Preparation and Performance Characterization of Exploding Foil Initiator Based on ODPA-ODA Polyimide Flyer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
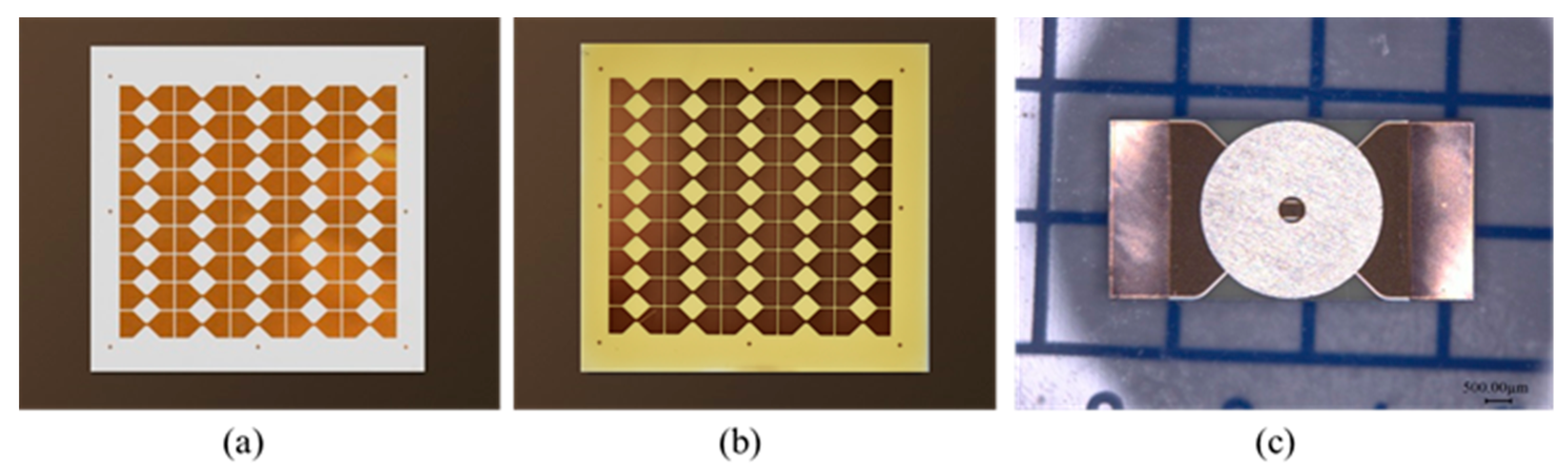
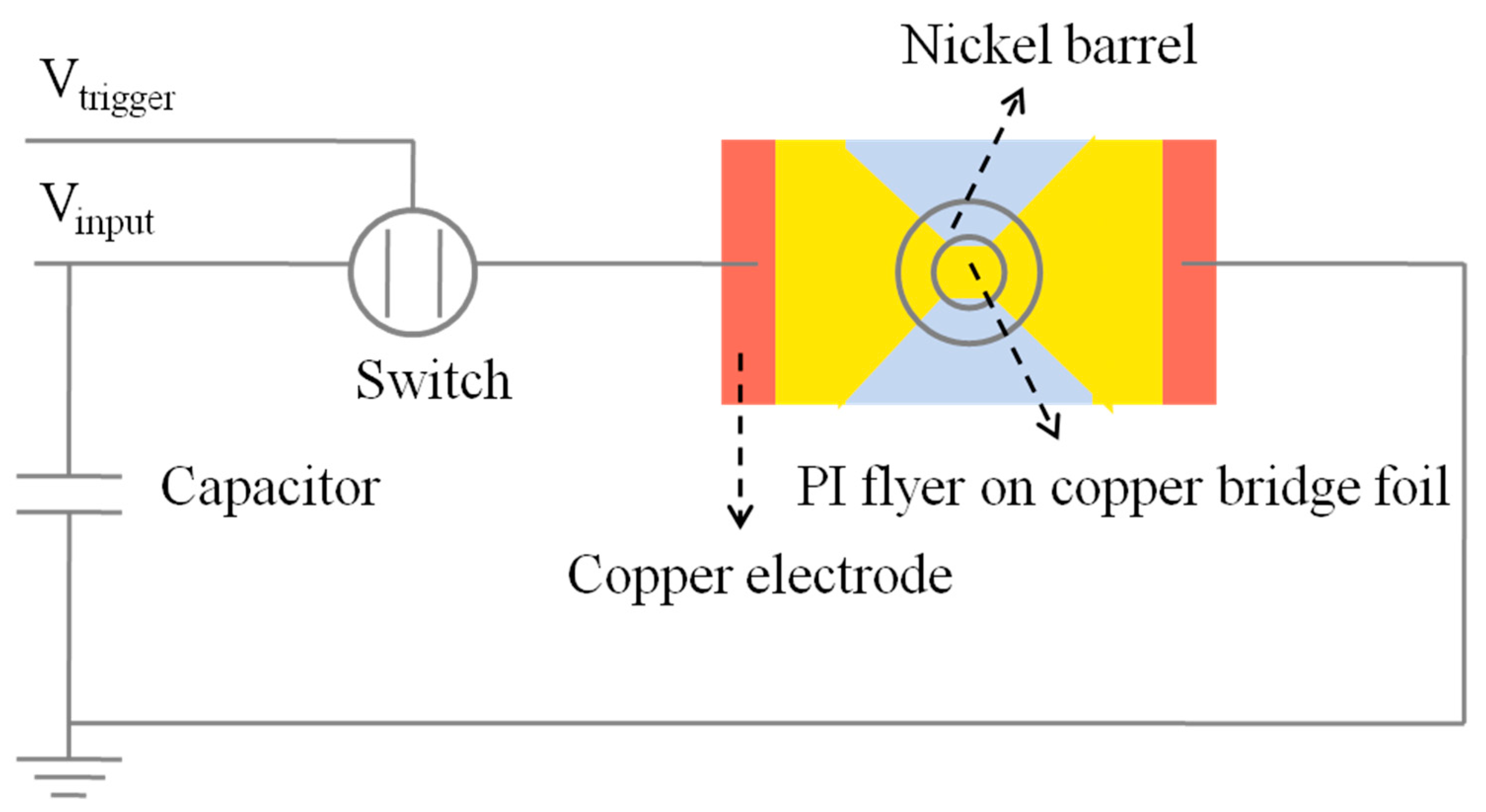
2.2. Fabrication of EFI Based on ODPA-ODA Polyimide Flyer
2.3. Characterization
2.4. Electric Explosion Experiment
3. Results and Discussion
3.1. The Properties of Polyimide Film
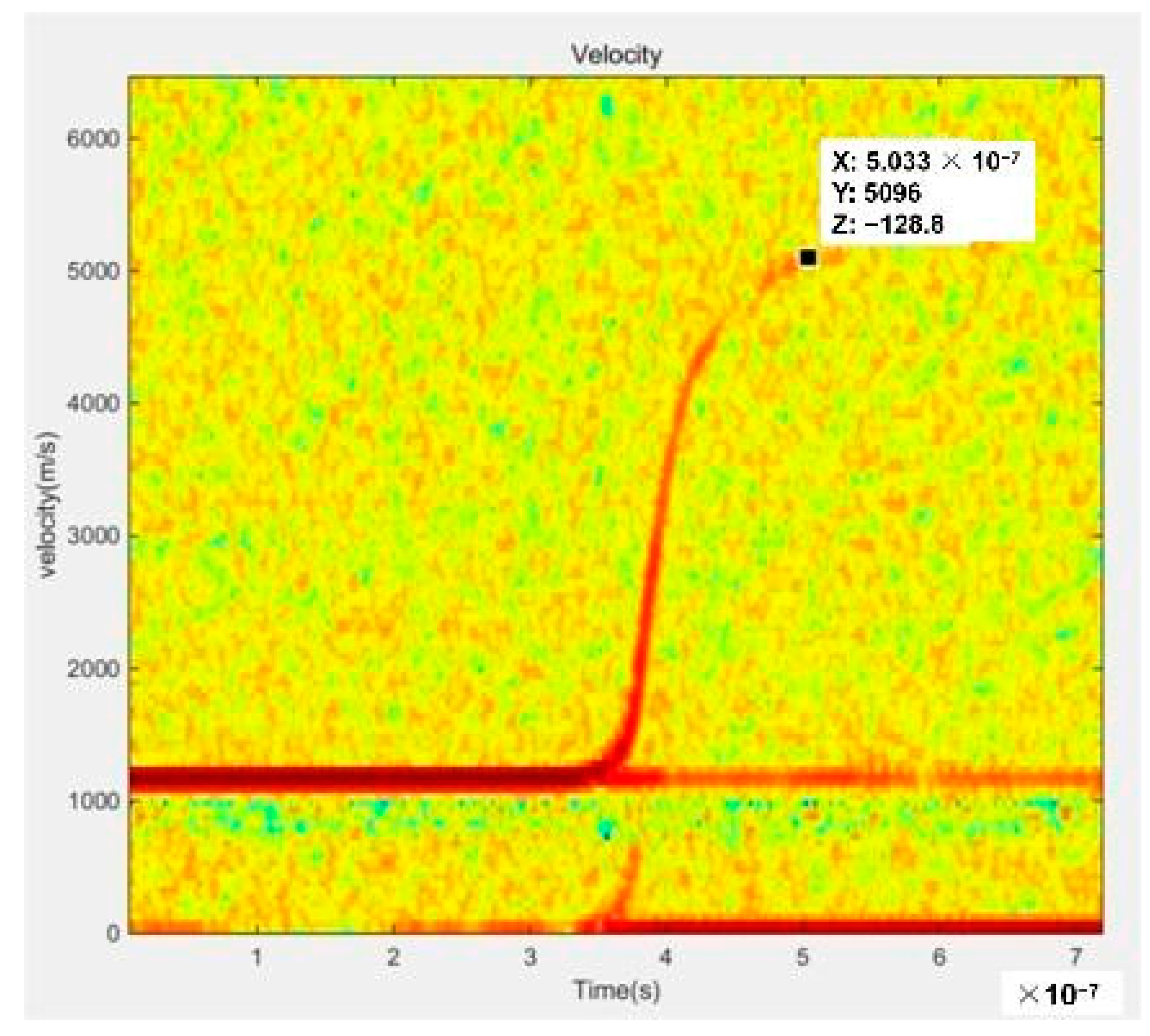
3.2. The Performance of the EFI
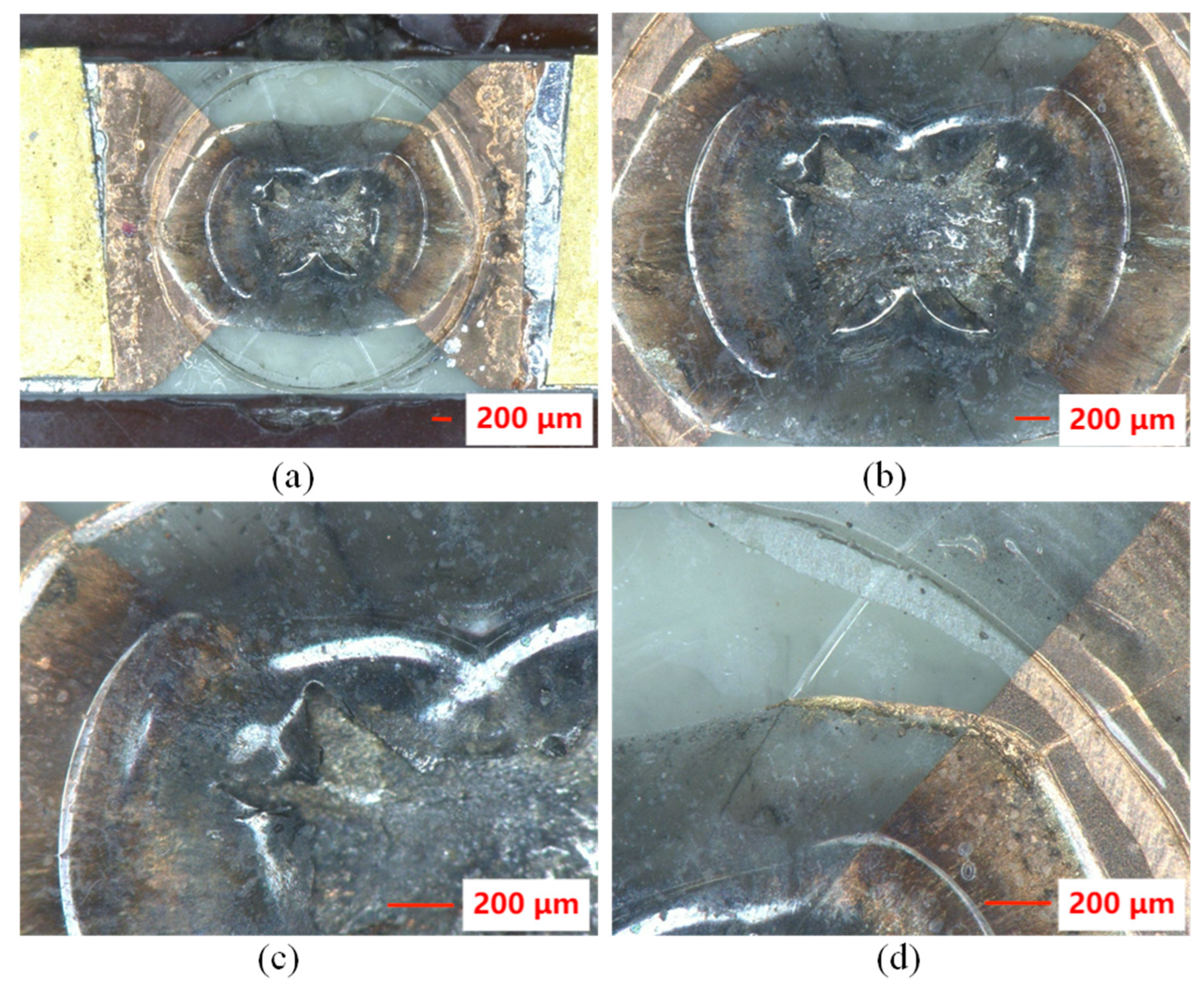
3.3. The Morphology of Flyer Plate after the Electric Explosion Test
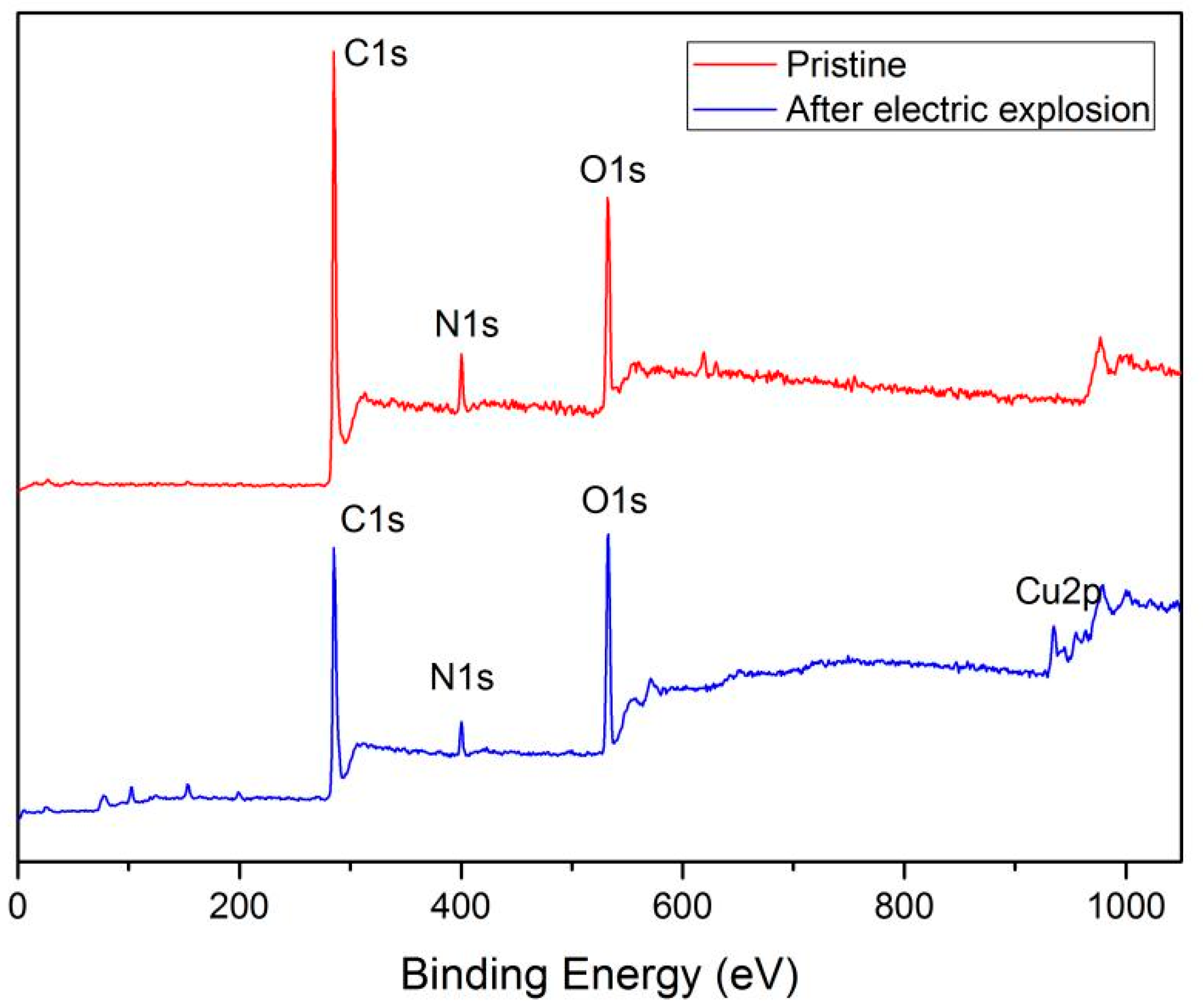
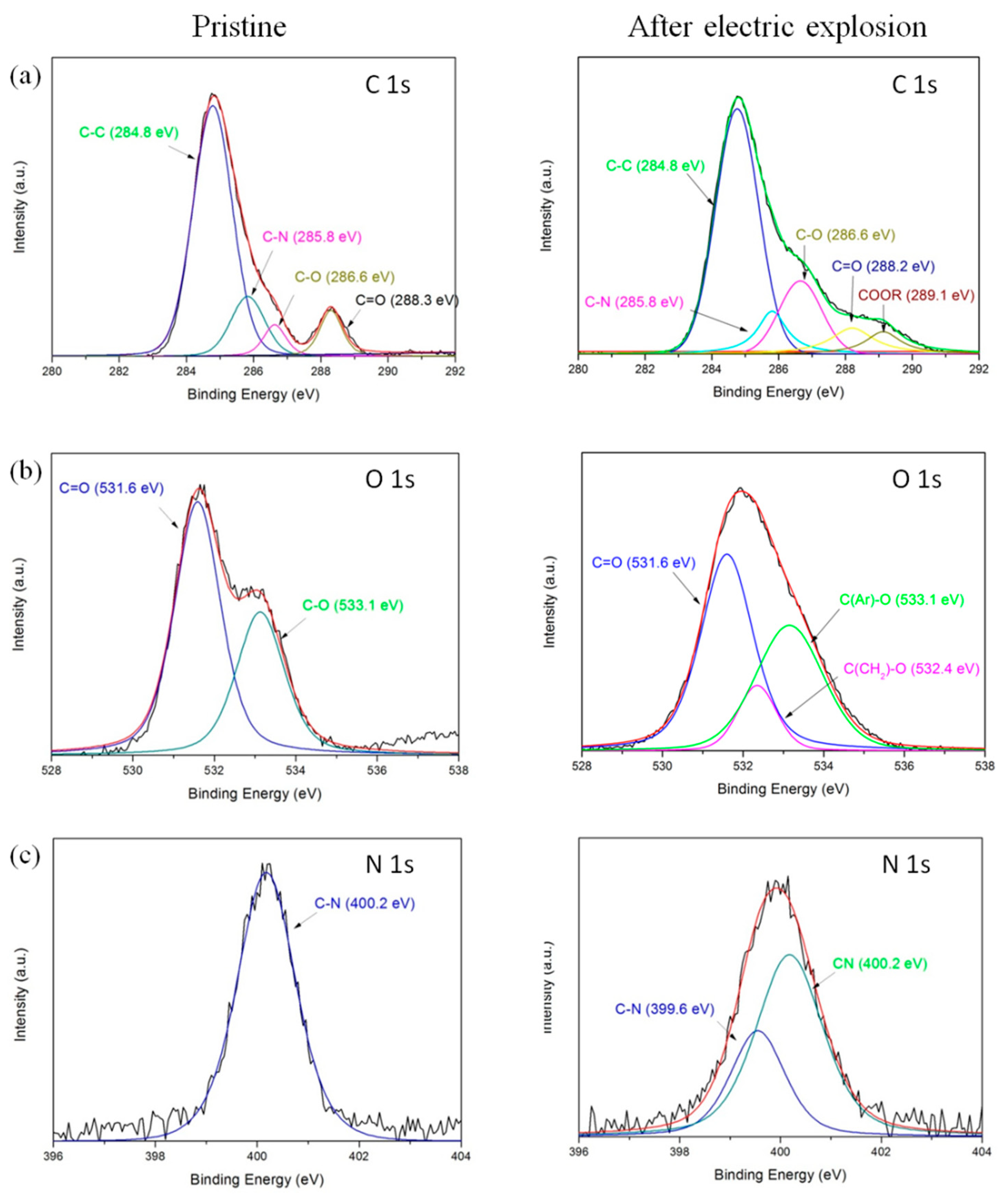
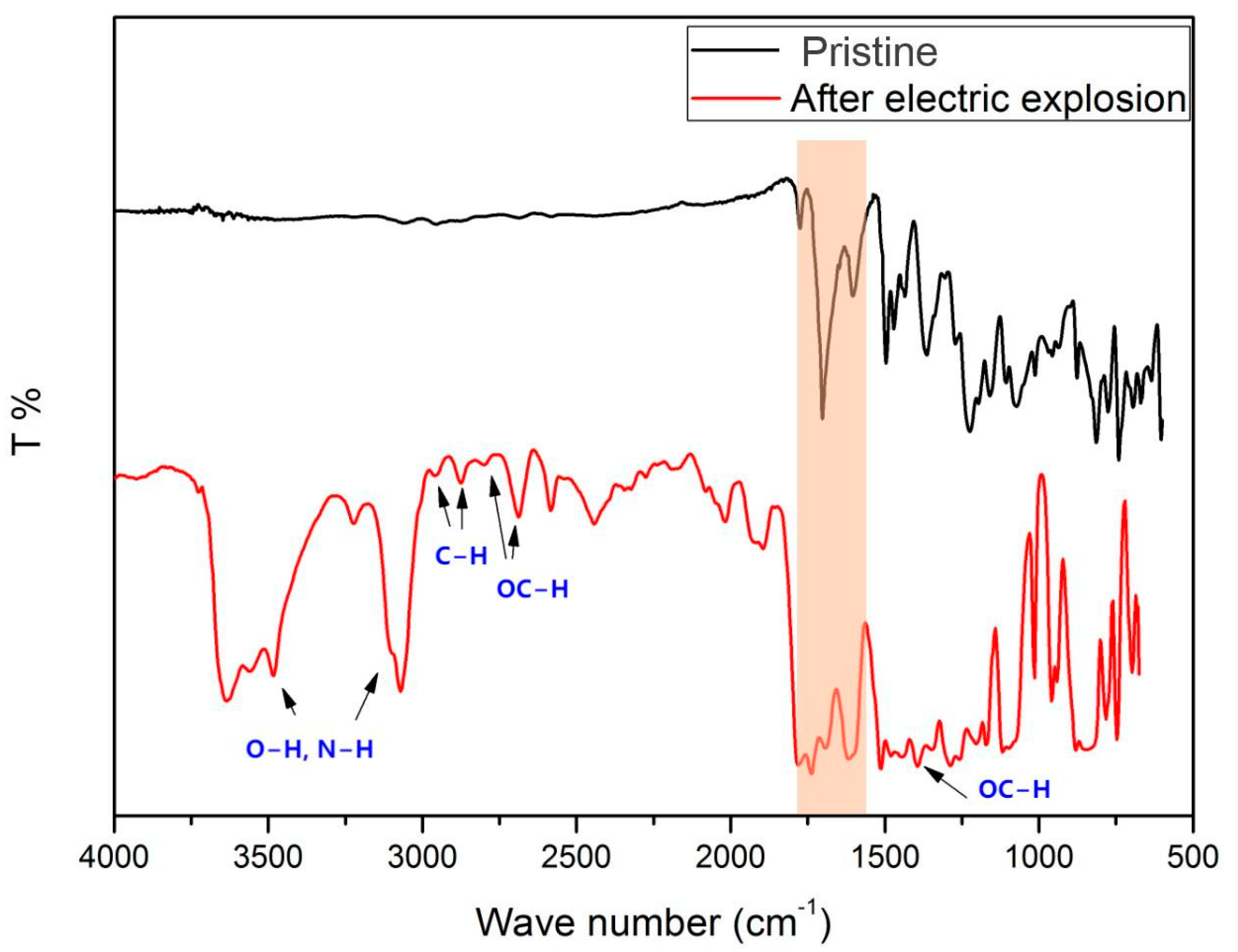
3.4. Characterization of Chemical Composition of Polyimide Film Flyer after the Electric Explosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Waschl, J.; Hatt, D. Characterization of a small-scale exploding bridge foil flyer generator. Int. J. Impact Eng. 1993, 14, 785–796. [Google Scholar] [CrossRef]
