Nano Catalysis of Biofuels and Biochemicals from Cotinus coggygria Scop. Wood for Bio-Oil Raw Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
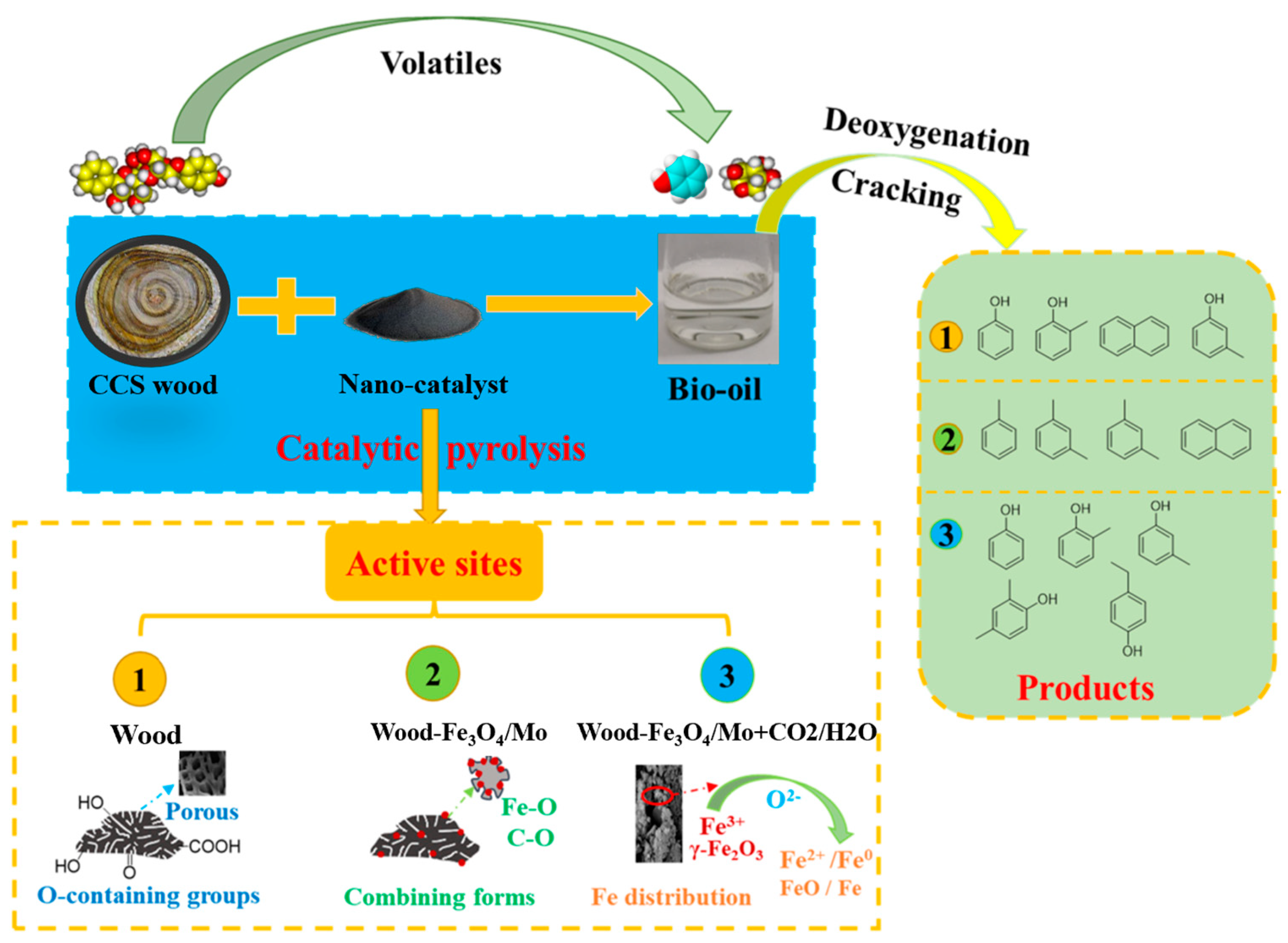
3. Results and Discussion
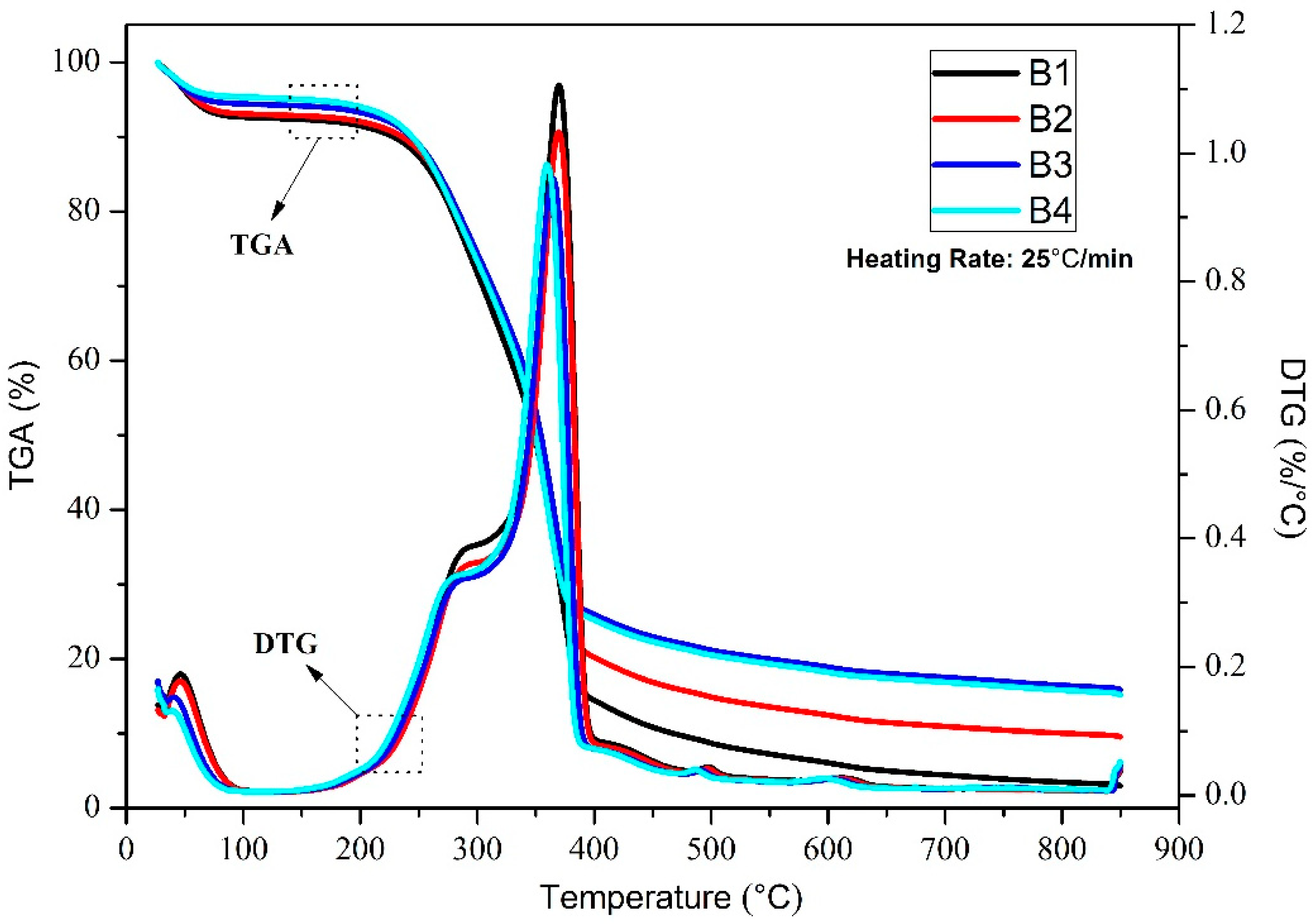
3.1. TG-DTG Analysis
3.2. TG-FTIR Analysis
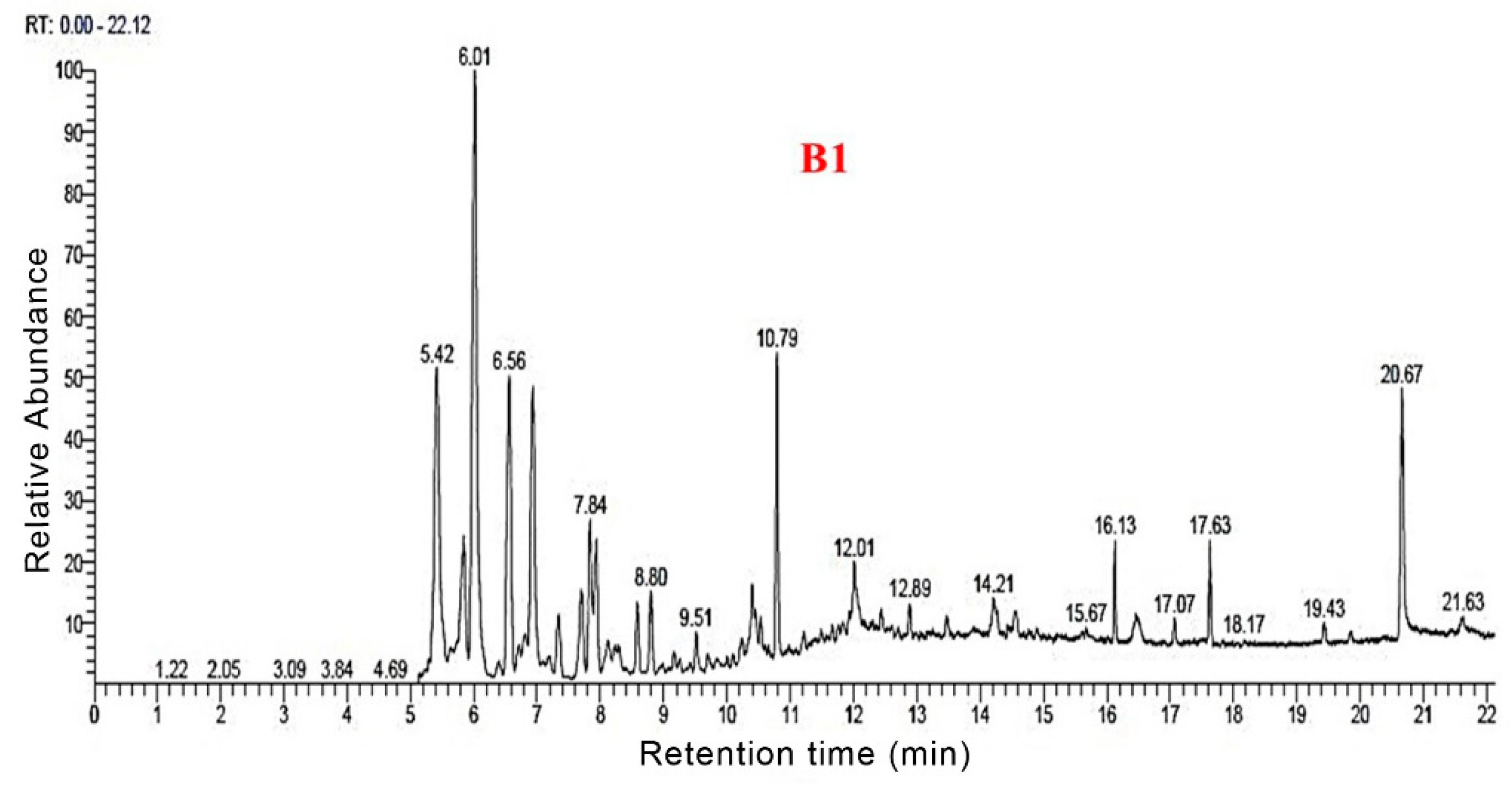
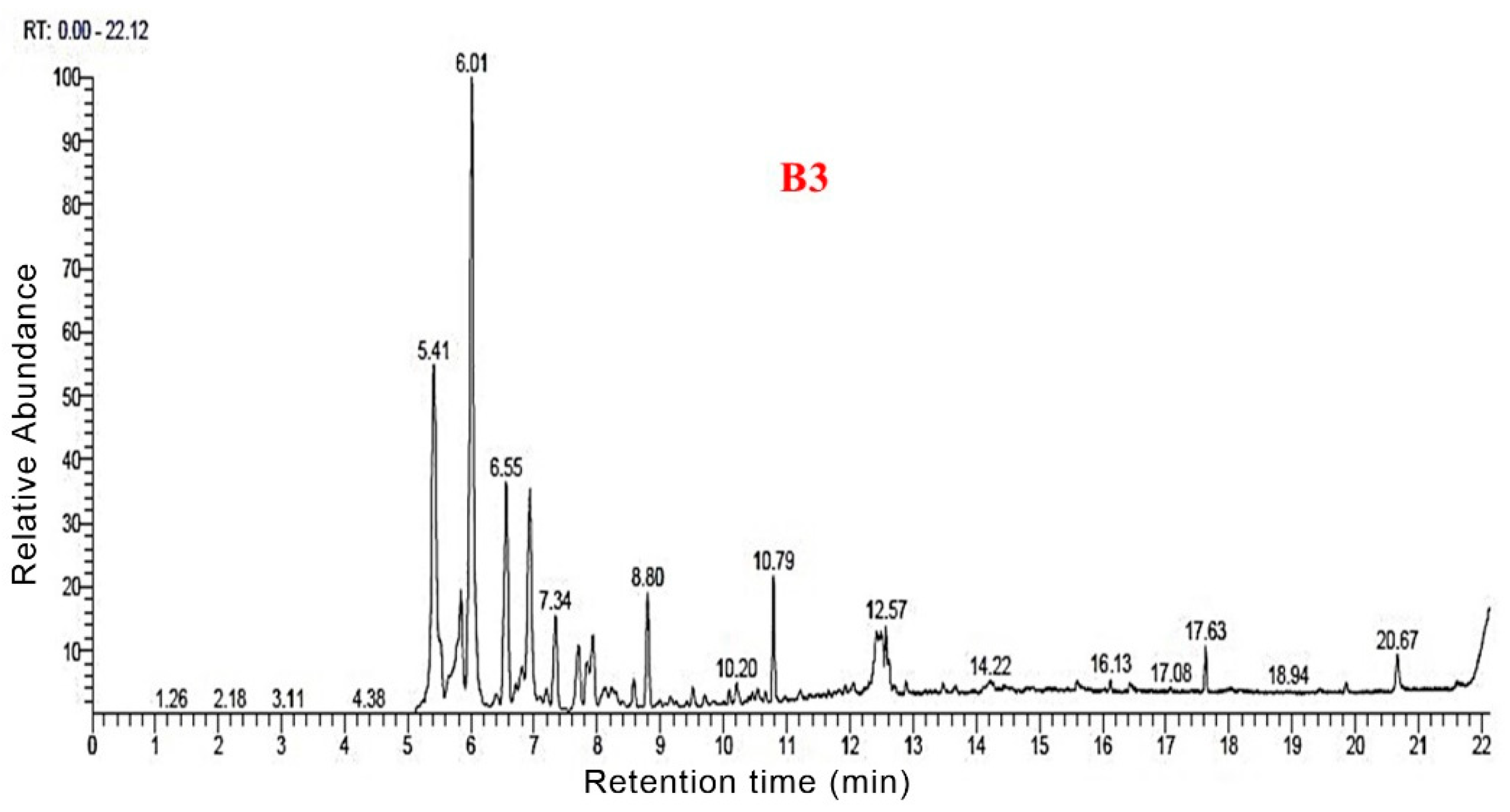
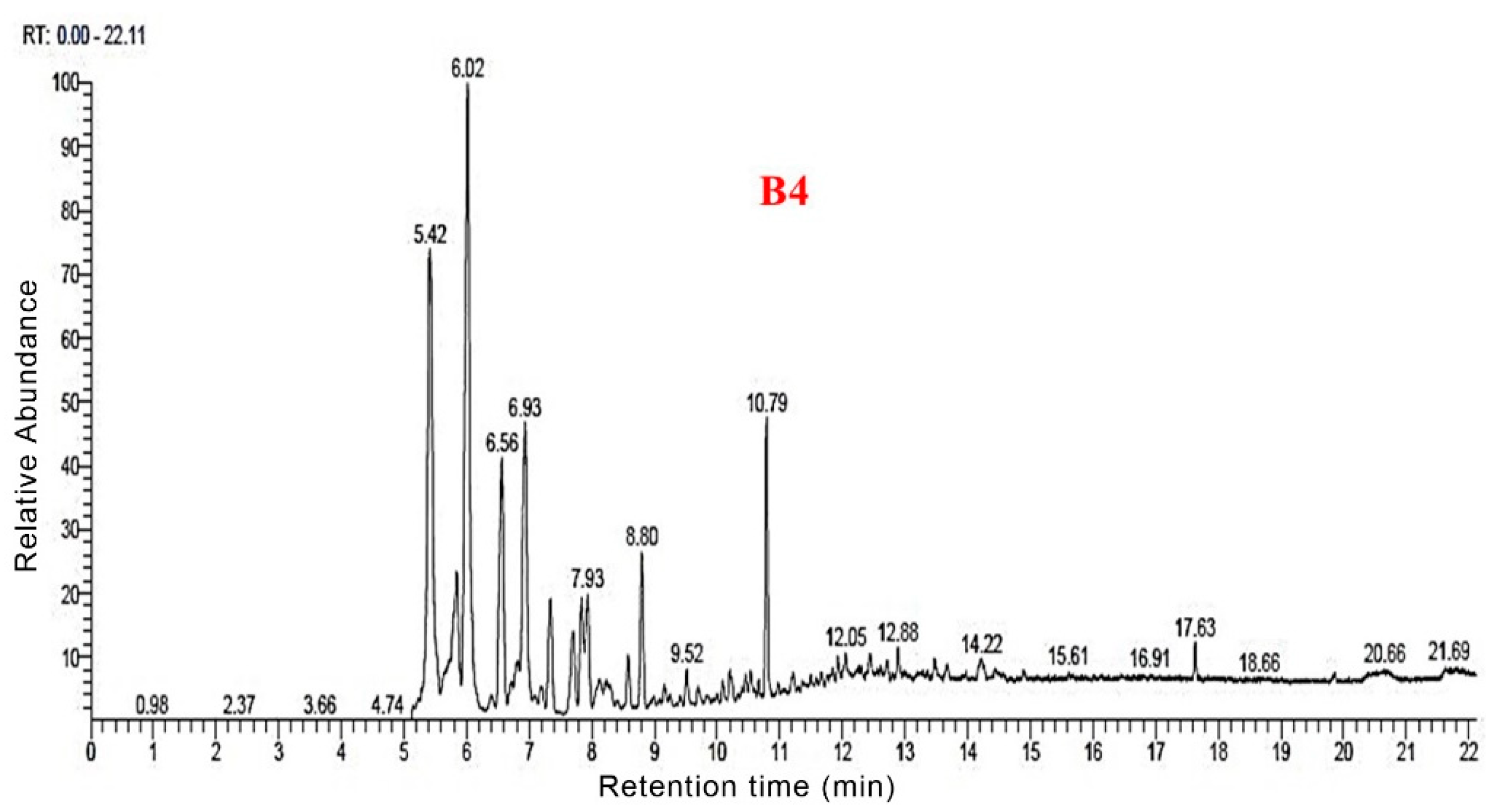
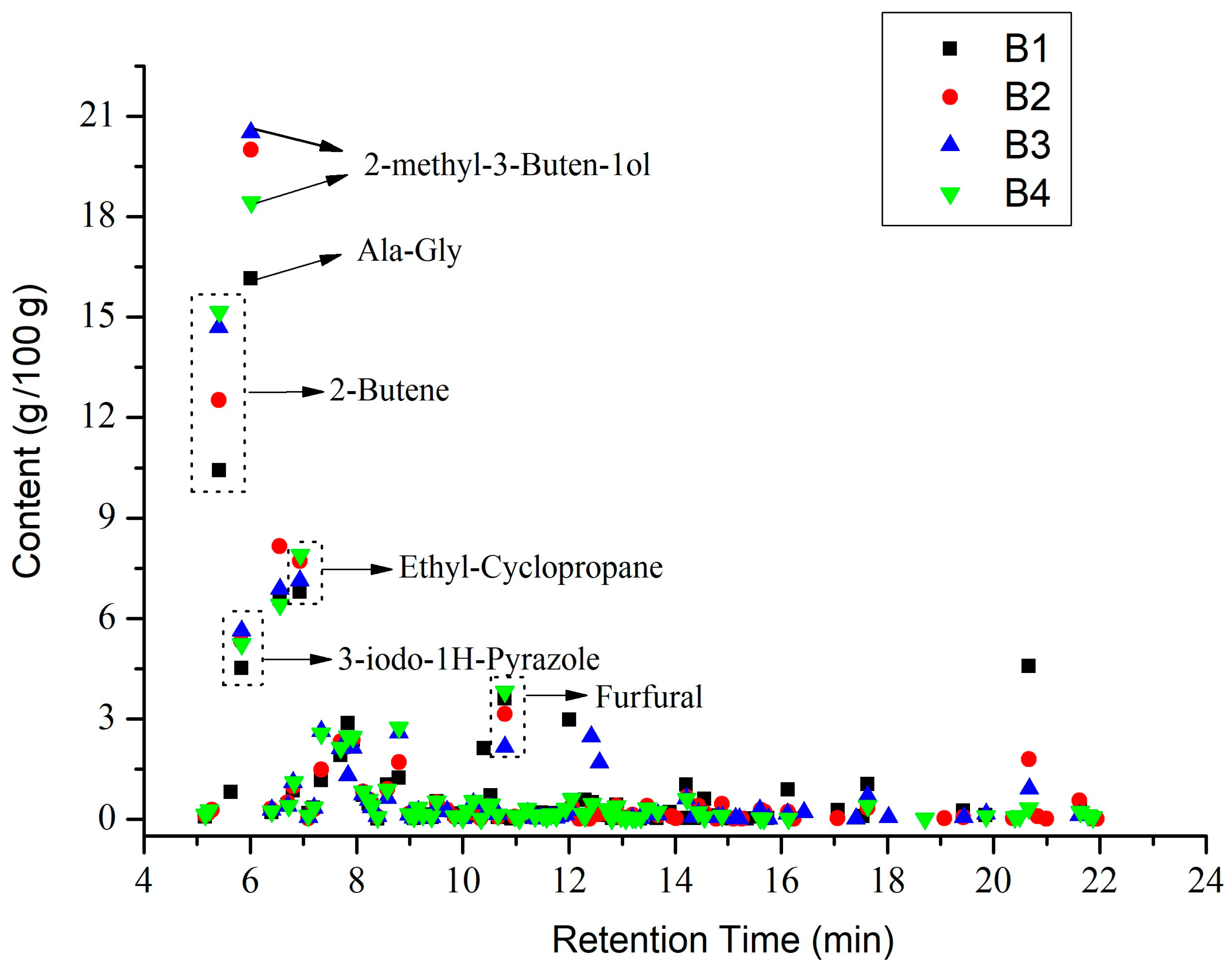
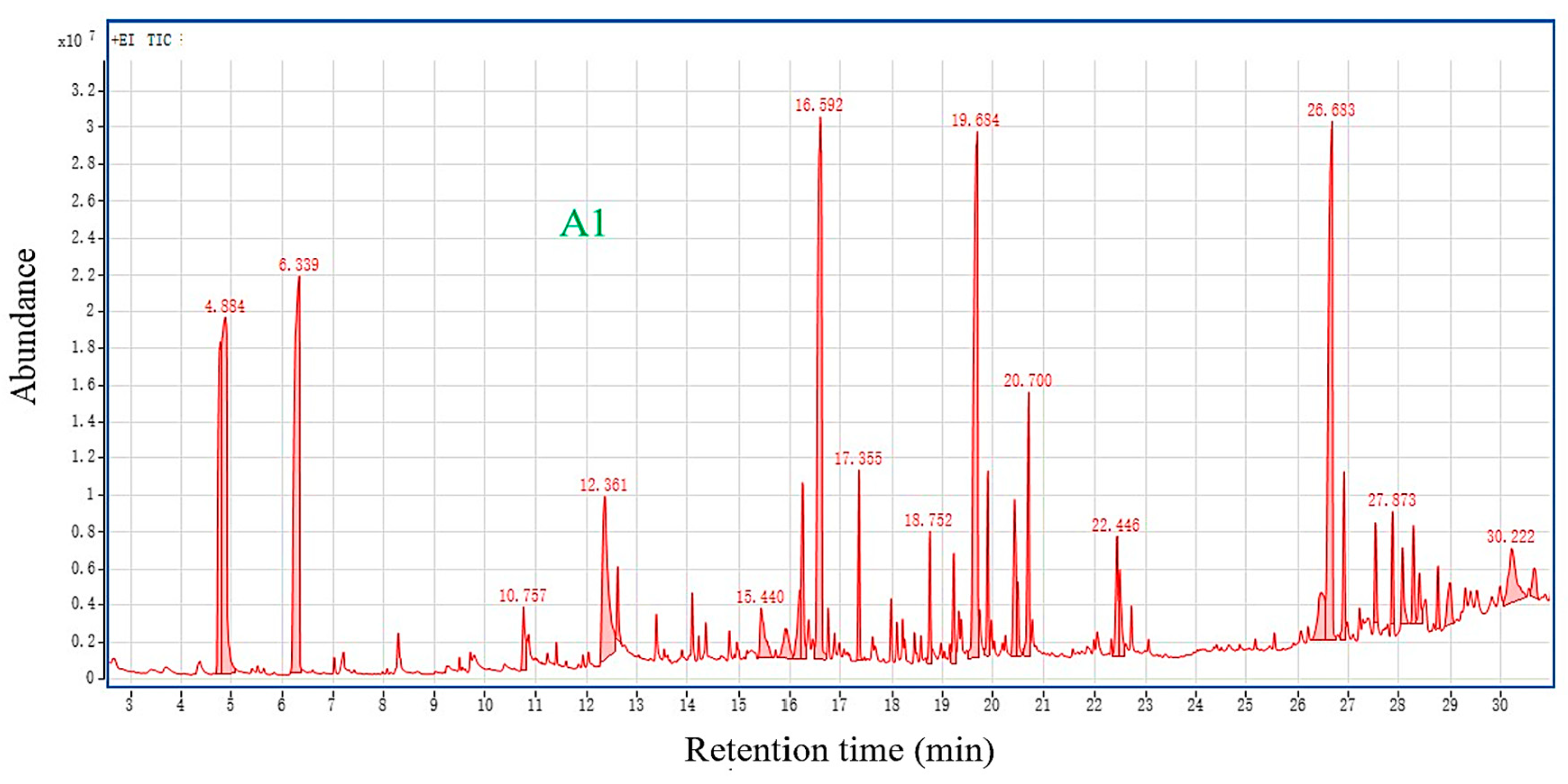
3.3. Py-GC/MS Analysis
3.4. FT-IR Analysis
3.5. GC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guner, S.; Tilki, F. Dormancy breaking in Cotinus coggygria scop. seeds of three provenances. Sci. Res. Essays. 2009, 4, 73–77. [Google Scholar]
- Lin, Q.L.; Su, H.R.; He, H. First Report of Alternaria alternata Causing Leaf Spot on Bruguiera gymnorrhiza in China. Plant Dis. 2015, 99, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.Y.; Li, Y.; Yang, J.; Mao, R.L. Landscape genomics reveal that ecological character determines adaptation: A case study in smoke tree (Cotinus coggygria Scop.). BMC Evol. Biol. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Shaboyan, N.K.; Ghazaryan, A.M.; Chichoyan, N.B. The analysis of chemical composition of smoke trees leaves (cotinuscoggygriascop.), growing in armenian flora by gc-ms method. Toxicon 2019, 159, S29. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Lakka, A.; Valianou, L.; Chryssoulakis, Y. High-performance liquid chromatographic determination of colouring matters in historical garments from the Holy Mountain of Athos. Microchim. Acta 2008, 160, 477–483. [Google Scholar] [CrossRef]
