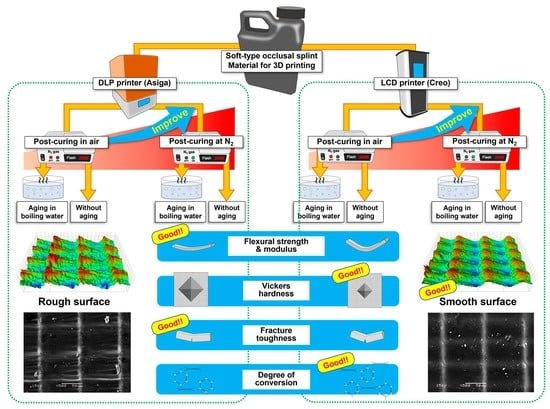
Effect of 3D Printer Type and Use of Protection Gas during Post-Curing on Some Physical Properties of Soft Occlusal Splint Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Fabrication
2.2. Flexural Strength and Modulus Testing
2.3. Vickers Hardness (VHN)
2.4. Fracture Toughness (KIC)
2.5. Degree of Double Bond Conversion (DC%)
2.6. Water Sorption (WSP) and Solubility (WSL)
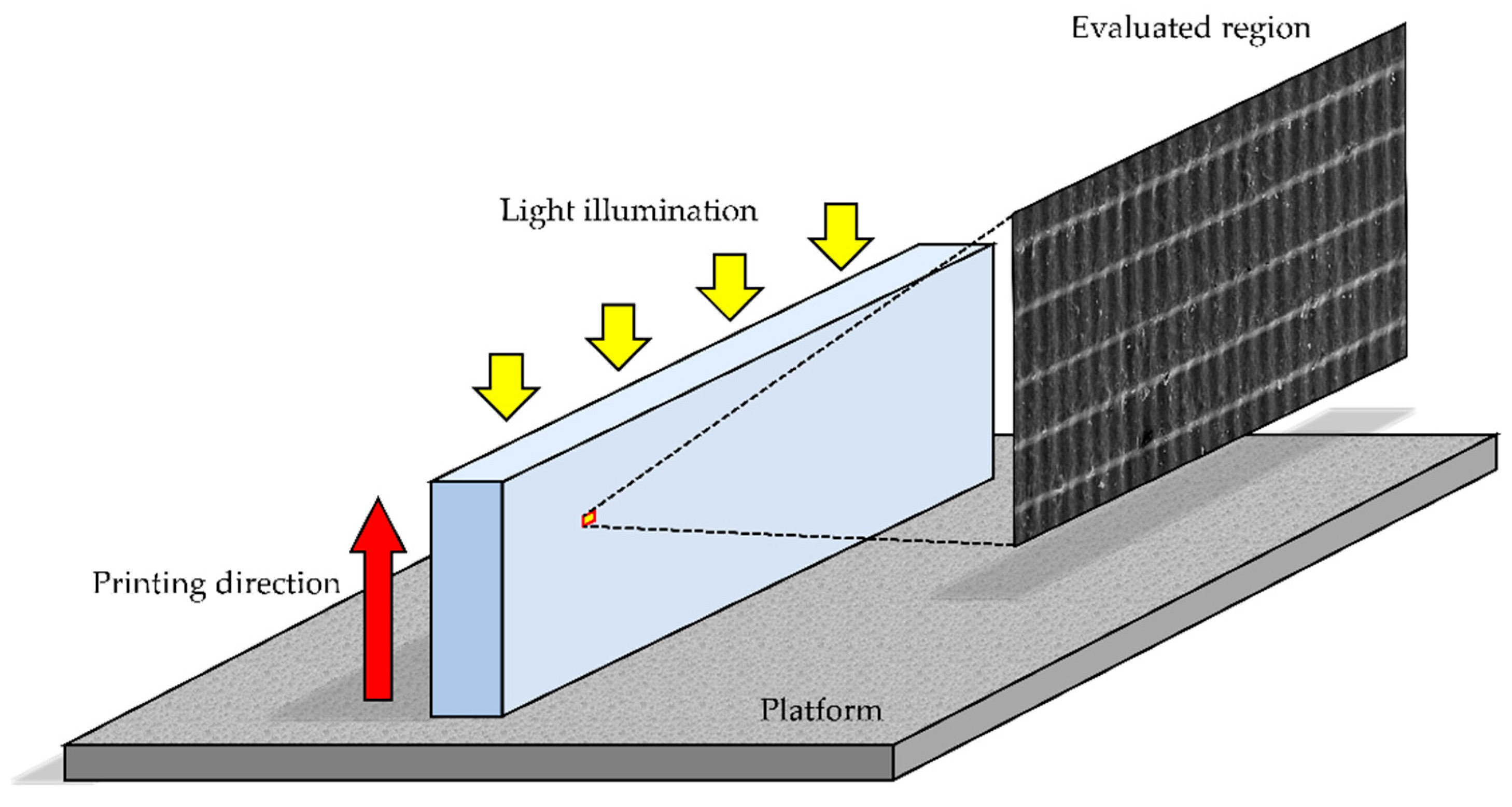
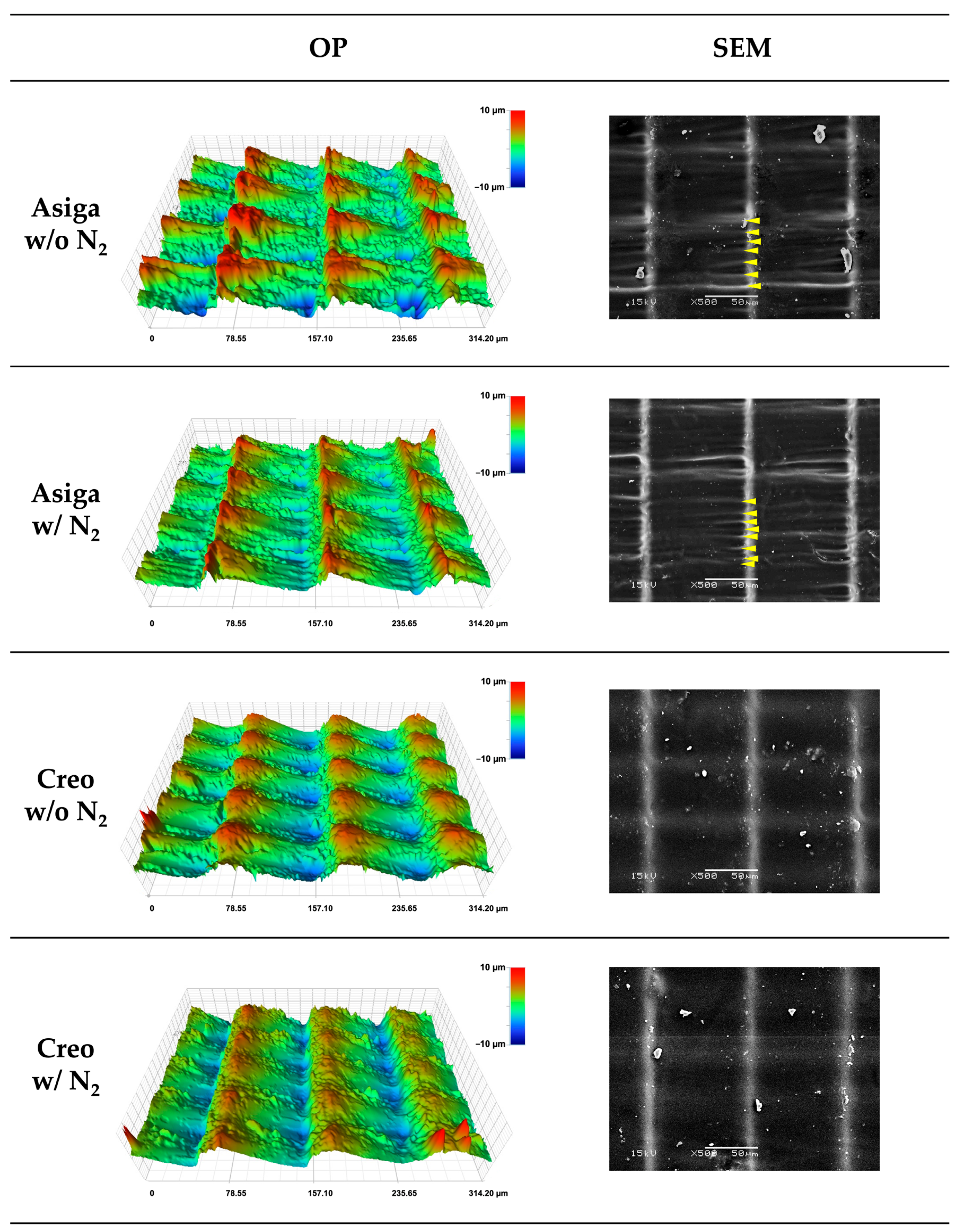
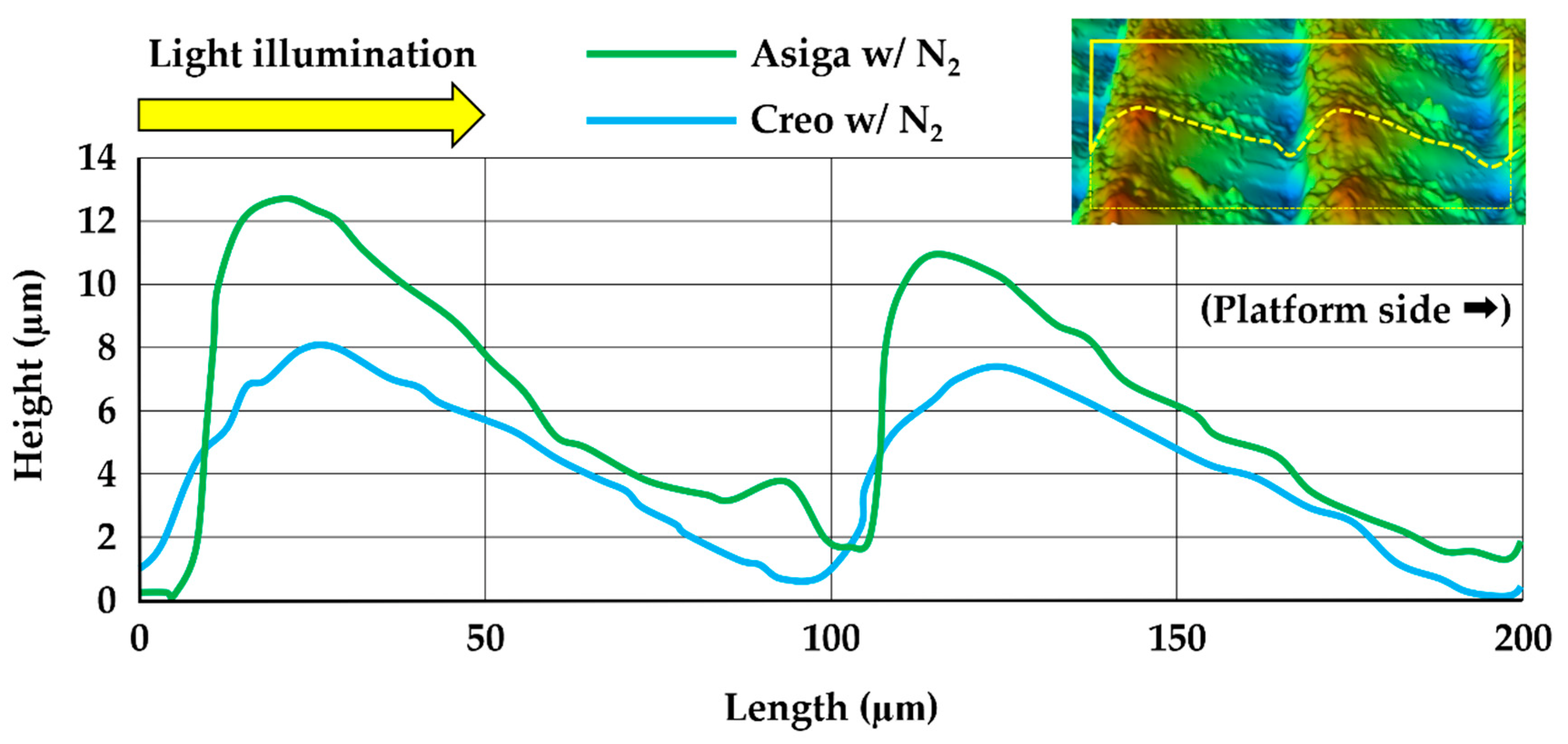
2.7. Three-Dimensional Microlayer Structure and Surface Condition
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weir, T. Clear aligners in orthodontic treatment. Aust. Dent. J. 2017, 62, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.G.; van Noort, R.; Found, M.S. Scale of protection and the various types of sports mouthguard. Br. J. Sports Med. 2005, 39, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Klasser, G.D.; Greene, C.S. Oral appliances in the management of temporomandibular disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Seifeldin, S.A.; Elhayes, K.A. Soft versus hard occlusal splint therapy in the management of temporomandibular disorders (TMDs). Saudi Dent. J. 2015, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Farea, R.; Qasem, K.A.; Al-Wadeai, M.S.; Al-Sabahi, M.E.; Al-Iryani, G.M. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: Network meta-analysis of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2020, 49, 1042–1056. [Google Scholar] [CrossRef]
- Goiato, M.C.; Sonego, M.V.; dos Santos, D.M.; da Silva, E.V. Implant rehabilitation in bruxism patient. BMJ Case Rep. 2014, 2014, bcr2014204080. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, T.; Zhang, L.; Bao, G.; Kang, H. Restoration of the dentition in a patient with a history of bruxism and amelogenesis imperfecta: A clinical report. Clin. Case Rep. 2020, 9, 898–905. [Google Scholar] [CrossRef]
