Chitosan Modified by Kombucha-Derived Bacterial Cellulose: Rheological Behavior and Properties of Convened Biopolymer Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chi/KBC Solutions and Films
2.3. Rheological Study
2.4. Atomic Force Microscope (AFM)
2.5. Fourier-Transformed Infrared Spectroscopy (FTIR)
2.6. Thermogravimetric Analysis
2.7. Mechanical Analysis
2.8. Statistical Analysis
3. Results
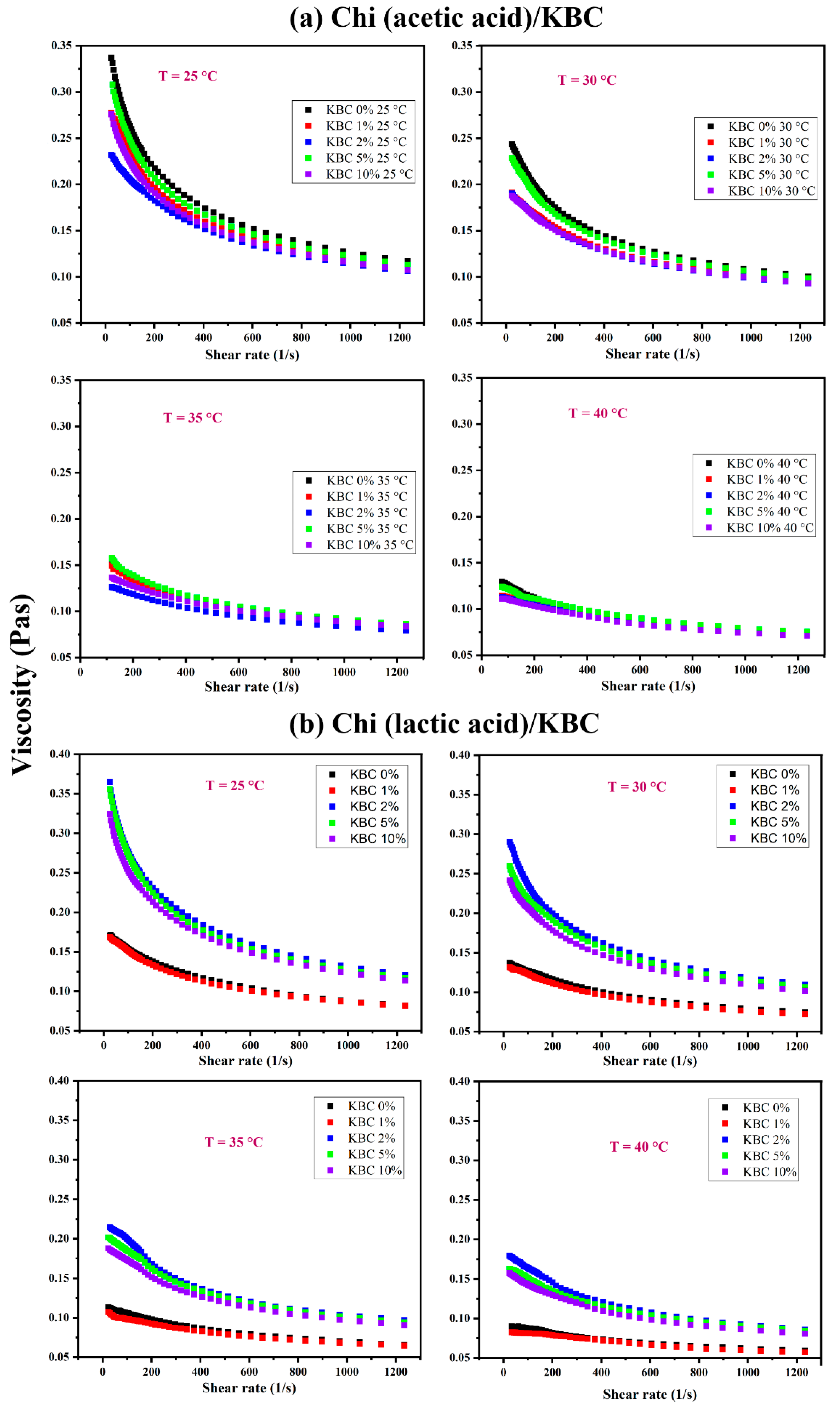
3.1. Rheological Data and Viscosity of Chi/KBC Film-Forming Solutions
3.2. Characterization of Chi/KBC Biopolymer Films
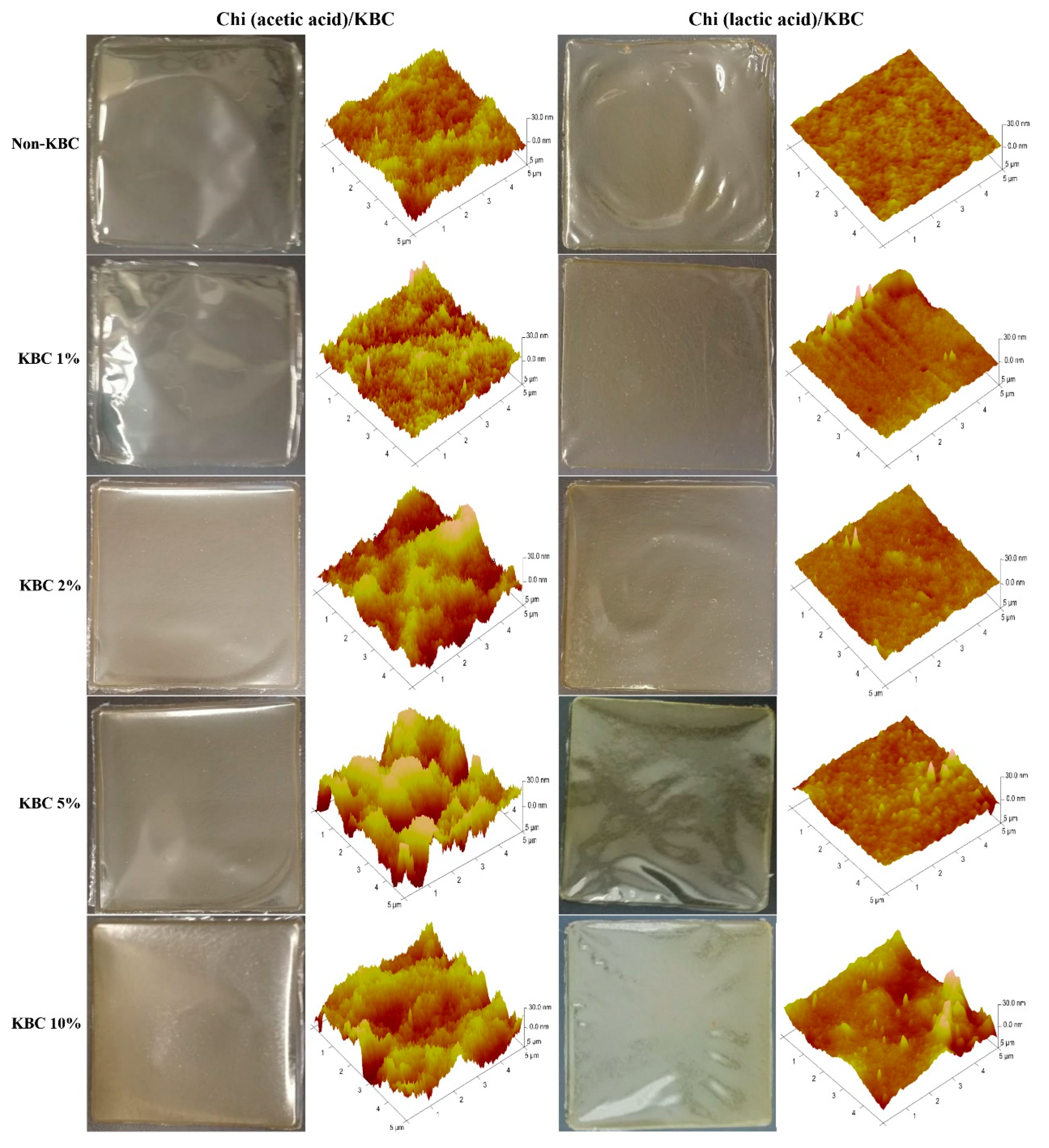
3.2.1. Morphology Analysis
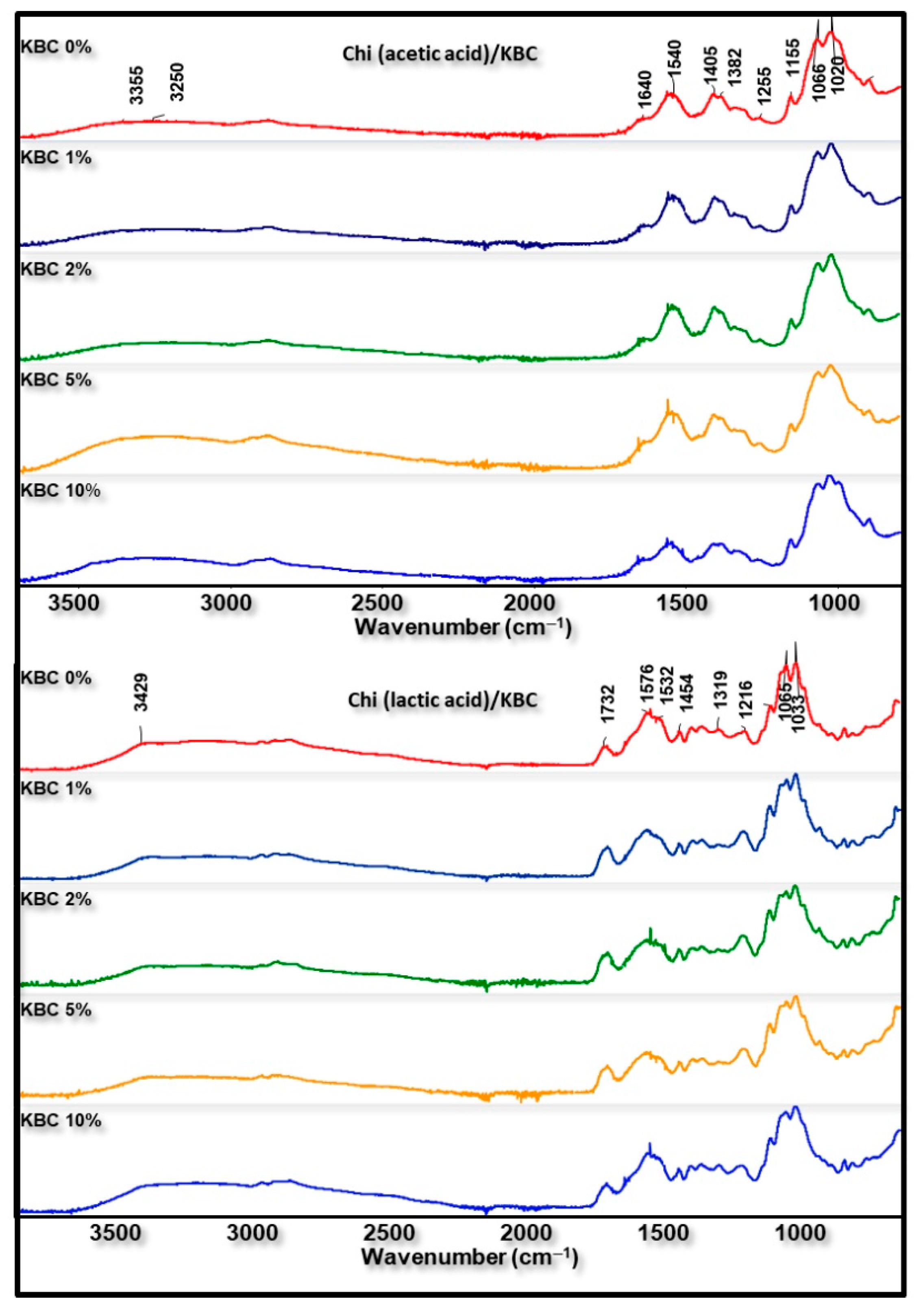
3.2.2. Chemical Structure Analysis
3.2.3. Thermogravimetric Analysis
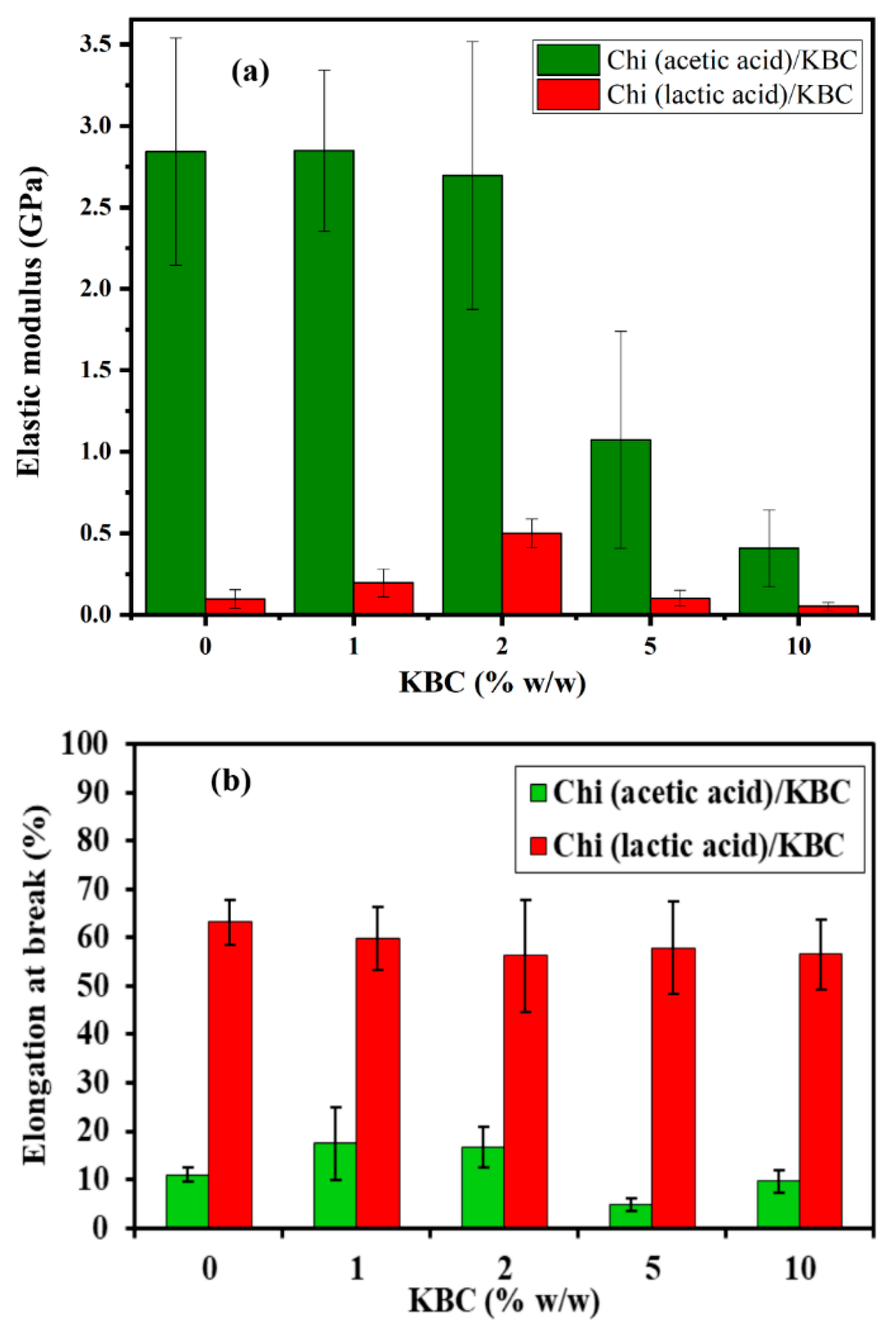
3.2.4. Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Zhang, L.; Zheng, S.; Chai, S.N.; Wei, J.L.; Zhong, L.L.; He, Y.; Xue, J. Bacteriostatic activity and cytotoxicity of bacterial cellulose-chitosan film loaded with in-situ synthesized silver nanoparticles. Carbohydr. Polym. 2022, 281, 11907. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D bacterial cellulose-chitosan-alginate-gelatin hydrogel scaffold for cartilage tissue engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Guo, C.; Zhang, C.; Liu, S.; Gao, J.; Lin, G.; Yang, H.; Xia, W. Effect of chitosan grafting oxidized bacterial cellulose on dispersion stability and modulability of biodegradable films. Int. J. Biol. Macromol. 2022, 204, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.R.; Sun, B.J.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef]
- Li, D.W.; Tian, X.J.; Wang, Z.Q.; Guan, Z.; Li, X.Q.; Qiao, H.; Ke, Z.; Luo, L.; Wi, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Follain, N.; Belbekhouche, S.; Marais, S.; Dufresne, A. Thermal and mechanical properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. Carbohydr. Polym. 2013, 91, 711–717. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Mouro, C.; Riool, M.; Gouveia, I.C. Antimicrobial Food Packaging Based on Prodigiosin-Incorporated Double-Layered Bacterial Cellulose and Chitosan Composites. Polymers 2022, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of acid type for chitosan dissolution on the characteristics and biodegradability of cornstarch/chitosan based films. Int. J. Biol. Macromol. 2019, 138, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.D.; Ma, X.G.; Wang, X.J.; Liu, L.B. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT-Food Sci. Technol. 2021, 135, 109984. [Google Scholar] [CrossRef]
- Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A.S. Bacterial cellulose as a potential biopolymer in biomedical applications: A state-of-the-art review. J. Mater. Chem. B 2022, 10, 3199–3241. [Google Scholar] [CrossRef]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The growing merits and dwindling limitations of bacterial cellulose-based tissue engineering scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Jang, W.D.; Hwang, J.H.; Kim, H.U.; Ryu, J.Y.; Lee, S.Y. Bacterial cellulose as an example product for sustainable production and consumption. Microb. Biotechnol. 2017, 10, 1181–1185. [Google Scholar] [CrossRef] [Green Version]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Coseri, S. Insights on Cellulose Research in the Last Two Decades in Romania. Polymers 2021, 13, 689. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y.H. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The remarkable three-dimensional network structure of bacterial cellulose for tissue engineering applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, F.; Li, X.; Ji, X.; Liu, S.; Han, X.; Yuah, Z.; Luo, J. Transcrystallization of isotactic polypropylene/bacterial cellulose hamburger composite. Polymers 2019, 11, 508. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.W.; Ul-Islam, M.; Khana, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, R.V.; Varley, A.J.; Strap, J.L. Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria. Front. Microbiol. 2015, 6, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerova, M.; Saha, P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Jozala, A.F.; Pertile, R.A.N.; dos Santos, C.A.; Santos-Ebinuma, V.D.; Seckler, M.M.; Gama, F.M.; Pessoa, A., Jr. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, A.; Banerjee, R. Statistical optimization of bacterial cellulose production by Leifsonia soli and its physico-chemical characterization. Process Biochem. 2020, 91, 297–302. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Fontao, A.I.; Coelho, A.; Leal, M.; da Silva, F.; Wan, Y.Z.; Dourado, F.; Gama, M. Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. New Biotechnol. 2019, 49, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.; Gabiatti, N.C.; De Souza, S.S. A review of culture media for bacterial cellulose production: Complex, chemically defined and minimal media modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Jahan, F.; Kumar, V.; Saxena, R.K. Distillery effluent as a potential medium for bacterial cellulose production: A biopolymer of great commercial importance. Bioresour. Technol. 2018, 250, 922–926. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; e Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Saha, N.; Ngwabebhoh, F.A.; Zandraa, O.; Saha, T.; Saha, P. Kombucha-derived bacterial cellulose from diverse wastes: A prudent leather alternative. Cellulose 2021, 28, 9335–9353. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Beaufort, S.; Bonneaud, D.; Souchard, J.P.; Taillandier, P. Physicochemical properties of bacterial cellulose obtained from different Kombucha fermentation conditions. J. Vinyl Addit. Technol. 2021, 27, 183–190. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Borges, O.M.A.; de Oliveira, D.; Poletto, P. Typical kombucha fermentation: Kinetic evaluation of beverage and morphological characterization of bacterial cellulose. J. Food Process. Preserv. 