Advances in Biodegradable Soft Robots
Abstract
:1. Introduction
2. Biodegradable Materials for Soft Robots
3. Fabrication Methods for Soft Robots
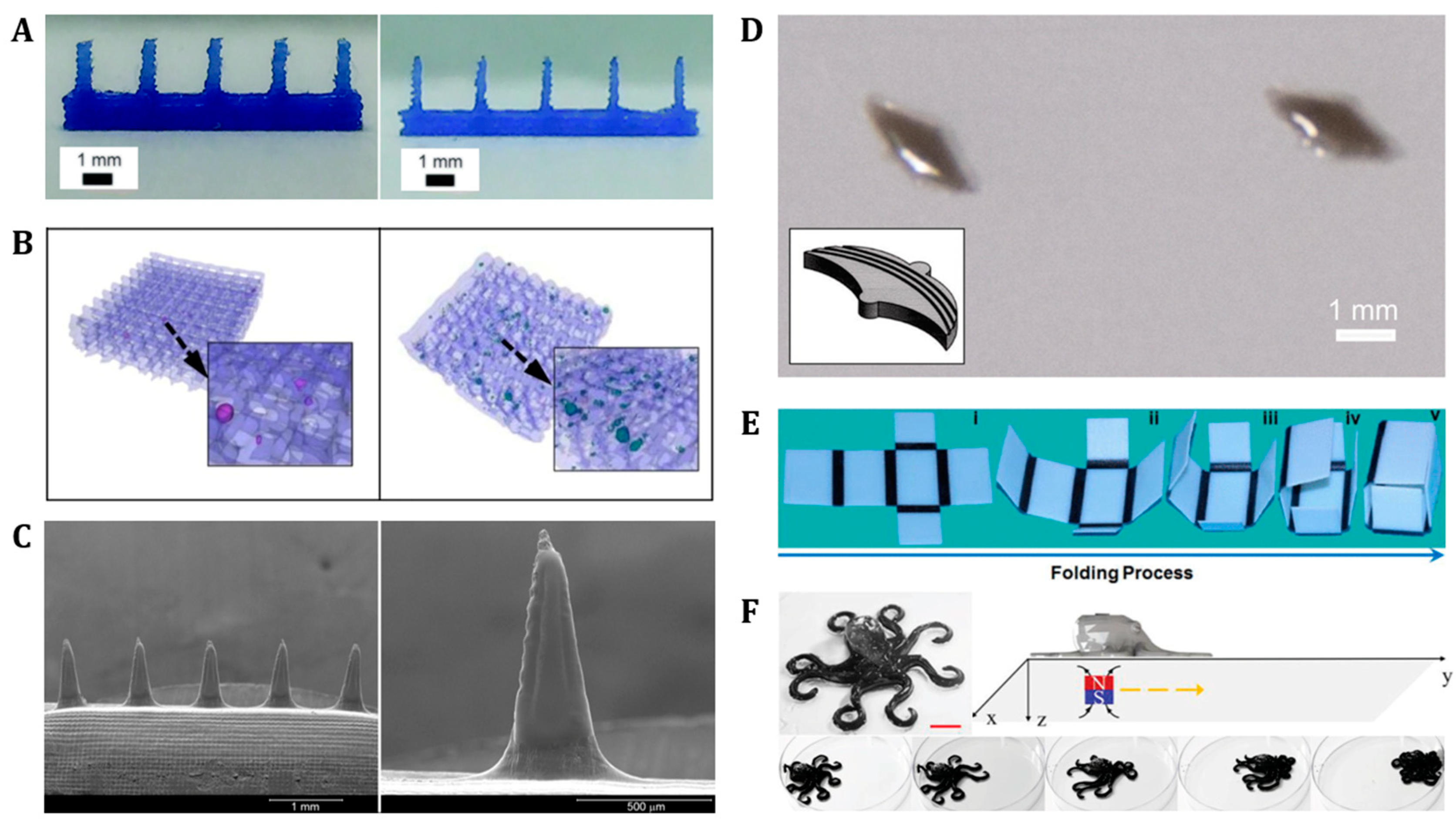
3.1. Photolithography
3.2. 3D/4D Printing
4. Applications of Biodegradable Soft Robots
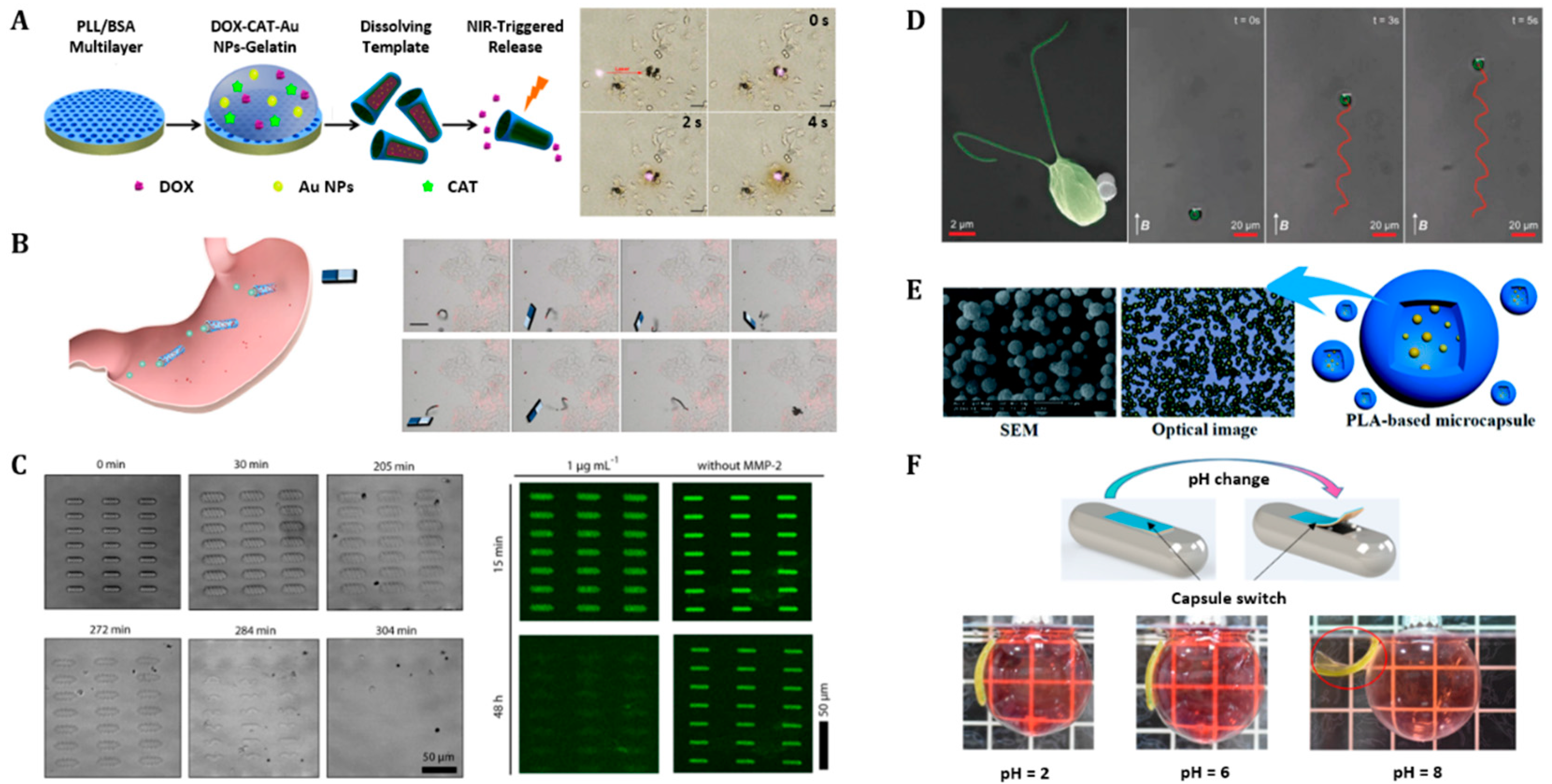
4.1. Drug Delivery Carriers
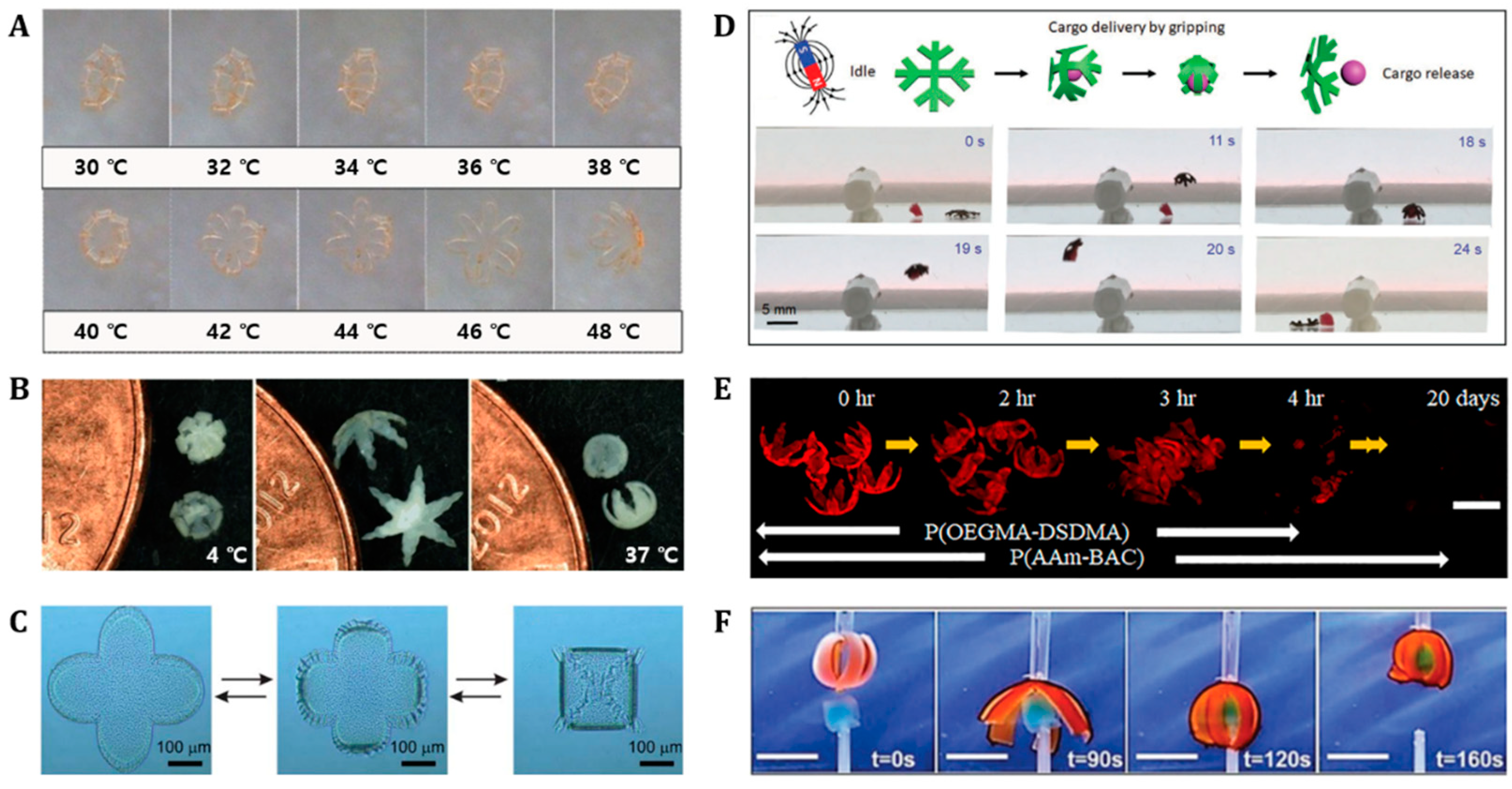
4.2. Grippers
4.3. Tissue Engineering
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel Machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, F.; Zhu, X.; Yong, K.-T.; Gu, G. Stimuli-Responsive Functional Materials for Soft Robotics. J. Mater. Chem. B 2020, 8, 8972–8991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Farino, C.; Yang, C.; Scott, T.; Browe, D.; Choi, W.; Freeman, J.W.; Lee, H. Soft Robotic Manipulation and Locomotion with a 3D Printed Electroactive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 17512–17518. [Google Scholar] [CrossRef]
- Goswami, D.; Liu, S.; Pal, A.; Silva, L.G.; Martinez, R.V. 3D-Architected Soft Machines with Topologically Encoded Motion. Adv. Funct. Mater. 2019, 29, 1808713. [Google Scholar] [CrossRef]
- Trimmer, B.A.; Lin, H.T.; Baryshyan, A.; Leisk, G.G.; Kaplan, D.L. Towards a Biomorphic Soft Robot: Design Constraints and Solutions. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 599–605. [Google Scholar] [CrossRef]
- Shintake, J. Green Robotics: Toward Realization of Environmentally Friendly Soft Robots. J. Robot. Mechatron. 2022, 34, 270–272. [Google Scholar] [CrossRef]
- Rossiter, J. Soft Robotics: The Route to True Robotic Organisms. Artif. Life Robot. 2021, 26, 269–274. [Google Scholar] [CrossRef]
- Sitti, M. Miniature soft robots—road to the clinic. Nat. Rev. Mater. 2018, 3, 74–75. [Google Scholar] [CrossRef]
- Gracias, D.H. Stimuli Responsive Self-Folding Using Thin Polymer Films. Curr. Opin. Chem. Eng. 2013, 2, 112–119. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.E.; Fu, X.; Li, K.; Wong, I.Y.; Chen, P.-Y. Multifunctional Soft Machines Based on Stimuli-Responsive Hydrogels: From Freestanding Hydrogels to Smart Integrated Systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Ahn, S.K.; Kasi, R.M.; Kim, S.C.; Sharma, N.; Zhou, Y. Stimuli-Responsive Polymer Gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-Isopropylacrylamide): Experiment, Theory and Application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Son, H.; Yoon, C. Advances in Stimuli-Responsive Soft Robots with Integrated Hybrid Materials. Actuators 2020, 9, 115. [Google Scholar] [CrossRef]
- Rogers, J.; Huang, Y.; Schmidt, O.G.; Gracias, D.H. Origami MEMS and NEMS. MRS Bull. 2016, 41, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D Bioprinting: The next-Generation Technology for Biofabrication Enabled by Stimuli-Responsive Materials. Biofabrication 2017, 9, 012001. [Google Scholar] [CrossRef]
- Randall, C.L.; Gultepe, E.; Gracias, D.H. Self-Folding Devices and Materials for Biomedical Applications. Trends Biotechnol. 2012, 30, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Rus, D.; Tolley, M.T. Design, Fabrication and Control of Origami Robots. Nat. Rev. Mater. 2018, 3, 101–112. [Google Scholar] [CrossRef]
- Bolaños Quiñones, V.A.; Zhu, H.; Solovev, A.A.; Mei, Y.; Gracias, D.H. Origami Biosystems: 3D Assembly Methods for Biomedical Applications. Adv. Biosyst. 2018, 2, 1800230. [Google Scholar] [CrossRef]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D Printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Ionov, L. 4D Biofabrication: Materials, Methods, and Applications. Adv. Healthc. Mater. 2018, 7, 1800412. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Qi, H.J.; Dunn, M.L. Active Materials by Four-Dimension Printing. Appl. Phys. Lett. 2013, 103, 131901. [Google Scholar] [CrossRef]