- Niciforovic, N.; Mihailovic, V.; Maskovic, P.; Solujic, S.; Stojkovic, A.; Muratspahic, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Pacholczak, A.Z.J.; Szydlo, W.I.S.; Lukaszewska, A.S. The effect of etiolation and shading of stock plants on rhizogenesis in stem cuttings of Cotinus coggygria. Acta Physiol. Plant. 2005, 27, 417–428. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Harrison-Murray, R.S.; Judd, H.L.; Marks, T.R.; Ford, Y.Y.; Bates, C.H.A. The effects of photoperiod and light spectrum on stock plant growth and rooting of cuttings of Cotinus coggygria ‘Royal Purple’. J. Horticult. Sci. Biotechnol. 2005, 80, 245–253. [Google Scholar] [CrossRef]
- Savikin, K.; Zdunic, G.; Jankovic, T.; Stanojkovic, T.; Juranic, Z.; Menkovic, N. In vitro cytotoxic and antioxidative activity of Cornus mas and Cotinus coggygria. Nat. Prod. Res. 2009, 23, 1731–1739. [Google Scholar] [CrossRef]
- Antal, D.S.; Schwaiger, S.; Ellmerer-Mueller, E.P.; Stuppner, H. Cotinus coggygria Wood: Novel Flavanone Dimer and Development of an HPLC/UV/MS Method for the Simultaneous Determination of Fourteen Phenolic Constituents. Planta Med. 2010, 76, 1765–1772. [Google Scholar] [CrossRef]
- Constantin, R.P.; Constantin, J.; Salgueiro Pagadigorria, C.L.; Ishii-Iwamoto, E.L.; Bracht, A.; Cardoso Ono, M.D.K.; Yamamoto, N.S. The actions of fisetin on glucose metabolism in the rat liver. Cell Biochem. Funct. 2010, 28, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Vuckovic, I.; Janackovic, P.; Sokovic, M.; Filipovic, A.; Tesevic, V.; Milosavljevic, S. Chemical composition, antibacterial and antifungal activity of the essential oils of Cotinus coggygria from Serbia. J. Serb. Chem. Soc. 2007, 72, 1045–1051. [Google Scholar] [CrossRef]
