J. Mol. Struct. 2021, 1242, 130750. [Google Scholar] [CrossRef]
- Sumana, V.S.; Sudhakarb, Y.N.; Varghese, A.; Nagaraja, G.K. Pt nanoflower-poly(aniline) electrode material with the synchronized concept of energy storage in supercapacitor. Appl. Surf. Sci. 2022, 589, 152994. [Google Scholar] [CrossRef]
- Radoičić, M.B.; Milošević, M.V.; Miličević, D.S.; Suljovrujić, E.H.; Marjanović, G.N.Ć.; Radetić, M.M.; Šaponjić, Z.V. Influence of TiO2 nanoparticles on formation mechanism of PANI/TiO2 nanocomposite coating on PET fabric and its structural and electrical properties. Surf. Coat. Technol. 2015, 278, 38–47. [Google Scholar] [CrossRef]
- Girimonte, R.; Testa, F.; Turano, M.; Leone, G.; Gallo, M.; Golemme, G. Amine-Functionalized Mesoporous Silica Adsorbent for CO2 Capture in Confined-Fluidized Bed: Study of the Breakthrough Adsorption Curves as a Function of Several Operating Variables. Processes 2022, 10, 422. [Google Scholar] [CrossRef]
- Toumi, I.; Djelad, H.; Chouli, F.; Benyoucef, A. Synthesis of PANI@ZnO Hybrid Material and Evaluations in Adsorption of Congo Red and Methylene Blue Dyes: Structural Characterization and Adsorption Performance. J. Inorg. Organomet. Polym. Mater. 2022, 32, 112–121. [Google Scholar] [CrossRef]
- Anandgaonker, P.; Kulkarni, G.; Gaikwad, S.; Rajbhoj, A. Synthesis of TiO2 nanoparticles by electrochemical method and their antibacterial application. Arab. J. Chem. 2019, 12, 1815–1822. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y. Hydrogen bond enforced polyaniline grown on activated carbon fibers substrate for wearable bracelet supercapacitor. J. Energy Storage 2022, 52, 105042. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Gleason, K.K. Recent Progress in Conjugated Conducting and Semiconducting Polymers for Energy Devices. Energies 2022, 15, 3661. [Google Scholar] [CrossRef]
- Radja, I.; Djelad, H.; Morallon, E.; Benyoucef, A. Characterization and electrochemical properties of conducting nanocomposites synthesized from p-anisidine and aniline with titanium carbide by chemical oxidative method. Synth. Met. 2015, 202, 25–32. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Fan, L.; Zhang, C.; Xiao, X.; Gogotsi, Y.; Yang, S. Foldable supercapacitors from triple networks of macroporous cellulose fibers, single-walled carbon nanotubes and polyaniline nanoribbons. Nano Energy 2015, 11, 568–578. [Google Scholar] [CrossRef]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, Y.; Zhang, M.; Zhang, L.; Li, H. Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors. Appl. Sci. 2021, 11, 8690. [Google Scholar] [CrossRef]
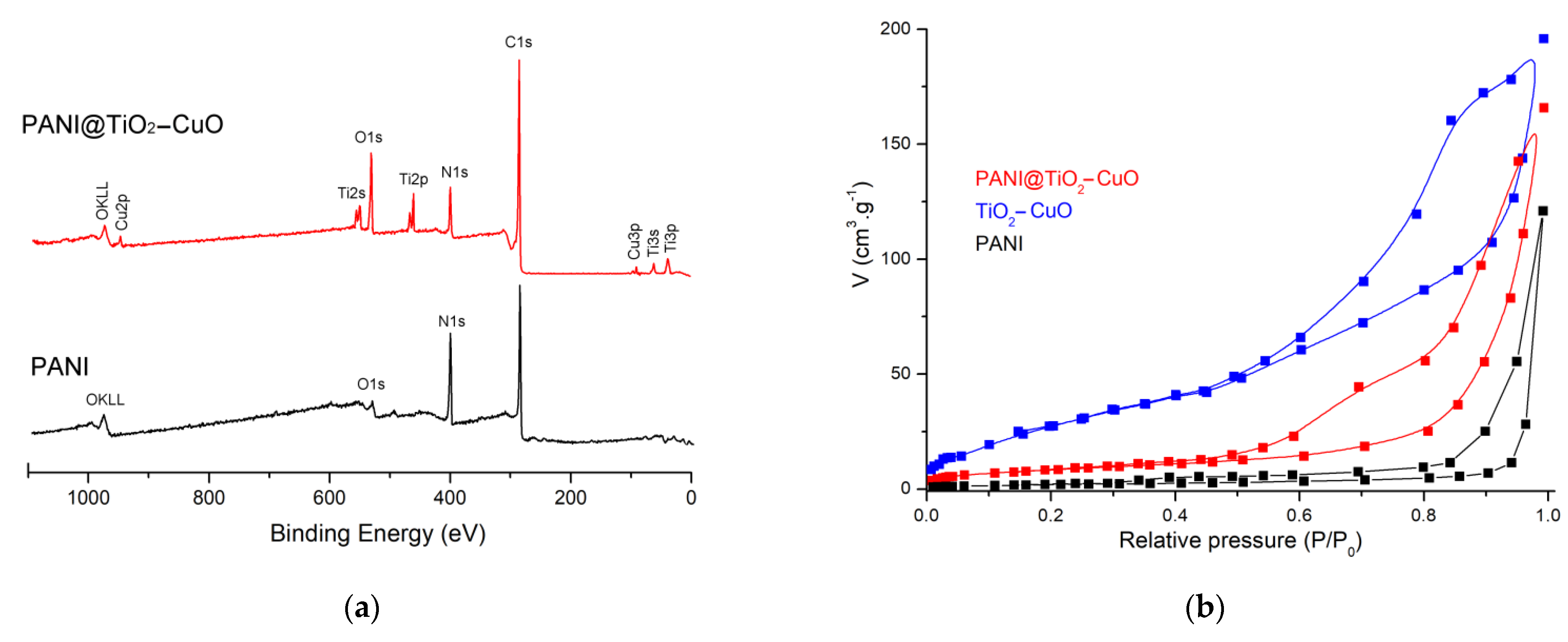

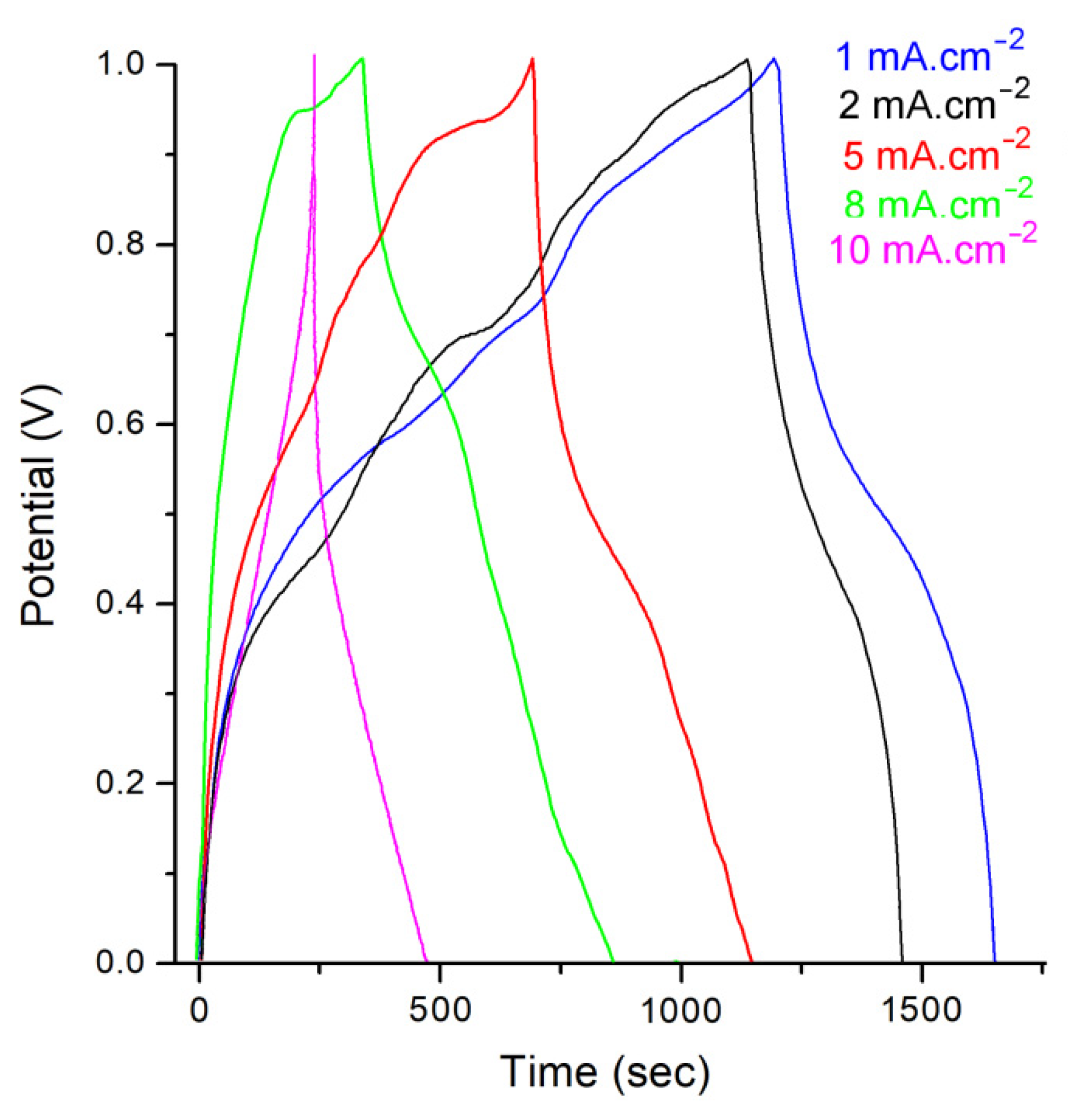
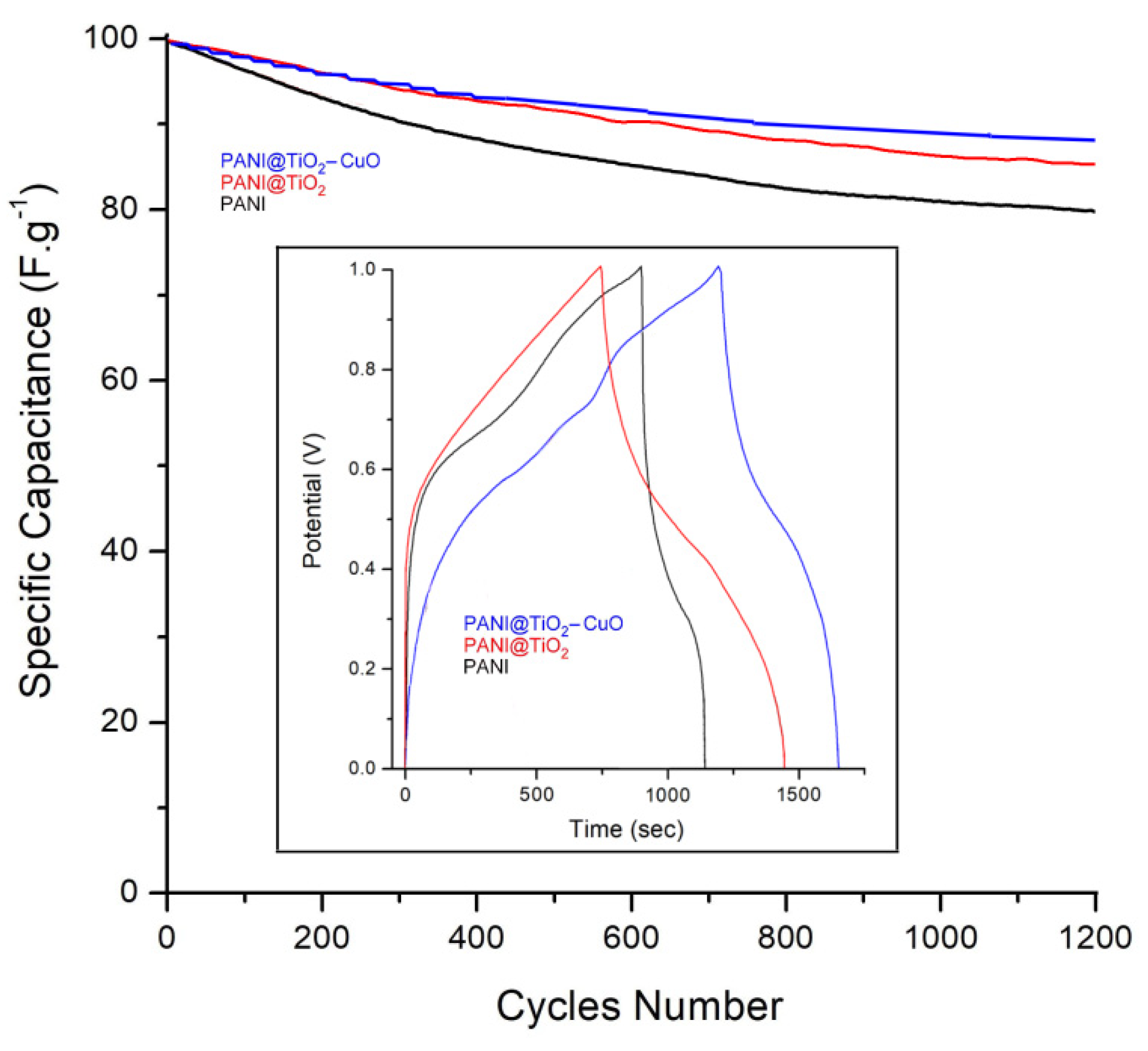
| Material | PANI | PANI@TiO2 | TiO2-CuO | PANI@TiO2-CuO |
|---|---|---|---|---|
| SBET/(m2 g−1) | 25 ± 0.5 | 53 ± 0.5 | 132 ± 0.5 | 108 ± 0.5 |
| VDR (N2)/(cm3 g−1) | 1.50 ± 0.01 | 1.32 ± 0.01 | 1.98 ± 0.01 | 1.94 ± 0.01 |
| Vmeso/(cm3 g−1) | 0.01 | 0.02 | 0.05 | 0.04 |
| Vmacro/(cm3 g−1) | 0.01 | 0.08 | 0.14 | 0.11 |
| Vtot pore/(cm3 g−1) | 0.02 | 0.10 | 0.19 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutaleb, N.; Dahou, F.Z.; Djelad, H.; Sabantina, L.; Moulefera, I.; Benyoucef, A. Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications. Polymers 2022, 14, 4562. https://doi.org/10.3390/polym14214562
Boutaleb N, Dahou FZ, Djelad H, Sabantina L, Moulefera I, Benyoucef A. Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications. Polymers. 2022; 14(21):4562. https://doi.org/10.3390/polym14214562
Chicago/Turabian StyleBoutaleb, Nadia, Fatima Zohra Dahou, Halima Djelad, Lilia Sabantina, Imane Moulefera, and Abdelghani Benyoucef. 2022. "Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications" Polymers 14, no. 21: 4562. https://doi.org/10.3390/polym14214562
APA StyleBoutaleb, N., Dahou, F. Z., Djelad, H., Sabantina, L., Moulefera, I., & Benyoucef, A. (2022). Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications. Polymers, 14(21), 4562. https://doi.org/10.3390/polym14214562