2021, 45, e16100. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.Y.; Zhang, J.C.; Cao, X.D. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Chen, S.Y.; Yang, J.X.; Li, Z.; Wang, H.P. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, R.; Chen, R.P. The Impact of Cross-linking Mode on the Physical and Antimicrobial Properties of a Chitosan/Bacterial Cellulose Composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, Y.; Cheng, Z.; Sheng, J.; Yang, R.D. Nano-sized fibrils dispersed from bacterial cellulose grafted with chitosan. Carbohydr. Polym. 2019, 214, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jin, J.N.; Kan, E.; Kim, K.J.; Lee, S.H. Bacterial Cellulose-chitosan Composite Hydrogel Beads for Enzyme Immobilization. Biotechnol. Bioprocess Eng. 2017, 22, 89–94. [Google Scholar] [CrossRef]
- Indriyati; Dara, F.; Primadona, I.; Srikandace, Y.; Karina, M. Development of bacterial cellulose/chitosan films: Structural, physicochemical and antimicrobial properties. J. Polym. Res. 2021, 28, 70. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Khattak, S.; Qin, X.T.; Huang, L.H.; Xie, Y.Y.; Jia, S.R.; Zhong, C. Preparation and characterization of antibacterial bacterial cellulose/chitosan hydrogels impregnated with silver sulfadiazine. Int. J. Biol. Macromol. 2021, 189, 483–493. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Zdravkovic, N.M.; Trisic, D.D.; Budimir, M.D.; Markovic, Z.M.; Kozyrovska, N.O.; Markovic, B.M.T. Chronic wound dressings-Pathogenic bacteria anti-biofilm treatment with bacterial cellulose-chitosan polymer or bacterial cellulose-chitosan dots composite hydrogels. Int. J. Biol. Macromol. 2021, 191, 315–323. [Google Scholar] [CrossRef]
- Kai, J.; Zhou, X.S. Preparation, Characterization, and Cytotoxicity Evaluation of Zinc Oxide-Bacterial Cellulose-Chitosan Hydrogels for Antibacterial Dressing. Macromol. Chem. Phys. 2020, 221, 2000257. [Google Scholar] [CrossRef]