- Ge, S.B.; Liang, Y.Y.; Zhou, C.X.; Sheng, Y.Q.; Zhang, M.L.; Cai, L.P.; Zhou, Y.H.; Huang, Z.H.; Manzo, M.; Wu, C.Y.; et al. The potential of Pinus armandii Franch for high-grade resource utilization. Biomass Bioenergy 2022, 158, 106345. [Google Scholar] [CrossRef]
- Ma, J.H.; Ma, N.L.; Zhang, D.Q.; Wu, N.P.; Liu, X.; Meng, L.; Ma, D.L.; Gao, X.Y.; Chu, Z.Q.; Zhang, P.P.; et al. Zero waste multistage utilization of Ginkgo biloba branches. Chemosphere 2022, 292, 133345. [Google Scholar] [CrossRef]
- Matic, S.; Stanic, S.; Bogojevic, D.; Solujic, S.; Grdovic, N.; Vidakovic, M.; Mihailovic, M. Genotoxic potential of Cotinus coggygria Scop. (Anacardiaceae) stem extract in vivo. Genet. Mol. Biol. 2011, 34, 298–303. [Google Scholar] [CrossRef]
- Bowen, J.L.; Valiela, I. Using delta N-15 to assess coupling between watersheds and estuaries in temperate and tropical regions. J. Coast. Res. 2008, 24, 804–813. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Z.; Wang, L.; Chang, J.; Gu, F.; Zhu, X. Molecular characteristics of Illicium verum extractives to activate acquired immune response. Saudi J. Biol. Sci. 2016, 23, 348–352. [Google Scholar] [CrossRef]
- Hrablay, I.; Jelemensky, L. Kinetic Study of the Thermochemical Degradation of Lignocellulosic Materials Based on TG-FTIR, Py-GC/MS and C-13 NMR Experiments. Chem. Biochem. Eng. Q. 2016, 30, 359–372. [Google Scholar] [CrossRef]