- Fricton, J.; Look, J.O.; Wright, E.; Alencar, F.G.P., Jr.; Chen, H.; Lang, M.; Ouyang, W.; Velly, A.M. Systematic review and meta-analysis of randomized controlled trials evaluating intraoral orthopedic appliances for temporomandibular disorders. J. Orofac. Pain 2010, 24, 237–254. [Google Scholar]
- Uchida, H.; Wada, J.; Watanabe, C.; Nagayama, T.; Mizutani, K.; Mikami, R.; Inukai, S.; Wakabayashi, N. Effect of night dentures on tooth mobility in denture wearers with sleep bruxism: A pilot randomized controlled trial. J. Prosthodont. Res. 2022, 66, 564–571. [Google Scholar] [CrossRef]
- Okeson, J.P. The effects of hard and soft occlusal splints on nocturnal bruxism. J. Am. Dent. Assoc. 1987, 114, 788–791. [Google Scholar] [CrossRef]
- Quayle, A.A.; Gray, R.J.; Metcalfe, R.J.; Guthrie, E.; Wastell, D. Soft occlusal splint therapy in the treatment of migraine and other headaches. J. Dent. 1990, 18, 123–129. [Google Scholar] [CrossRef]
- Cruz-Reyes, R.A.; Martínez-Aragón, I.; Guerrero-Arias, R.E.; García-Zura, D.A.; González-Sánchez, L.E. Influence of occlusal stabilization splints and soft occlusal splints on the electromyographic pattern, in basal state and at the end of six weeks treatment in patients with bruxism. Acta Odontol. Latinoam. 2011, 24, 66–74. [Google Scholar]
- Silva, C.; Grossi, M.L.; Araldi, J.C.; Corso, L.L. Can hard and/or soft occlusal splints reduce the bite force transmitted to the teeth and temporomandibular joint discs? A finite element method analysis. Cranio 2020. ahead of print. [Google Scholar] [CrossRef]
- Halachmi, M.; Gavish, A.; Gazit, E.; Winocur, E.; Brosh, T. Splints and stress transmission to teeth: An in vitro experiment. J. Dent. 2000, 28, 475–480. [Google Scholar] [CrossRef]