- Stanescu, P.O.; Radu, I.C.; Alexa, R.L.; Hudita, A.; Tanasa, E.; Ghitman, J.; Stoian, O.; Tsatsakis, A.; Ginghina, O.; Zaharia, C.; et al. Novel chitosan and bacterial cellulose biocomposites tailored with polymeric nanoparticles for modern wound dressing development. Drug Deliv. 2021, 28, 1932–1950. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Zhang, F.L.; Duan, J.F.; Jiang, J.X. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef] [PubMed]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gomez-Guillen, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Lipovka, A.; Kharchenko, A.; Dubovoy, A.; Filipenko, M.; Stupak, V.; Mayorov, A.; Fomenko, V.; Geydt, P.; Parshin, D. The effect of adding modified chitosan on the strength properties of bacterial cellulose for clinical applications. Polymers 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Windhab, E.J. Rheology of food materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 36–40. [Google Scholar] [CrossRef]
- Kalyani, P.; Khandelwal, M. Modulation of morphology, water uptake/retention, and rheological properties by in-situ modification of bacterial cellulose with the addition of biopolymers. Cellulose 2021, 28, 11025–11036. [Google Scholar] [CrossRef]
- Song, S.; Liu, X.Y.; Ding, L.; Abubaker, M.A.; Zhang, J.; Huang, Y.L.; Yang, S.; Fan, Z. Conformational and rheological properties of bacterial cellulose sulfate. Int. J. Biol. Macromol. 2021, 183, 2326–2336. [Google Scholar] [CrossRef]
- Lakehal, I.; Montembault, A.; David, L.; Perrier, A.; Vibert, R.; Duclaux, L.; Reinert, L. Prilling and characterization of hydrogels and derived porous spheres from chitosan solutions with various organic acids. Int. J. Biol. Macromol. 2019, 129, 68–77. [Google Scholar] [CrossRef]
- de Souza Soares, L.; Perim, R.B.; de Alvarenga, E.S.; de Moura Guimarães, L.; de Carvalho Teixeira, A.V.N.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Insights on physicochemical aspects of chitosan dispersion in aqueous solutions of acetic, glycolic, propionic or lactic acid. Int. J. Biol. Macromol. 2019, 128, 140–148. [Google Scholar] [CrossRef]