- Yu, H.; Kim, B.; Jang, S.-G.; Kim, K.-H.; Yoh, J.J. Performance characterization of a miniaturized exploding foil initiator via modified VISAR interferometer and shock wave analysis. J. Appl. Phys. 2017, 121, 215901. [Google Scholar] [CrossRef] [Green Version]
- Willey, T.M.; Champley, K.; Hodgin, R.; Lauderbach, L.; Bagge-Hansen, M.; May, C.; Sanchez, N.; Jensen, B.J.; Iverson, A.; van Buuren, T. X-ray imaging and 3D reconstruction of in-flight exploding foil initiator flyers. J. Appl. Phys. 2016, 119, 235901. [Google Scholar] [CrossRef]
- Ichihara, D.; Fukushima, G.; Kuwabara, D.; Sasoh, A. Energy conversion efficiency of electrical exploding foil accelerators. AIP Adv. 2021, 11, 095203. [Google Scholar] [CrossRef]
- Borman, A.J.; Dowding, C.F. Characterization of Plasma Formation and Mass Ejection in Exploding Foil Initiators. IEEE Trans. Plasma Sci. 2021, 49, 1159–1165. [Google Scholar] [CrossRef]
- Wang, K.; Xu, C.; Zhu, P.; Zhang, Q.; Yang, Z.; Shen, R. Strategy for increasing flyer launching capacity of electro-explosively actuator by coupling electric explosion and plasma discharge. Sens. Actuators A Phys. 2021, 322, 112609. [Google Scholar] [CrossRef]