- Ariji, Y.; Koyama, S.; Sakuma, S.; Nakayama, M.; Ariji, E. Regional brain activity during jaw clenching with natural teeth and with occlusal splints: A preliminary functional MRI study. Cranio 2016, 34, 188–194. [Google Scholar] [CrossRef]
- Sriharsha, P.; Gujjari, A.K.; Dhakshaini, M.R.; Prashant, A. Comparative evaluation of salivary cortisol levels in bruxism patients before and after using soft occlusal splint: An in vivo study. Contemp. Clin. Dent. 2018, 9, 182–187. [Google Scholar]
- Dedem, P.; Türp, J.C. Digital Michigan splint—From intraoral scanning to plasterless manufacturing. Int. J. Comput. Dent. 2016, 19, 63–76. [Google Scholar]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L. Evaluation of the mechanical properties and degree of conversion of 3D printed splint material. J. Mech. Behav. Biomed. Mater. 2021, 115, 104254. [Google Scholar] [CrossRef]
- Wada, J.; Wada, K.; Gibreel, M.; Wakabayashi, N.; Iwamoto, T.; Vallittu, P.K.; Lassila, L. Effect of nitrogen gas post-curing and printer type on the mechanical properties of 3D-printed hard occlusal splint material. Polymers 2022, 14, 3971. [Google Scholar] [CrossRef]
- Lauren, M.; McIntyre, F. A new computer-assisted method for design and fabrication of occlusal splints. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S130–S135. [Google Scholar] [CrossRef]
- Salmi, M.; Paloheimo, K.S.; Tuomi, J.; Ingman, T.; Mäkitie, A. A digital process for additive manufacturing of occlusal splints: A clinical pilot study. J. R. Soc. Interface 2013, 10, 20130203. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, C.; Kleven, M.; Heian, M.; Hjortsjö, C. Clinical comparison of conventional and additive manufactured stabilization splints. Acta Biomater. Odontol. Scand. 2018, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.M.M.; Fonseca, A.C.; Domingos, M.; Gloria, A.; Serra, A.C.; Coelho, J.F.J. The potential of unsaturated polyesters in biomedicine and tissue engineering: Synthesis, structure-properties relationships and additive manufacturing. Prog. Polym. Sci. 2017, 68, 1–34. [Google Scholar] [CrossRef]