- Kjm, K.M.; Son, J.H.; Kim, S.K.; Weller, C.L.; Hanna, M.A. Properties of chitosan films as a function of pH and solvent type. J. Food Sci. 2006, 71, E119–E124. [Google Scholar]
- Nguyen, H.T.; Ngwabebhoh, F.A.; Saha, N.; Zandraa, O.; Saha, T.; Saha, P. Development of novel biocomposites based on the clean production of microbial cellulose from dairy waste (sour whey). J. Appl. Polym. Sci. 2022, 139, 51433. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting. In Standard D882 Annual Book of American Standard Testing Methods; American Society for Testing and Materials: Philadelphia, PA, USA, 2001; pp. 162–170. [Google Scholar]
- Velásquez-Cock, J.; Ramírez, E.; Betancourt, S.; Putaux, J.-L.; Osorio, M.; Castro, C.; Gañán, P.; Zuluaga, R. Influence of the acid type in the production of chitosan films reinforced with bacterial nanocellulose. Int. J. Biol. Macromol. 2014, 69, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Liu, X.L.; Jiang, Q.X.; Yu, D.W.; Xu, Y.S.; Wang, B.; Xia, W. Development and properties of bacterial cellulose, curcumin, and chitosan composite biodegradable films for active packaging materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef] [PubMed]

| Materials | Preparation Method | Enhanced Properties | Desired Application | Ref. |
|---|---|---|---|---|
| Chi/KBC | Impregnation | Water vapor permeability; antioxidant activity; against ultraviolet | Active food packaging | [5] |
| Chi/BC | Casting | Mechanical properties | Food packaging | [6] |
| Chi/BC/metal-organic framework | Impregnation | Water stability | Wastewater treatment | [7] |
| Chi/BC/magnetic attapulgite | Blending | Adsorption capability | Water treatment | [8] |
| Chi/BC | Grafting | Uniform; acid and temperature stability | Papermaking; Food packaging; Textiles; Medical | [43] |