- Ge, Q.; Sakhaei, A.H.; Lee, H.; Dunn, C.K.; Fang, N.X.; Dunn, M.L. Multimaterial 4D Printing with Tailorable Shape Memory Polymers. Sci. Rep. 2016, 6, 31110. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.-J.; Hauser, A.W.; Hayward, R.C. Shape-Morphing Materials from Stimuli-Responsive Hydrogel Hybrids. Acc. Chem. Res. 2017, 50, 161–169. [Google Scholar] [CrossRef]
- Kirillova, A.; Ionov, L. Shape-Changing Polymers for Biomedical Applications. J. Mater. Chem. B 2019, 7, 1597–1624. [Google Scholar] [CrossRef]
- Panchal, S.S.; Vasava, D.V. Biodegradable Polymeric Materials: Synthetic Approach. ACS Omega 2020, 5, 4370–4379. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, J.; Winfield, J.; Ieropoulos, I. Here Today, Gone Tomorrow: Biodegradable Soft Robots. Electroact. Polym. Actuators Devices 2016, 9798, 97981S. [Google Scholar]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, X.; Zhu, K.; Guo, J.; Zhang, L. Bilayer Hydrogel Actuators with Tight Interfacial Adhesion Fully Constructed from Natural Polysaccharides. Soft Matter 2017, 13, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhu, W.L. Viscosity Properties of Sodium Carboxymethylcellulose Solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and Gelling Properties of Gelatin from Goat Skin as Affected by Drying Methods. J. Food Sci. Technol. 2017, 54, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 529. [Google Scholar] [CrossRef]
- Ghosh, T.; Deveswaran, R.; Bharath, S. Copper Crosslinked Carboxymethyl Chitosan–Gelatin Scaffolds: A Potential Antibacterial and Cytocompatible Material for Biomedical Applications. Mater. Today Proc. 2022, 59, 31–38. [Google Scholar] [CrossRef]
- Joo, G.; Park, M.; Park, S.S.; Tripathi, G.; Lee, B.T. Tailored Alginate/PCL-Gelatin-β-TCP Membrane for Guided Bone Regeneration. Biomed. Mater. 2022, 17, 045011. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet Entirely Degradable Gelatin-Based Biogels for Soft Robots and Electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Dias, P.P.; Vasconcelos, R.O.; Taboga, S.R.; Canola, P.A.; Chinelatto, M.A.; Pereira, G.T.; Ferraz, G.C. Biocompatibility and Biodegradation of Poly(Lactic Acid) (PLA) and an Immiscible PLA/Poly(ε-Caprolactone) (PCL) Blend Compatibilized by Poly(ε-Caprolactone-b-Tetrahydrofuran) Implanted in Horses. Polym. J. 2020, 52, 629–643. [Google Scholar] [CrossRef]
- Nonato, R.C.; Mei, L.H.I.; Bonse, B.C.; Leal, C.V.; Levy, C.E.; Oliveira, F.A.; Delarmelina, C.; Duarte, M.C.T.; Morales, A.R. Nanocomposites of PLA/ZnO Nanofibers for Medical Applications: Antimicrobial Effect, Thermal, and Mechanical Behavior under Cyclic Stress. Polym. Eng. Sci. 2022, 62, 1147–1155. [Google Scholar] [CrossRef]
- Lovald, S.T.; Khraishi, T.; Wagner, J.; Baack, B. Mechanical Design Optimization of Bioabsorbable Fixation Devices for Bone Fractures. J. Craniofac. Surg. 2009, 20, 389–398. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Mulinti, P.; Brooks, J.E.; Lervick, B.; Pullan, J.E.; Brooks, A.E. 10-Strategies to Improve the Hemocompatibility of Biodegradable Biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 253–278. [Google Scholar]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-L-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and Mechanical Properties of Poly(d,l-Lactic Acid)/Poly(ε-Caprolactone) Blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Galgali, P.; Varma, A.J.; Puntambekar, U.S.; Gokhale, D.V. Towards Biodegradable Polyolefins: Strategy of Anchoring Minute Quantities of Monosaccharides and Disaccharides onto Functionalized Polystyrene, and Their Effect on Facilitating Polymer Biodegradation. Chem. Commun. 2002, 2, 2884–2885. [Google Scholar] [CrossRef] [PubMed]
- Galgali, P.; Puntambekar, U.S.; Gokhale, D.V.; Varma, A.J. Fungal Degradation of Carbohydrate-Linked Polystyrenes. Carbohydr. Polym. 2004, 55, 393–399. [Google Scholar] [CrossRef]
- Xuan, Y.; Jiang, G.; Li, Y.; Yang, L.; Zhang, X. Biodegradable Oligo (Poly-l-lysine) as a High-Performance Hydration Inhibitor for Shale. RSC Adv. 2015, 5, 84947–84958. [Google Scholar] [CrossRef]