- Väyrynen, V.O.; Tanner, J.; Vallittu, P.K. The anisotropicity of the flexural properties of an occlusal device material processed by stereolithography. J. Prosthet. Dent. 2016, 116, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Zinser, M.J.; Mischkowski, R.A.; Sailer, H.F.; Zöller, J.E. Computer-assisted orthognathic surgery: Feasibility study using multiple CAD/CAM surgical splints. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 673–687. [Google Scholar] [CrossRef]
- Wickström, H.; Hilgert, E.; Nyman, J.O.; Desai, D.; Karaman, D.Ş.; de Beer, T.; Sandler, N.; Rosenholm, J.M. Inkjet printing of drug-loaded mesoporous silica nanoparticles-a platform for drug development. Molecules 2017, 22, 2020. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Lutz, A.M.; Hampe, R.; Roos, M.; Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Fracture resistance and 2-body wear of 3-dimensional-printed occlusal devices. J. Prosthet. Dent. 2019, 121, 166–172. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Tulcan, A.; Vasilescu, M.D.; Tulcan, L. Study of the influence of technological parameters on generating flat part with cylindrical features in 3D printing with resin cured by optical processing. Polymers 2020, 2, 1941. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, D.H.; Huang, S.C.; Lin, Y.M. Comparison of flexural properties and cytotoxicity of interim materials printed from mono-LCD and DLP 3D printers. J. Prosthet. Dent. 2021, 126, 703–708. [Google Scholar] [CrossRef]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef]