- Fang, S.; Yu, Z.; Ma, X.; Lin, Y.; Chen, L.; Liao, Y. Analysis of catalytic pyrolysis of municipal solid waste and paper sludge using TG-FTIR, Py-GC/MS and DAEM (distributed activation energy model). Energy 2018, 143, 517–532. [Google Scholar] [CrossRef]
- Kok, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Thermal characterization of crude oils in the presence of limestone matrix by TGA-DTG-FTIR. J. Pet. Sci. Eng. 2017, 154, 495–501. [Google Scholar]
- Onal, E.; Uzun, B.B.; Putun, A.E. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers. Manag. 2014, 78, 704–710. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J. Hazard. Mater. 2010, 173, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Yan, J.H.; Jiang, X.G.; Lai, Y.E.; Cen, K.F. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. J. Hazard. Mater. 2008, 153, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Kaal, J.; Nierop, K.G.J.; Kraal, P.; Preston, C.M. A first step towards identification of tannin-derived black carbon: Conventional pyrolysis (Py-GC-MS) and thermally assisted hydrolysis and methylation (THM-GC-MS) of charred condensed tannins. Org. Geochem. 2012, 47, 99–108. [Google Scholar] [CrossRef]
- Xue, J.; Zhuo, J.; Liu, M.; Chi, Y.; Zhang, D.; Yao, Q. Synergetic Effect of Co-pyrolysis of Cellulose and Polypropylene over an All-Silica Mesoporous Catalyst MCM-41 Using Thermogravimetry-Fourier Transform Infrared Spectroscopy and Pyrolysis Gas Chromatography-Mass Spectrometry. Energy Fuels 2017, 31, 9576–9584. [Google Scholar] [CrossRef]