- Stamou, A.; Iatrou, H.; Tsitsilianis, C. NIPAm-Based Modification of Poly(l-Lysine): A PH-Dependent LCST-Type Thermo-Responsive Biodegradable Polymer. Polymers 2022, 14, 802. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable Protein-Based Rockets for Drug Transportation and Light-Triggered Release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef]
- Godbey, W.T. Chapter 13-Gene Delivery. In An Introduction to Biotechnology, 1st ed.; Academic Press Inc.: London, UK, 2014; pp. 275–312. [Google Scholar]
- Reineke, T.M.; Davis, M.E. 9.26-Nucleic Acid Delivery via Polymer Vehicles. In Polymer Science: A Comprehensive Reference, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 497–527. [Google Scholar]
- Wang, Y.; Chang, Y.C. Synthesis and Conformational Transition of Surface-Tethered Polypeptide: Poly(l-Lysine). Macromolecules 2003, 36, 6511–6518. [Google Scholar] [CrossRef]
- Thapa, B.; Narain, R. 1-Mechanism, Current Challenges and New Approaches for Non Viral Gene Delivery. In Polymers and Nanomaterials for Gene Therapy, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 1–27. [Google Scholar]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.W. Effects of Gelatin Methacrylate Bio-Ink Concentration on Mechano-Physical Properties and Human Dermal Fibroblast Behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.H.; Hu, C.; Terzopoulou, A.; Chen, X.Z.; Huang, T.Y.; Maniura-Weber, K.; Pané, S.; Nelson, B.J. 3D Printed Enzymatically Biodegradable Soft Helical Microswimmers. Adv. Funct. Mater. 2018, 28, 1804107. [Google Scholar] [CrossRef]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable Untethered Magnetic Hydrogel Milli-Grippers. Adv. Funct. Mater. 2020, 30, 2004975. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 6–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sun, M.; Liu, T.; Hou, M.; Yang, H. Effect of Different Additives on the Mechanical Properties of Gelatin Methacryloyl Hydrogel: A Meta-Analysis. ACS Omega 2021, 6, 9112–9128. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Noreen, A.; Tabasum, S.; Ghaffar, S.; Somi, T.; Sultan, N.; Aslam, N.; Naseer, R.; Ali, I.; Anwar, F. Chapter 12-Protein-Based Bionanocomposites. In Bionanocomposites: Green Synthesis and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–320. [Google Scholar]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar]
- Fukunishi, T.; Shoji, T.; Shinoka, T. 18-Nanofiber Composites in Vascular Tissue Engineering. In Nanofiber Composites for Biomedical Applications, 1st ed.; Elsevier Inc.: Columbus, OH, USA, 2017; pp. 455–481. [Google Scholar]
- Alihosseini, F. 10-Plant-Based Compounds for Antimicrobial Textiles. In Antimicrobial Textiles, 1st ed.; Woodhead Publising: Cambridge, UK, 2016; pp. 155–195. [Google Scholar]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray Biodegradable Microcapsules Loaded with Curcumin for Drug Delivery Systems with High Bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(Lactic Acid) Fiber: An Overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Noh, S.; Jeon, S.; Kim, E.; Oh, U.; Park, D.; Park, S.H.; Kim, S.W.; Pané, S.; Nelson, B.J.; Kim, J.Y.; et al. A Biodegradable Magnetic Microrobot Based on Gelatin Methacrylate for Precise Delivery of Stem Cells with Mass Production Capability. Small 2022, 18, 2107888. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Ono, K.; Sumiya, M.; Yoshinobu, N.; Dode, T.; Katayama, T.; Ueda, N.; Nagahama, K. Angiogenesis Promotion by Combined Administration of DFO and Vein Endothelial Cells Using Injectable, Biodegradable, Nanocomposite Hydrogel Scaffolds. ACS Appl. Bio Mater. 2022, 5, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the In Vivo Degradation Mechanism of PEGDA Hydrogels. J. Biomed. Mater. Res.-Part A 2014, 102, 4244–4251. [Google Scholar]