- Zinelis, S.; Panayi, N.; Polychronis, G.; Papageorgiou, S.N.; Eliades, T. Comparative analysis of mechanical properties of orthodontic aligners produced by different contemporary 3D printers. Orthod. Craniofac. Res. 2022, 25, 336–341. [Google Scholar] [CrossRef]
- Kunjan, C.; Jawahar, N.; Chandrasekhar, U. Influence of layer thickness on mechanical properties in stereolithography. Rapid Prototyp. J. 2006, 12, 106–113. [Google Scholar]
- Al Mortadi, N.; Eggbeer, D.; Lewis, J.; Williams, R.J. CAD/CAM/AM applications in the manufacture of dental appliances. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 727–733. [Google Scholar] [CrossRef]
- Li, P.; Lambart, A.L.; Stawarczyk, B.; Reymus, M.; Spintzyk, S. Postpolymerization of a 3D-printed denture base polymer: Impact of post-curing methods on surface characteristics, flexural strength, and cytotoxicity. J. Dent. 2021, 115, 103856. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico-mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N.; Stawarczyk, B. 3D-printed material for temporary restorations: Impact of print layer thickness and post-curing method on degree of conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar] [PubMed]
- Soto-Montero, J.; de Castro, E.F.; Romano, B.C.; Nima, G.; Shimokawa, C.A.K.; Giannini, M. Color alterations, flexural strength, and microhardness of 3D printed resins for fixed provisional restoration using different post-curing times. Dent. Mater. 2022, 38, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- © Keystone Industries, Keyprint, Frequently Asked Questions, KeySplint Soft and KeySplint Soft Clear for Carbon Printers. Q: If I Wanted to Make Post-Printing Adjustments by Adding Traditional Acrylic to the KeySplint Soft Device, Will It Bond Well? Available online: https://keyprint.keystoneindustries.com/faq/ (accessed on 14 October 2022).