- Zhi Yu, J.; Yong, Y.; Ru Yi, Z.; Xin Cheng, L.; Yu Fen, Z. Synthesis and thermal stability of a novel phosphorus-nitrogen containing intumescent flame retardant. Chin. Chem. Lett. 2008, 19, 277–278. [Google Scholar]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Poletto, M.; Dettenborn, J.; Pistor, V.; Zeni, M.; Zattera, A.J. Materials Produced from Plant Biomass. Part I: Evaluation of Thermal Stability and Pyrolysis of Wood. Mater. Res.-Ib.-Am. J. Mater. 2010, 13, 375–379. [Google Scholar] [CrossRef]
- Clauss, S.; Joscak, M.; Niemz, P. Thermal stability of glued wood joints measured by shear tests. Eur. J. Wood Wood Prod. 2011, 69, 101–111. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Khelfa, A.; Finqueneisel, G.; Auber, M.; Weber, J.V. Influence of some minerals on the cellulose thermal degradation mechanisms–Thermogravimetic and pyrolysis-mass spectrometry studies. J. Therm. Anal. Calorim. 2008, 92, 795–799. [Google Scholar] [CrossRef]
- Yan, G.; Jiang, Y.; Kuang, C.; Wang, S.; Liu, H.; Zhang, Y.; Wang, J. Nano-Fe2O3-catalyzed direct borylation of arenes. Chem. Commun. 2010, 46, 3170–3172. [Google Scholar] [CrossRef] [PubMed]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch reaction on free nano-Fe2O3 catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, X.; Wang, Z.; Huang, H.; Ma, C.; Qin, Y. Effect of the NO plus CO reaction on the consumption of carbon supports: An in situ TG-FTIR analysis. Chem. Eng. J. 2018, 352, 90–102. [Google Scholar] [CrossRef]
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M.A. Interaction and kinetic analysis for coal and biomass co-gasification by TG-FTIR. Bioresour. Technol. 2014, 154, 313–321. [Google Scholar] [CrossRef]