- Takeda, N.; Komatsu, H.; Takahashi, K. Spall fracture characterization of thermosetting and thermoplastic polymer matrix composite plates. Adv. Compos. Mater. 1993, 3, 85–97. [Google Scholar] [CrossRef]
- Wang, G.; He, J.; Zhao, J.; Tan, F.; Sun, C.; Mo, J.; Xong, X.; Wu, G. The techniques of metallic foil electrically exploding driving hy-pervelocity flyer to more than 10 km/s for shock wave physics experiments. Rev. Sci. Instrum. 2011, 82, 095105. [Google Scholar] [CrossRef]
- Fu, Q.B.; Wang, Y.; Wang, M.; Qin, W.Z.; Guo, F. Experimental Study on Velocity of Flyer Based on Silicon. Key Eng. Mater. 2015, 645–646, 766–770. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, P.; Chen, K.; Zhang, W.; Shen, R.; Ye, Y. A Highly Integrated Conjoined Single Shot Switch and Exploding Foil Initiator Chip Based on MEMS Technology. IEEE Electron Device Lett. 2017, 38, 1610–1613. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocom posites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in Aromatic Polyimide Films for Electronic Applications: Preparation, Structure and Properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Ruan, K.; Guo, Y.; Gu, J. Liquid Crystalline Polyimide Films with High Intrinsic Thermal Conductivities and Robust Toughness. Macromolecules 2021, 54, 4934–4944. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Sanchez, N.J.; Jensen, B.J.; Neal, W.D.; Iverson, A.J.; Carlson, C.A. Dynamic exploding foil initiator imaging at the advanced photon source. AIP Conf. Proc. 2018, 1979, 160023. [Google Scholar] [CrossRef]
- Sun, L.; Zhan, Z.; Wu, Z.; Zhou, R.; Zhu, Y.; Yi, T.; Luo, J.; Niu, G.; Lei, F.; Wang, J.; et al. Optimizing cure temperature of polyimide flyer for exploding foil initiator applications. J. Appl. Polym. Sci. 2022, 139, e53072. [Google Scholar] [CrossRef]
- Han, K.; Deng, P.; Chu, E.; Jiao, Q. Effect of Grain Size and Micromorphology of Cu Foil on the Velocity of Flyer of Exploding Foil Detonator. Appl. Sci. 2021, 11, 6598. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Xu, C.; Zhang, Q.; Qin, X.; Shen, R. Review on Micro Chip Exploding Foil Initiator and Its Planar High-Voltage Switch. Chin. J. Eng. Mater. 2019, 27, 167–176. [Google Scholar]
- Desai, A. Efficient Exploding Foil Initiator and Process for Making Same. U.S. Patent US7938065, 10 May 2011. [Google Scholar]
- Xu, C.; Zhu, P.; Wang, K.; Qin, X.; Zhang, Q.; Yang, Z.; Shen, R. An electro-explosively actuated mini-flyer launcher. Sens. Actuators A Phys. 2019, 292, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Wu, Y.; Wang, D.; Wang, T. Facile preparation of ODPA-ODA type polyetherimide-based carbon membranes by chemical crosslinking. J. Appl. Polym. Sci. 2017, 134, 44889. [Google Scholar] [CrossRef]
- Chen, K.-M.; Wang, T.-H.; King, J.-S.; Hung, A. Effect of imidization temperature on properties of polymide films. J. Appl. Polym. Sci. 1993, 48, 291–297. [Google Scholar] [CrossRef]
- Taylor, M.J. Formation of plasma around wire fragments created by electrically exploded copper wire. J. Phys. D Appl. Phys. 2002, 35, 700–709. [Google Scholar] [CrossRef]