- Park, J.; Jin, C.; Lee, S.; Kim, J.Y.; Choi, H. Magnetically Actuated Degradable Microrobots for Actively Controlled Drug Release and Hyperthermia Therapy. Adv. Healthc. Mater. 2019, 8, e1900213. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.Z.; Dean, D. Poly(Propylene Fumarate)-Based Materials: Synthesis, Functionalization, Properties, Device Fabrication and Biomedical Applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef]
- Timmer, M.D.; Shin, H.; Horch, R.A.; Ambrose, C.G.; Mikos, A.G. In Vitro Cytotoxicity of Injectable and Biodegradable Poly(Propylene Fumarate)-Based Networks: Unreacted Macromers, Cross-Linked Networks, and Degradation Products. Biomacromolecules 2003, 4, 1026–1033. [Google Scholar] [CrossRef]
- Peter, S.J.; Lu, L.; Kim, D.J.; Mikos, A.G. Marrow Stromal Osteoblast Function on a Poly(Propylene Fumarate)/β-Tricalcium Phosphate Biodegradable Orthopaedic Composite. Biomaterials 2000, 21, 1207–1213. [Google Scholar] [CrossRef]
- Kim, K.; Dean, D.; Mikos, A.G.; Fisher, J.P. Effect of Initial Cell Seeding Density on Early Osteogenic Signal Expression of Rat Bone Marrow Stromal Cells Cultured on Cross-Linked Poly(Propylene Fumarate) Disks. Biomacromolecules 2009, 10, 1810–1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the In Vitro Cytotoxicity of Cross-Linked Biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Malachowski, K.; Breger, J.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Selaru, F.M.; Gracias, D.H. Stimuli-Responsive Theragrippers for Chemomechanical Controlled Release. Angew. Chem.-Int. Ed. 2014, 53, 8045–8049. [Google Scholar] [CrossRef]
- Breger, J.C.; Yoon, C.; Xiao, R.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-Folding Thermo-Magnetically Responsive Soft Microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405. [Google Scholar] [CrossRef]
- Zhou, M.; Hou, T.; Li, J.; Yu, S.; Xu, Z.; Yin, M.; Wang, J.; Wang, X. Self-Propelled and Targeted Drug Delivery of Poly(Aspartic Acid)/Iron-Zinc Microrocket in the Stomach. ACS Nano 2019, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Blakey, I.; Little, P.J.; Ta, H.T. Hydrogels Based on Poly(Aspartic Acid): Synthesis and Applications. Front. Chem. 2019, 7, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Chapter 4-Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–172. [Google Scholar]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual PH-Responsive Hydrogel Actuator for Lipophilic Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of Several Properties and Consequences on Their Applications. Polym. Test. 2001, 99, 483. [Google Scholar] [CrossRef]
- Meng, Z.; He, J.; Cai, Z.; Wang, F.; Zhang, J.; Wang, L.; Ling, R.; Li, D. Design and Additive Manufacturing of Flexible Polycaprolactone Scaffolds with Highly-Tunable Mechanical Properties for Soft Tissue Engineering. Mater. Des. 2020, 189, 108508. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic Actuated PH-Responsive Hydrogel-Based Soft Micro-Robot for Targeted Drug Delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Fusco, S.; Sakar, M.S.; Kennedy, S.; Peters, C.; Bottani, R.; Starsich, F.; Mao, A.; Sotiriou, G.A.; Pané, S.; Pratsinis, S.E.; et al. An Integrated Microrobotic Platform for On-Demand, Targeted Therapeutic Interventions. Adv. Mater. 2014, 26, 952–957. [Google Scholar] [CrossRef]
- Peters, C.; Hoop, M.; Pané, S.; Nelson, B.J.; Hierold, C. Degradable Magnetic Composites for Minimally Invasive Interventions: Device Fabrication, Targeted Drug Delivery, and Cytotoxicity Tests. Adv. Mater. 2016, 28, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. In Vitro Degradation of a Poly(Propylene Fumarate)-Based Composite Material. Biomaterials 1996, 17, 2127–2130. [Google Scholar] [CrossRef]