- Oudghiri, F.; Allali, N.; Quiroga, J.M.; Rocio Rodriguez-Barroso, M. TG-FTIR analysis on pyrolysis and combustion of marine sediment. Infrared Phys. Technol. 2016, 78, 268–274. [Google Scholar] [CrossRef]
- Dziwitiski, E.J.; Ilowska, J.; Gniady, J. Py-GC/MS analyses of poly(ethylene terephthalate) film without and with the presence of tetramethylammonium acetate reagent. Comparative study. Polym. Test. 2018, 65, 111–115. [Google Scholar] [CrossRef]
- Chen, D.Y.K.; Pouwer, R.H.; Richard, J.-A. Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev. 2012, 41, 4631–4642. [Google Scholar] [CrossRef]
- Sazinsky, M.H.; Bard, J.; Di Donato, A.; Lippard, S.J. Crystal structure of the toluene/o-xylene monooxygenase hydroxylase from Pseudomonas stutzeri OX1–Insight into the substrate specificity, substrate channeling, and active site tuning of multicomponent monooxygenases. J. Biol. Chem. 2004, 279, 30600–30610. [Google Scholar] [CrossRef]
- Liu, S.S.; Wu, G.; Syed-Hassan, S.S.A.; Li, B.; Hu, X.; Zhou, J.B.; Huang, Y.; Zhang, S.; Zhang, H. Catalytic pyrolysis of pine wood over char-supported Fe: Bio-oil upgrading and catalyst regeneration by CO2/H2O. Fuel 2022, 307, 121778. [Google Scholar] [CrossRef]
- Gao, Z.; Li, N.; Yin, S.; Yi, W. Pyrolysis behavior of cellulose in a fixed bed reactor: Residue evolution and effects of parameters on products distribution and bio-oil composition. Energy 2019, 175, 1067–1074. [Google Scholar] [CrossRef]
- Andini, S.; Castronuovo, G.; Elia, V.; Pignone, A.; Velleca, F. Chiral recognition in aqueous solutions: On the role of urea in hydrophilic and hydrophobic interactions of unsubstituted α-amino acids. J. Solut. Chem. 1996, 25, 837–848. [Google Scholar] [CrossRef]