- Lamb, R.N.; Baxter, J.; Grunze, M.; Kong, C.W.; Unertl, W.N. An XPS study of the composition of thin polyimide films formed by vapor deposition. Langmuir 1988, 4, 249–256. [Google Scholar] [CrossRef]
- Ektessabi, A.; Hakamata, S. XPS study of ion beam modified polyimide films. Thin Solid Films 2000, 377–378, 621–625. [Google Scholar] [CrossRef]
- Dong, S.-S.; Shao, W.-Z.; Yang, L.; Ye, H.-J.; Zhen, L. Surface characterization and degradation behavior of polyimide films induced by coupling irradiation treatment. RSC Adv. 2018, 8, 28152–28160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Bu, J.; Liu, W.; Niu, H.; Qi, S.; Tian, G.; Wu, D. Surface modification of polyimide fibers by oxygen plasma treatment and interfacial adhesion behavior of a polyimide fiber/epoxy composite. Sci. Eng. Compos. Mater. 2017, 24, 477–484. [Google Scholar] [CrossRef]



| Property | Test Data |
|---|---|
| Modulus | 4.42 Gpa |
| Hardness | 0.37 Gpa |
| Dielectric constant | 3.29 (1 MHz, 18 °C) |
| Voltage breakdown field strength | 3.9 × 106 V/m |
| Leakage current density | 1.2 × 10−4 A/cm2 |