- Domb, A.J.; Manor, N.; Elmalak, O. Biodegradable Bone Cement Compositions Based on Acrylate and Epoxide Terminated Poly(Propylene Fumarate) Oligomers and Calcium Salt Compositions. Biomaterials 1996, 17, 411–417. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.D.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Synthesis of Biodegradable Poly(Propylene Fumarate) Networks with Poly(Propylene Fumarate)-Diacrylate Macromers as Crosslinking Agents and Characterization of Their Degradation Products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Tabata, K.; Kasuya, K.I.; Abe, H.; Masuda, K.; Doi, Y. Poly(Aspartic Acid) Degradation by a Sphingomonas Sp. Isolated from Freshwater. Appl. Environ. Microbiol. 1999, 65, 4268–4270. [Google Scholar] [CrossRef] [Green Version]
- Mehtani, D.; Seth, A.; Sharma, P.; Maheshwari, N.; Kapoor, D.; Shrivastava, S.K.; Tekade, R.K. 4-Biomaterials for Sustained and Controlled Delivery of Small Drug Molecules. In Biomaterials and Bionanotechnology, 1st ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 89–152. [Google Scholar]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Azam, A.; Laflin, K.E.; Jamal, M.; Fernandes, R.; Gracias, D.H. Self-Folding Micropatterned Polymeric Containers. Biomed. Microdevices 2011, 13, 51–58. [Google Scholar] [CrossRef]
- Zakharchenko, S.; Puretskiy, N.; Stoychev, G.; Stamm, M.; Ionov, L. Temperature Controlled Encapsulation and Release Using Partially Biodegradable Thermo-Magneto-Sensitive Self-Rolling Tubes. Soft Matter 2010, 6, 2633–2636. [Google Scholar] [CrossRef]
- Kobayashi, K.; Oh, S.H.; Yoon, C.K.; Gracias, D.H. Multitemperature Responsive Self-Folding Soft Biomimetic Structures. Macromol. Rapid Commun. 2018, 39, 2107888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mitomo, H.; Yosh, F. Synthesis of PH-Sensitive and Biodegradable CM-Cellulose/Chitosan Polyampholytic Hydrogels with Electron Beam Irradiation. J. Bioact. Compat. Polym. 2008, 23, 319–333. [Google Scholar] [CrossRef]
- Seunarine, K.; Meredith, D.O.; Riehle, M.O.; Wilkinson, C.D.W.; Gadegaard, N. Biodegradable Polymer Tubes with Lithographically Controlled 3D Micro- and Nanotopography. Microelectron. Eng. 2008, 85, 1350–1354. [Google Scholar] [CrossRef]
- Xiao, H. 6-Photolithography. In Introduction to Semiconductor Manufacturing Technology; Prentice-Hall Inc.: Hoboken, NJ, USA, 2001; pp. 181–228. [Google Scholar]
- Vasiev, I.; Greer, A.I.M.; Khokhar, A.Z.; Stormonth-Darling, J.; Tanner, K.E.; Gadegaard, N. Self-Folding Nano- and Micropatterned Hydrogel Tissue Engineering Scaffolds by Single Step Photolithographic Process. Microelectron. Eng. 2013, 108, 76–81. [Google Scholar] [CrossRef]
- Huang, Y.; Fitzpatrick, V.; Zheng, N.; Cheng, R.; Huang, H.; Ghezzi, C.; Kaplan, D.L.; Yang, C. Self-Folding 3D Silk Biomaterial Rolls to Facilitate Axon and Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 2000530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hou, Y.; Lin, J. A Review on the Processing Accuracy of Two-Photon Polymerization. AIP Adv. 2015, 5, 030701. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, C.; Gritsenko, D.; Zhou, R.; Xu, J. Soft Lithography Based on Photolithography and Two-Photon Polymerization. Microfluid. Nanofluid. 2018, 22, 97. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136. [Google Scholar] [CrossRef]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing-Characterization and Fabrication. Materials 2019, 16, 887. [Google Scholar] [CrossRef] [Green Version]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D Printed Polymer Microneedles for Transdermal Drug Delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McBride, S.; Tellis, B.; Alvarez-Urena, P.; Song, Y.H.; Dean, D.D.; Sylvia, V.L.; Elgendy, H.; Ong, J.; Hollinger, J.O. Rapid-Prototyped PLGA/β-TCP/Hydroxyapatite Nanocomposite Scaffolds in a Rabbit Femoral Defect Model. Biofabrication 2012, 4, 025003. [Google Scholar] [CrossRef]