- Fischer, E.; Sauer, U. Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur. J. Biochem. 2003, 270, 880–891. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Carrillo, A.; Colom, X.; Sunol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Ni, Y.; Liu, Z.; Chen, J.; Liang, X. Temperature- and pH-dependent morphology and FT-IR analysis of magnesium carbonate hydrates. J. Phys. Chem. B 2006, 110, 12969–12973. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, J.S.; Choi, H.; Sohn, D.; Ahn, D.J. Rational design and in-situ FTIR analyses of colorimetrically reversibe polydiacetylene supramolecules. Macromolecules 2005, 38, 9366–9376. [Google Scholar] [CrossRef]
- Ma, X.F.; Yu, J.G.; Jin, F. Urea and formamide as a mixed plasticizer for thermoplastic starch. Polym. Int. 2004, 53, 1780–1785. [Google Scholar]
- Whone, A.L.; Watts, R.L.; Stoessl, A.J.; Davis, M.; Reske, S.; Nahmias, C.; Lang, A.E.; Rascol, O.; Ribeiro, M.J.; Remy, P.; et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann. Neurol. 2003, 54, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Hauser, R.A.; Gauger, L.; Malapira, T.; Koller, W.; Hubble, J.; Bushenbark, K.; Lilienfeld, D.; Esterlitz, J. The effect of deprenyl and levodopa on the progression of Parkinson’s disease. Ann. Neurol. 1995, 38, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Katan, M.B. Hydrogenation alternatives: Effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. J. Lipid Res. 1992, 33, 399–410. [Google Scholar] [CrossRef]