| Sample | Atomic Concentration (%) | |||
|---|---|---|---|---|
| C | O | N | Cu | |
| Pristine polyimide film | 72.85 | 20.87 | 6.29 | - |
| Polyimide film after the electric explosion test | 70.51 | 22.5 | 6.91 | 0.08 |
| Sample | Atomic Concentration (%) | |||
|---|---|---|---|---|
| C 1s | O 1s | N 1s | ||
| Pristine polyimide film | 75.41 | 17.63 | 6.96 | |
| Polyimide film after the electric explosion | P1 | 70.52 | 24.06 | 5.42 |
| P2 | 64.58 | 30.36 | 5.07 | |
| P3 | 70.4 | 24.67 | 4.93 | |
| Peaks | Bonds | Ebind (eV) | Proportion (%) | |
|---|---|---|---|---|
| Pristine | after The Electric Explosion | |||
| C1s | C–C | 284.8 | 70.93 | 57.49 |
| C–N | 285.8 | 14.89 | 10.2 | |
| C–O | 286.6 | 5.77 | 16.88 | |
| C=O | 288.3 | 8.41 | 9.1 | |
| COOR | 289.1 | 0 | 6.33 | |
| O1s | C=O | 531.6 | 62.89 | 50.49 |
| CH2–O | 532.4 | 0 | 11.41 | |
| Ar–O | 533.1 | 37.11 | 38.1 | |
| N1s | C–N | 400.2 | 100 | 63.1 |