- Dávila, J.L.; Freitas, M.S.; Inforçatti Neto, P.; Silveira, Z.C.; Silva, J.V.L.; D’Ávila, M.A. Fabrication of PCL/β-TCP Scaffolds by 3D Mini-Screw Extrusion Printing. J. Appl. Polym. Sci. 2016, 133, 15. [Google Scholar] [CrossRef]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet Printing for Biosensor Fabrication: Combining Chemistry and Technology for Advanced Manufacturing. Lab Chip 2015, 15, 2538–2558. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.H.; Lee, J.H.; Kim, G.H. 3D Bioprinting and Its in Vivo Applications. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet Printing for Pharmaceutical Applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Sung, Y.-L.; Jeang, J.; Lee, C.-H.; Shih, W.-C. Fabricating Optical Lenses by Inkjet Printing and Heat-Assisted In Situ Curing of Polydimethylsiloxane for Smartphone Microscopy. J. Biomed. Opt. 2015, 20, 047005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, A.T.; Meisel, N.A.; Williams, C.B.; Guest, J.K. Multiple-Material Topology Optimization of Compliant Mechanisms Created Via PolyJet Three-Dimensional Printing. J. Manuf. Sci. Eng. Trans. ASME 2014, 136, 061015. [Google Scholar] [CrossRef] [Green Version]
- Parmar, J.; Ma, X.; Katuri, J.; Simmchen, J.; Stanton, M.M.; Trichet-Paredes, C.; Soler, L.; Sanchez, S. Nano and Micro Architectures for Self-Propelled Motors. Sci. Technol. Adv. Mater. 2015, 16, 14802. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, S.; Ke, Y.; Ding, L.; Zeng, X.; Magdassi, S.; Long, Y. 4D Printed Hydrogels: Fabrication, Materials, and Applications. Adv. Mater. Technol. 2020, 5, 2000034. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, V.S. PREFACE. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. xi–xii. [Google Scholar]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yoon, C.; Oh, S.H.; Pagaduan, J.V.; Gracias, D.H. Biodegradable Thermomagnetically Responsive Soft Untethered Grippers. ACS Appl. Mater. Interfaces 2019, 11, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yasa, O.; Erkoc, P.; Alapan, Y.; Sitti, M. Microalga-Powered Microswimmers toward Active Cargo Delivery. Adv. Mater. 2018, 30, 1804130. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, V.; Boi, S.; Read, J.; Guillemet, R.; Zhang, J.; Udalov, A.; Shesterikov, E.; Tverdokhlebov, S.; Pastorino, L.; Gould, D.J.; et al. Biodegradable Defined Shaped Printed Polymer Microcapsules for Drug Delivery. ACS Appl. Mater. Interfaces 2021, 13, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.-I.; Taboryski, R. Recent Advances in Microswimmers for Biomedical Applications. Micromachines 2020, 11, 1048. [Google Scholar] [CrossRef]
- Hartmann, F.; Baumgartner, M.; Kaltenbrunner, M. Becoming Sustainable, The New Frontier in Soft Robotics. Adv. Mater. 2021, 33, 2004413. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Jang, B.; Ahmed, D.; Hu, C.; De Marco, C.; Hoop, M.; Mushtaq, F.; Nelson, B.J.; Pané, S. Small-Scale Machines Driven by External Power Sources. Adv. Mater. 2018, 30, 1705061. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.W.; Singh, A.V.; Sitti, M. Bioengineered and Biohybrid Bacteria-Based Systems for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-Mediated Delivery of Nanoparticles and Cargo into Cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, S.-H.; Cho, S.; Kim, D.-M.; Lee, Y.; Ko, S.Y.; Hong, Y.; Choy, H.E.; Min, J.-J.; Park, J.-O.; et al. New Paradigm for Tumor Theranostic Methodology Using Bacteria-Based Microrobot. Sci. Rep. 2013, 3, 3394. [Google Scholar] [CrossRef] [Green Version]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Zhong Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-Aerotactic Bacteria Deliver Drug-Containing Nanoliposomes to Tumour Hypoxic Regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Du Nguyen, V.; Han, J.-W.; Choi, Y.J.; Cho, S.; Zheng, S.; Ko, S.Y.; Park, J.-O.; Park, S. Active Tumor-Therapeutic Liposomal Bacteriobot Combining a Drug (Paclitaxel)-Encapsulated Liposome with Targeting Bacteria (Salmonella Typhimurium). Sens. Actuators B Chem. 2016, 224, 217–224. [Google Scholar] [CrossRef]