| Chi/BC | Blending | Thermal stability | Immobilize proteins | [44] |
| Chi/BC/glycerol /carboxymethyl cellulose | Casting | Water vapor transmission rate; tensile strength | Antimicrobial films | [45] |
| Chi/BC/ ciprofloxacin | Impregnation | Antimicrobial activity | Wound dressing | [46] |
| Chi/BC/silver sulfadiazine | Impregnation | Mechanical and antibacterial properties | Food packaging, Tissue engineering, Drug delivery, Biomedical | [47] |
| Chi/BC | Impregnation | Antimicrobial activity; porosity; migration of cell | Chronic wound healing agents | [48] |
| Chi/BC/ZnO | Blending | antimicrobial activity; thermal stability; Compressive strength | Antibacterial dressing | [49] |
| Chi/BC/poly(N-isopropylacrylamide/polyvinyl alcohol/methyl oleate/silver sulfadiazine | Blending | Mechanical strength and biocompatibility | Wound dressing materials | [50] |
| Chi/BC/Poly(vinyl alcohol) | Casting | Tensile strength and antibacterial properties | Food packaging | [51] |
| Chi/BC/collagen | Impregnation | Breathability and antibacterial properties | Wound dressing | [52] |
| KBC (% w/w) | Viscosity Reduction Percentage (%) | |
|---|---|---|
| Chi (Acetic Acid)/KBC | Chi (Lactic Acid)/KBC | |
| 0 | 60.53 | 51.38 |
| 1 | 58.22 | 51.54 |
| 2 | 57.84 | 53.43 |
| 5 | 57.81 | 54.21 |
| 10 | 59.42 | 54.63 |
| KBC (% w/w) | Chi (Acetic Acid)/KBC | Chi (Lactic Acid)/KBC | ||||
|---|---|---|---|---|---|---|
| Ea (kJ/mol) | A (1/s) | r2 | Ea (kJ/mol) | A (1/s) | r2 | |
| 0 | 13.67 | 0.054 × 10−6 | 0.9989 | 10.80 | 2.46 × 10−6 | 0.9994 |
| 1 | 11.95 | 0.25 × 10−6 | 0.9955 | 10.26 | 1.55 × 10−6 | 0.9996 |
| 2 | 11.43 | 0.28 × 10−6 | 0.9874 | 11.75 | 1.23 × 10−6 | 0.9977 |
| 5 | 12.13 | 0.22 × 10−6 | 0.9999 | 11.72 | 4.43 × 10−6 | 0.9962 |
| 10 | 11.79 | 0.28 × 10−6 | 0.9972 | 11.52 | 4.99 × 10−6 | 0.9997 |
| Chi (Acetic Acid)/KBC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KBC (% w/w) | 25 °C | 30 °C | 35 °C | 40 °C | ||||||||
| k | n | r2 | k | n | r2 | k | n | r2 | k | n | r2 | |