| C≡N | 399.6 | 0 | 36.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Lei, F.; Zhan, Z.; Luo, J.; Niu, G.; Li, Z.; Yi, T.; Chen, S.; Yang, B.; Fu, Q.; et al. Preparation and Performance Characterization of Exploding Foil Initiator Based on ODPA-ODA Polyimide Flyer. Polymers 2022, 14, 4604. https://doi.org/10.3390/polym14214604
Wu Z, Lei F, Zhan Z, Luo J, Niu G, Li Z, Yi T, Chen S, Yang B, Fu Q, et al. Preparation and Performance Characterization of Exploding Foil Initiator Based on ODPA-ODA Polyimide Flyer. Polymers. 2022; 14(21):4604. https://doi.org/10.3390/polym14214604
Chicago/Turabian StyleWu, Zhiqing, Fan Lei, Zhiqiang Zhan, Jiangshan Luo, Gao Niu, Zhaoguo Li, Tao Yi, Shufan Chen, Bo Yang, Qiubo Fu, and et al. 2022. "Preparation and Performance Characterization of Exploding Foil Initiator Based on ODPA-ODA Polyimide Flyer" Polymers 14, no. 21: 4604. https://doi.org/10.3390/polym14214604
APA StyleWu, Z., Lei, F., Zhan, Z., Luo, J., Niu, G., Li, Z., Yi, T., Chen, S., Yang, B., Fu, Q., & Zhang, Z. (2022). Preparation and Performance Characterization of Exploding Foil Initiator Based on ODPA-ODA Polyimide Flyer. Polymers, 14(21), 4604. https://doi.org/10.3390/polym14214604