- Yang, J.Y.; Park, J.H.; Chung, N.; Lee, H.S. Inhibitory Potential of Constituents from Osmanthus fragrans and Structural Analogues Against Advanced Glycation End Products, alpha-Amylase, alpha-Glucosidase, and Oxidative Stress. Sci. Rep. 2017, 7, 45746. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.D.; Muller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
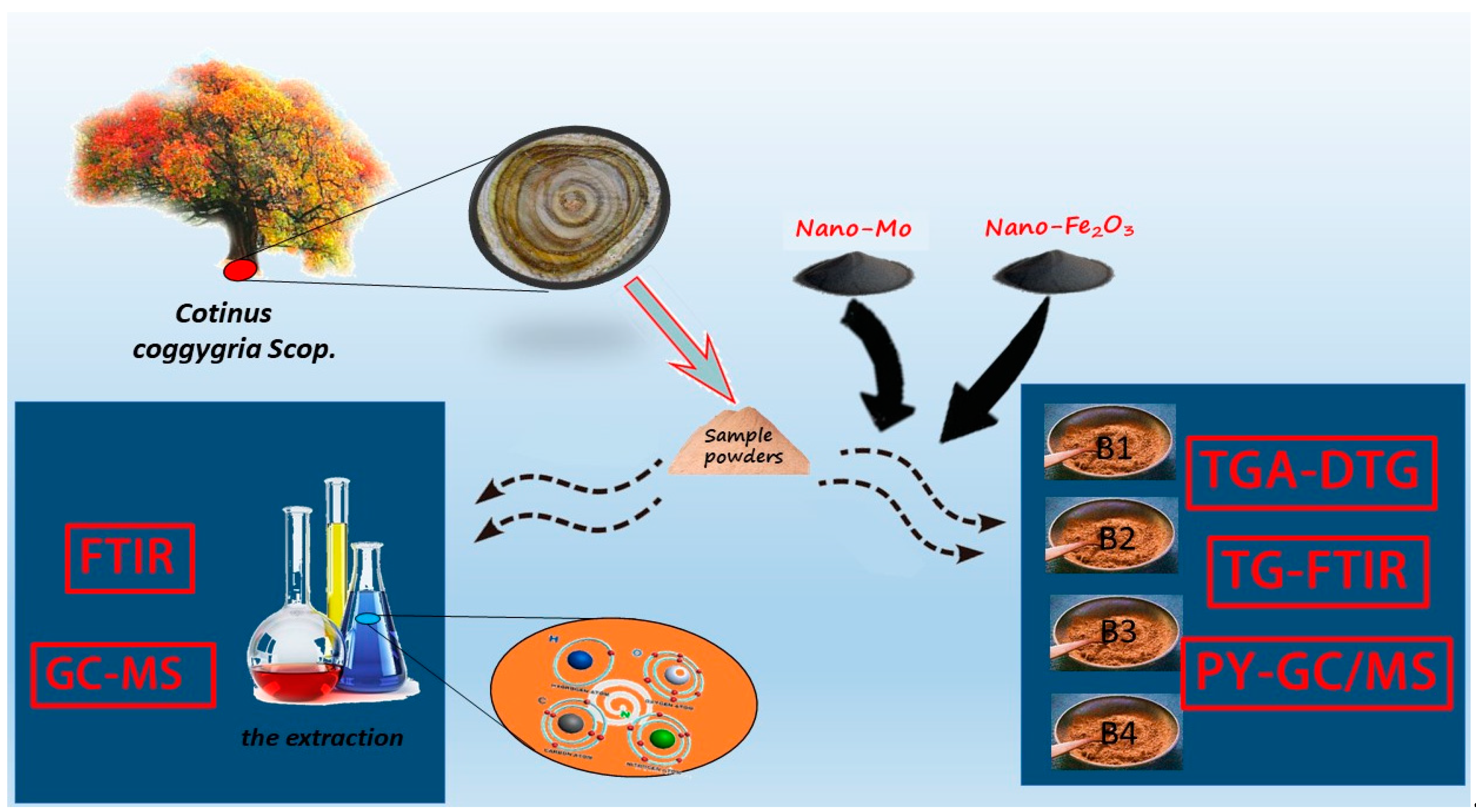






| No. | Material | Catalyst |
|---|---|---|
| B1 | Wood sample (5 g) | - |
| B2 | Wood sample (5 g) | Mo (0.05 g) |
| B3 | Wood sample (5 g) | Fe2O3 (0.05 g) |
| B4 | Wood sample (5 g) | Mo (0.025 g)/Fe2O3 (0.025 g) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Li, G.; Chen, X.; Li, Z.; Gu, H.; Chen, H.; Peng, W. Nano Catalysis of Biofuels and Biochemicals from Cotinus coggygria Scop. Wood for Bio-Oil Raw Material. Polymers 2022, 14, 4610. https://doi.org/10.3390/polym14214610
Yue X, Li G, Chen X, Li Z, Gu H, Chen H, Peng W. Nano Catalysis of Biofuels and Biochemicals from Cotinus coggygria Scop. Wood for Bio-Oil Raw Material. Polymers. 2022; 14(21):4610. https://doi.org/10.3390/polym14214610
Chicago/Turabian StyleYue, Xiaochen, Guanyan Li, Xiangmeng Chen, Zhaolin Li, Haiping Gu, Huiling Chen, and Wanxi Peng. 2022. "Nano Catalysis of Biofuels and Biochemicals from Cotinus coggygria Scop. Wood for Bio-Oil Raw Material" Polymers 14, no. 21: 4610. https://doi.org/10.3390/polym14214610
APA StyleYue, X., Li, G., Chen, X., Li, Z., Gu, H., Chen, H., & Peng, W. (2022). Nano Catalysis of Biofuels and Biochemicals from Cotinus coggygria Scop. Wood for Bio-Oil Raw Material. Polymers, 14(21), 4610. https://doi.org/10.3390/polym14214610