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Dendrimer/methyl methacrylate co-polymers: Residual methyl methacrylate and degree of conversion. J. Biomater. Sci. Polym. Ed. 2005, 16, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Figuerôa, R.M.S.; Conterno, B.; Arrais, C.A.G.; Sugio, C.Y.C.; Urban, V.M.; Neppelenbroek, K.H. Porosity, water sorption and solubility of denture base acrylic resins polymerized conventionally or in microwave. J. Appl. Oral Sci. 2018, 26, e20170383. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, M.; Perea-Lowery, L.; Vallittu, P.K.; Lassila, L. Characterization of occlusal splint materials: CAD-CAM versus conventional resins. J. Mech. Behav. Biomed. Mater. 2021, 124, 104813. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, L.; Jian, M.; Mao, Y.; Yu, M.; Guo, Z. EHMP-DLP: Multi-projector DLP with energy homogenization for large-size 3D printing. Rapid Prototyp. J. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Ozcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.A.; Bottino, M.A. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar] [CrossRef]
- Brendeke, J.; Ozcan, M. Effect of physicochemical aging conditions on the composite-composite repair bond strength. J. Adhes. Dent. 2007, 9, 399–406. [Google Scholar]
- Kim, D.; Shim, J.S.; Lee, D.; Shin, S.H.; Nam, N.E.; Park, K.H.; Shim, J.S.; Kim, J.E. Effects of post-curing time on the mechanical and color properties of three-dimensional printed crown and bridge materials. Polymers 2020, 12, 2762. [Google Scholar] [CrossRef]
- Song, G.; Son, J.W.; Jang, J.H.; Choi, S.H.; Jang, W.H.; Lee, B.N.; Park, C. Comparing volumetric and biological aspects of 3D-printed interim restorations under various post-curing modes. J. Adv. Prosthodont. 2021, 13, 71–78. [Google Scholar] [CrossRef]
- Shawkat, E.S.; Shortall, A.C.; Addison, O.; Palin, W.M. Oxygen inhibition and incremental layer bond strengths of resin composites. Dent. Mater. 2009, 25, 1338–1346. [Google Scholar] [CrossRef]
- Bechtle, S.; Fett, T.; Rizzi, G.; Habelitz, S.; Schneider, G.A. Mixed-mode stress intensity factors for kink cracks with finite kink length loaded in tension and bending: Application to dentin and enamel. J. Mech. Behav. Biomed. Mater. 2010, 3, 303–312. [Google Scholar] [CrossRef]
- Arima, T.; Murata, H.; Hamada, T. The effects of cross-linking agents on the water sorption and solubility characteristics of denture base resin. J. Oral Rehabil. 1996, 23, 476–480. [Google Scholar] [CrossRef]




| Variable | Flexural Strength a | Flexural Modulus a | Vickers Hardness Number (VHN) a | Fracture Toughness a | Degree of Double Bond Conversion (DC%) b | Water Sorption (WSP) b | Water Solubility (WSL) b | Size of Three-Dimensional (3D) Microlayer Structural Unit b | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Width | Length | Height | ||||||||
| Printer type | <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.015 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| Post-curing method | <0.001 * | <0.001 * | <0.001 * | 0.484 | <0.001 * | 0.457 | <0.001 * | 0.922 | 0.898 | 0.638 |