| 0 | 0.89 | 0.73 | 0.9952 | 0.57 | 0.77 | 0.9946 | 0.57 | 0.77 | 0.9946 | 0.24 | 0.85 | 0.9952 |
| 1 | 0.69 | 0.76 | 0.9944 | 0.29 | 0.88 | 0.997 | 0.14 | 0.99 | 0.9963 | 0.18 | 0.90 | 0.9954 |
| 2 | 0.59 | 0.80 | 0.9939 | 0.38 | 0.81 | 0.9952 | 0.23 | 0.87 | 0.9964 | 0.18 | 0.89 | 0.9954 |
| 5 | 0.81 | 0.74 | 0.9958 | 0.52 | 0.78 | 0.995 | 0.35 | 0.82 | 0.9923 | 0.25 | 0.85 | 0.9954 |
| 10 | 0.67 | 0.76 | 0.9961 | 0.37 | 0.82 | 0.9951 | 0.26 | 0.86 | 0.9953 | 0.18 | 0.88 | 0.9962 |
| Chi(Lactic Acid)/KBC | ||||||||||||
| 0 | 0.35 | 0.81 | 0.9944 | 0.26 | 0.84 | 0.996 | 0.26 | 0.84 | 0.996 | 0.15 | 0.88 | 0.9977 |
| 1 | 0.35 | 0.81 | 0.9952 | 0.200 | 0.89 | 0.9981 | 0.12 | 0.96 | 0.999 | 0.12 | 0.91 | 0.9963 |
| 2 | 0.98 | 0.72 | 0.9953 | 0.73 | 0.75 | 0.9946 | 0.51 | 0.78 | 0.9908 | 0.35 | 0.82 | 0.9917 |
| 5 | 0.99 | 0.71 | 0.9944 | 0.59 | 0.78 | 0.994 | 0.43 | 0.80 | 0.9924 | 0.32 | 0.83 | 0.9948 |
| 10 | 0.84 | 0.73 | 0.9957 | 0.55 | 0.78 | 0.9944 | 0.38 | 0.82 | 0.9923 | 0.29 | 0.84 | 0.9942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Sionkowska, A.; Lewandowska, K.; Brudzyńska, P.; Szulc, M.; Saha, N.; Saha, T.; Saha, P. Chitosan Modified by Kombucha-Derived Bacterial Cellulose: Rheological Behavior and Properties of Convened Biopolymer Films. Polymers 2022, 14, 4572. https://doi.org/10.3390/polym14214572
Nguyen HT, Sionkowska A, Lewandowska K, Brudzyńska P, Szulc M, Saha N, Saha T, Saha P. Chitosan Modified by Kombucha-Derived Bacterial Cellulose: Rheological Behavior and Properties of Convened Biopolymer Films. Polymers. 2022; 14(21):4572. https://doi.org/10.3390/polym14214572
Chicago/Turabian StyleNguyen, Hau Trung, Alina Sionkowska, Katarzyna Lewandowska, Patrycja Brudzyńska, Marta Szulc, Nabanita Saha, Tomas Saha, and Petr Saha. 2022. "Chitosan Modified by Kombucha-Derived Bacterial Cellulose: Rheological Behavior and Properties of Convened Biopolymer Films" Polymers 14, no. 21: 4572. https://doi.org/10.3390/polym14214572