- Singh, A.V.; Hosseinidoust, Z.; Park, B.W.; Yasa, O.; Sitti, M. Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery. ACS Nano 2017, 11, 9759–9769. [Google Scholar] [CrossRef]
- Joshi, S.J.; Abed, R.M.M. Biodegradation of Polyacrylamide and Its Derivatives. Environ. Process. 2017, 4, 463–476. [Google Scholar] [CrossRef]
- Yoon, C.K. Advances in Biomimetic Stimuli Responsive Soft Grippers. Nano Converg. 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-Folding All-Polymer Thermoresponsive Microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- McClelland, R.; Dennis, R.; Reid, L.M.; Palsson, B.; Macdonald, J.M. 7-TISSUE ENGINEERING. In Introduction to Biomedical Engineering, 2nd ed.; Academic Press: Boston, MA, USA, 2005; pp. 313–402. [Google Scholar]
- Berthiaume, F.; Yarmush, M.L. Tissue Engineering. In Encyclopedia of Physical Science and Technology, 3rd ed.; Elsevier Science Ltd.: San Diego, CA, USA, 2003; pp. 817–842. [Google Scholar]
- Wei, T.; Liu, J.; Li, D.; Chen, S.; Zhang, Y.; Li, J.; Fan, L.; Guan, Z.; Lo, C.M.; Wang, L.; et al. Development of Magnet-Driven and Image-Guided Degradable Microrobots for the Precise Delivery of Engineered Stem Cells for Cancer Therapy. Small 2020, 16, 1906908. [Google Scholar] [CrossRef]
- Huang, K.; Zhu, T.; Nie, J.; Du, J.; Liu, Y.; Bao, Y.; Chen, S.; Hu, S. Microporous Spongy Scaffolds Based on Biodegradable Elastic Polyurethanes for the Migration and Growth of Host Cells. ACS Appl. Polym. Mater. 2022, 4, 3942–3951. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Xiao, X.; Chen, S.; Wang, Y. Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-Lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds. Polymers 2019, 11, 1927. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.-T.; Dai, N.-T.; Hsu, S. Biodegradable Water-Based Polyurethane Scaffolds with a Sequential Release Function for Cell-Free Cartilage Tissue Engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, 2005759. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Su, W.; Zhang, X.; Chen, C.; Zhao, S.; Yan, X.; Jiang, J.; Zhu, T.; Zhao, J. Cowpea-like Bi-Lineage Nanofiber Mat for Repairing Chronic Rotator Cuff Tear and Inhibiting Fatty Infiltration. Chem. Eng. J. 2020, 392, 123671. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, L.; Yang, Q.; Huang, S.; Shi, H.; Long, Q.; Qian, B.; Liu, Z.; Guan, Q.; Liu, M.; et al. Self-Healing Polyurethane-Elastomer with Mechanical Tunability for Multiple Biomedical Applications in Vivo. Nat. Commun. 2021, 12, 4395. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Shrestha, B.K.; Lee, J.; Joong, O.K.; Kim, B.-S.; Park, C.H.; Kim, C.S. A Conducting Neural Interface of Polyurethane/Silk-Functionalized Multiwall Carbon Nanotubes with Enhanced Mechanical Strength for Neuroregeneration. Mater. Sci. Eng. C 2019, 102, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Gögele, C.; Müller, S.; Belov, S.; Pradel, A.; Wiltzsch, S.; Lenhart, A.; Hornfeck, M.; Kerling, V.; Rübling, A.; Kühl, H.; et al. Biodegradable Poly(d-l-Lactide-Co-Glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells 2022, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.; Geurts, J.A.P.; Arts, J.J.; Lindfors, N.C. 3-Biomaterials in Treatment of Orthopedic Infections. In Management of Periprosthetic Joint Infections (PJIs), 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 41–68. [Google Scholar]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.M.; Day, D.; Sakiyama-Elbert, S.E.; Harkins, A.B. Effects of Borate-Based Bioactive Glass on Neuron Viability and Neurite Extension. J. Biomed. Mater. Res. Part A 2014, 102, 2767–2775. [Google Scholar] [CrossRef]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peştean, C.; Nagy, A.; Tăbăran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin Wound Regeneration with Bioactive Glass-Gold Nanoparticles Ointment. Biomed. Mater. 2019, 14, 25011. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-Incorporated Bioactive Glass-Ceramics Inducing Anti-Inflammatory Phenotype and Regeneration of Cartilage/Bone Interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, D. Cartilage Tissue Engineering Using PHBV and PHBV/Bioglass Scaffolds. Mol. Med. Rep. 2014, 10, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xue, K.; Li, H.; Sun, J.; Liu, K. Improvement of PHBV Scaffolds with Bioglass for Cartilage Tissue Engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef] [PubMed]