| Aging in boiling water | <0.001 * | <0.001 * | <0.001 * | <0.001 * | - | - | - | - | - | - |
| Printer Type | Post- Curing | Aging in BW | Flexural Strength (MPa) | Flexural Modulus (GPa) | VHN | Fracture Toughness (MPa m1/2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | # | # | # | |||||||
| Asiga | w/o N2 | − | 31.1 ± 1.5 | a | 0.73 ± 0.06 | ab | 5.15 ± 0.25 | a | 2.66 ± 0.10 | a |
| + | 22.1 ± 1.0 | b | 0.48 ± 0.03 | c | 4.28 ± 0.26 | b | 1.49 ± 0.11 | b | ||
| w/N2 | − | 34.1 ± 1.4 | c | 0.94 ± 0.03 | d | 5.85 ± 0.25 | c | 2.60 ± 0.11 | a | |
| + | 24.4 ± 0.9 | d | 0.89 ± 0.03 | e | 5.19 ± 0.23 | a | 1.44 ± 0.05 | b | ||
| Creo | w/o N2 | − | 28.3 ± 1.9 | e | 0.68 ± 0.07 | a | 5.89 ± 0.29 | c | 2.03 ± 0.10 | c |
| + | 18.8 ± 1.2 | f | 0.37 ± 0.06 | f | 4.90 ± 0.28 | d | 1.05 ± 0.13 | d | ||
| w/N2 | − | 32.1 ± 1.4 | a | 0.77 ± 0.06 | b | 6.26 ± 0.24 | e | 2.08 ± 0.07 | c | |
| + | 18.6 ± 1.7 | f | 0.36 ± 0.06 | f | 5.19 ± 0.25 | a | 1.18 ± 0.08 | d | ||
| Printer Type | Post- Curing | DC% (%) | WSP (%) | WSL (%) | Size of 3D Microlayer Structural Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Width (μm) | Length (μm) | Height (μm) | |||||||||||
| # | # | # | # | # | # | ||||||||
| Asiga | w/o N2 | 75.7 ± 4.3 | a | 1.174 ± 0.004 | a | 0.470 ± 0.004 | a | 61.2 ± 0.5 | a | 101.3 ± 0.5 | a | 10.5 ± 1.3 | a |
| w/N2 | 84.9 ± 5.9 | bc | 1.173 ± 0.004 | a | 0.356 ± 0.024 | b | 61.3 ± 0.5 | a | 101.2 ± 1.0 | a | 10.2 ± 0.7 | a | |
| Creo | w/o N2 | 80.8 ± 3.7 | ab | 1.159 ± 0.008 | b | 0.447 ± 0.011 | c | 51.3 ± 0.4 | b | 97.5 ± 0.5 | b | 6.0 ± 0.4 | b |
| w/N2 | 91.6 ± 5.1 | c | 1.158 ± 0.004 | b | 0.432 ± 0.017 | d | 51.3 ± 0.5 | b | 97.6 ± 0.4 | b | 6.1 ± 0.2 | b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, J.; Wada, K.; Gibreel, M.; Wakabayashi, N.; Iwamoto, T.; Vallittu, P.K.; Lassila, L. Effect of 3D Printer Type and Use of Protection Gas during Post-Curing on Some Physical Properties of Soft Occlusal Splint Material. Polymers 2022, 14, 4618. https://doi.org/10.3390/polym14214618
Wada J, Wada K, Gibreel M, Wakabayashi N, Iwamoto T, Vallittu PK, Lassila L. Effect of 3D Printer Type and Use of Protection Gas during Post-Curing on Some Physical Properties of Soft Occlusal Splint Material. Polymers. 2022; 14(21):4618. https://doi.org/10.3390/polym14214618
Chicago/Turabian StyleWada, Junichiro, Kanae Wada, Mona Gibreel, Noriyuki Wakabayashi, Tsutomu Iwamoto, Pekka K. Vallittu, and Lippo Lassila. 2022. "Effect of 3D Printer Type and Use of Protection Gas during Post-Curing on Some Physical Properties of Soft Occlusal Splint Material" Polymers 14, no. 21: 4618. https://doi.org/10.3390/polym14214618
APA StyleWada, J., Wada, K., Gibreel, M., Wakabayashi, N., Iwamoto, T., Vallittu, P. K., & Lassila, L. (2022). Effect of 3D Printer Type and Use of Protection Gas during Post-Curing on Some Physical Properties of Soft Occlusal Splint Material. Polymers, 14(21), 4618. https://doi.org/10.3390/polym14214618