- Hirenkumar, M.; Steven, S. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar]
- Li, R.; Wang, L.; Yin, L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials 2018, 11, 2108. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.M.; Hou, X.F.; Bisoyi, H.K.; Feng, W.J.; Cao, Q.; Huang, S.; Yang, H.; Chen, D.; Li, Q. Light-Fueled Transient Supramolecular Assemblies in Water as Fluorescence Modulators. Nat. Commun. 2021, 12, 4993. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Feng, W.J.; Bisoyi, H.K.; Zhang, S.; Chen, X.; Yang, H.; Li, Q. Light-Activated Photodeformable Supramolecular Dissipative Self-Assemblies. Nat. Commun. 2022, 13, 3216. [Google Scholar] [CrossRef]
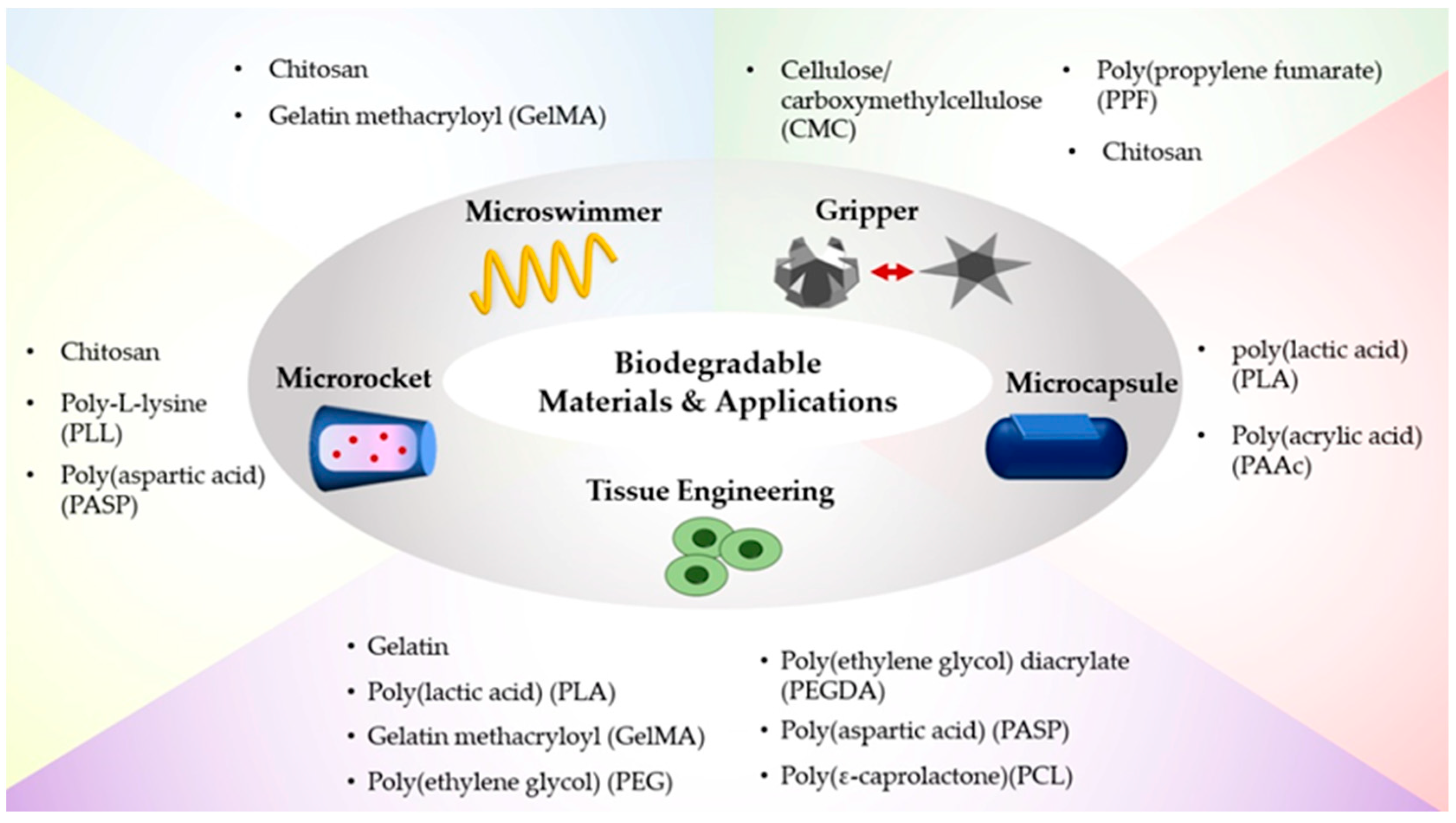


| Type | Material | Advantage | Disadvantage | Application |
|---|---|---|---|---|
| Natural polymer | Chitosan | Enzymatically degraded by lysozyme and chitosanase enzymes [35] | Water-insoluble, unstable, toxic at hydrogel phase [70] | Targeted drug/cell delivery [32] Gripper [33] |
| Cellulose/carboxymethylcellulose (CMC) | Biocompatible, soft, transparency, high viscosity at low concentrations, swelling at high pH [32,37] | Weak mechanical properties [71] | Gripper [33] | |
| Gelatin | Low gelation temperature: 22.4–25.2 °C [38,39] Non-toxic, high water absorption, biocompatible [40,41,42] | Weak mechanical properties [72] | Tissue engineering [73] Drug delivery [74] | |
| Synthetic polymer | Poly(lactic acid) (PLA) | Degraded by the hydrolysis of ester bonds without requiring any enzymes [44] | Slow degradation rate, hydrophobicity, low impact toughness [44] | Drug delivery [75] Surgical implant [76] Tissue engineering [76] |
| Poly-L-lysine (PLL) | Hydrophilic, biocompatible [56] | Cytotoxicity increases with its molecular weight [62] | Gene delivery [59,62] | |
| Gelatin methacryloyl (GelMA) | Degraded by cell-released enzymes [64] | Low mechanical strength (~50 to 150 KPa), short degradation time (~7 to 14 days) [63] | Drug delivery [64,65] Tissue engineering [69,77] | |
| Poly(ethylene glycol) (PEG) | Non-ionic, low inflammation [78] | Low mechanical strength [79] | Tissue engineering [80] | |
| Poly(ethylene glycol) diacrylate (PEGDA) | Mechanical stability [79] | Slow degradation rate in vivo [81] | Drug delivery [82] Tissue engineering [68] | |
| Poly(propylene fumarate) (PPF) | Biocompatible, non-toxic [83,84,85,86,87] | Mechanical strength loss, brittleness during degradation [83] | Gripper [88,89] | |
| Poly(aspartic acid) (PASP) | Smooth, intact, robust [90] | Complex synthesis [91] | Drug delivery [90] Tissue engineering [91] | |
| Poly(acrylic acid) (PAAc) | Water-soluble, high molecular-weight, pH-responsive [92] | Low mechanical strength [92] | Drug delivery [93] | |
| Poly(ε-caprolactone) (PCL) | Semi-rigid at room temperature [94] | Slow degradation rate, low stiffness [95] | Tissue engineering [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, H.; Yoon, C. Advances in Biodegradable Soft Robots. Polymers 2022, 14, 4574. https://doi.org/10.3390/polym14214574
Kim J, Park H, Yoon C. Advances in Biodegradable Soft Robots. Polymers. 2022; 14(21):4574. https://doi.org/10.3390/polym14214574
Chicago/Turabian StyleKim, Jiwon, Harim Park, and ChangKyu Yoon. 2022. "Advances in Biodegradable Soft Robots" Polymers 14, no. 21: 4574. https://doi.org/10.3390/polym14214574
APA StyleKim, J., Park, H., & Yoon, C. (2022). Advances in Biodegradable Soft Robots. Polymers, 14(21), 4574. https://doi.org/10.3390/polym14214574











