UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water
Abstract
1. Introduction
| Hydrogel | Active Agent | Ion/Ions Adsorbed | Solution and pH Range | Mechanical Properties | Reusability | Reference |
|---|---|---|---|---|---|---|
| Chitosan-based hydrogel beads | Chitosan | Cu(II) | 0.157 mmolCu/dm3 and 15.7 mmolCu/dm3 CuSO4 pH = 3.5 and 5 | - | - | [32] |
| Chitosan/PVA hydrogel beads | Chitosan | Pb(II) | 30 mg/L PbCl2 pH range 2–8 | - | 2 sets of adsorption experiments | [53] |
| Chitosan hydrogel beads | Chitosan | Pb(II) Experiments regarding co-adsorption of Pb(II) and humic acid show a decrease in adsorption of Pb(II) | 15 mg/L Pb(NO3)2 pH = 5.0, 6.5 and 7.5 | - | - | [54] |
| Chitosan and aminated chitosan beads | Chitosan | Hg(II) | 2–100 ppm Hg(NO3)2 pH range 3–9 | - | - | [55] |
| CR-impregnated chitosan hydrogel | Chitosan | Cu(II) | 317.5 mg/L C4H8CuO5 pH 2.0–6.1 | - | 3 cycles of adsorption-desorption | [56] |
| UV-cured chitosan-based hydrogel | Chitosan | As(V), Pb(II) | 10–20 mg/L Na2HAsO4·7H2O, 50–75 mg/L Pb(NO3)2 pH range 2–9 | - | - | [57] |
| Collagen/cellulose hydrogel beads | Collagen | Cu(II) | 4.5 mmol/L CuSO4·6H2O pH range 1–6 | - | 4 cycles of adsorption-desorption | [58] |
| Tannic acid-based hydrogel | Tannic acid | Cu(II) | CuSO4·5H2O | - | - | [59] |
| Collagen tannin resin | Cu(II) | 1 mmol/L CuSO4·6H2O pH range 2–5.5 | - | - | [60] | |
| Hydroxyethyl cellulose hydrogel modified with tannic acid | Tannic acid | Methylene blue | 200–1200 mg/L methylene blue pH range 2–12 | - | 5 cycles of adsorption-desorption | [46] |
| PVA/alginate-based hydrogel with green tea waste | Polyphenols | Cu(II), Cr(VI) | 2–100 mg/L CuSO4, 2–200 mg/mL K2Cr2O7 pH range 2–6 | - | - | [61] |
| Tannin-immobilized cellulose hydrogel | Tannins | Methylene blue | 10–80 mg/L methylene blue pH range 2–8 | - | 6 recycling events | [47] |
| Chitin-based composite hydrogel reinforced by tannic acid functionalized graphene (TRGO) | Tannic acid | Congo red | 100–400 mg/L Congo red pH range 4.5–9.0 | Compressive strength: 22.7 kPa (without TRGO) 72.3 kPa (with 7% of TRGO) | - | [62] |
2. Materials and Methods
2.1. Materials and Chemicals
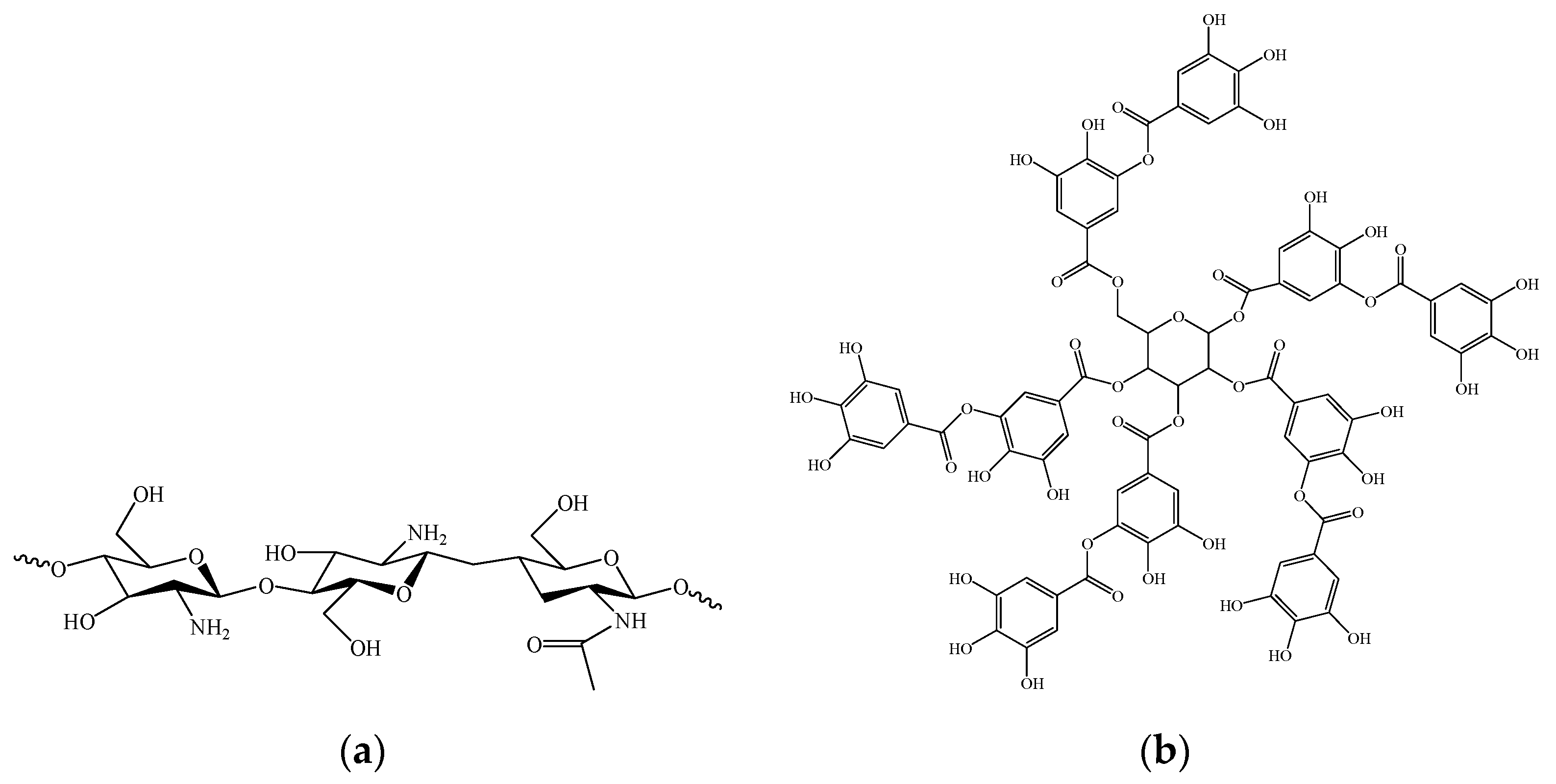
2.2. Preparation of Hydrogels
2.2.1. Synthesis of Methacrylated Chitosan (MCH)
2.2.2. UV-Curing of Chitosan-Based Hydrogel (CHg)
2.2.3. Preparation of CHg Containing TA (CHg-TA)
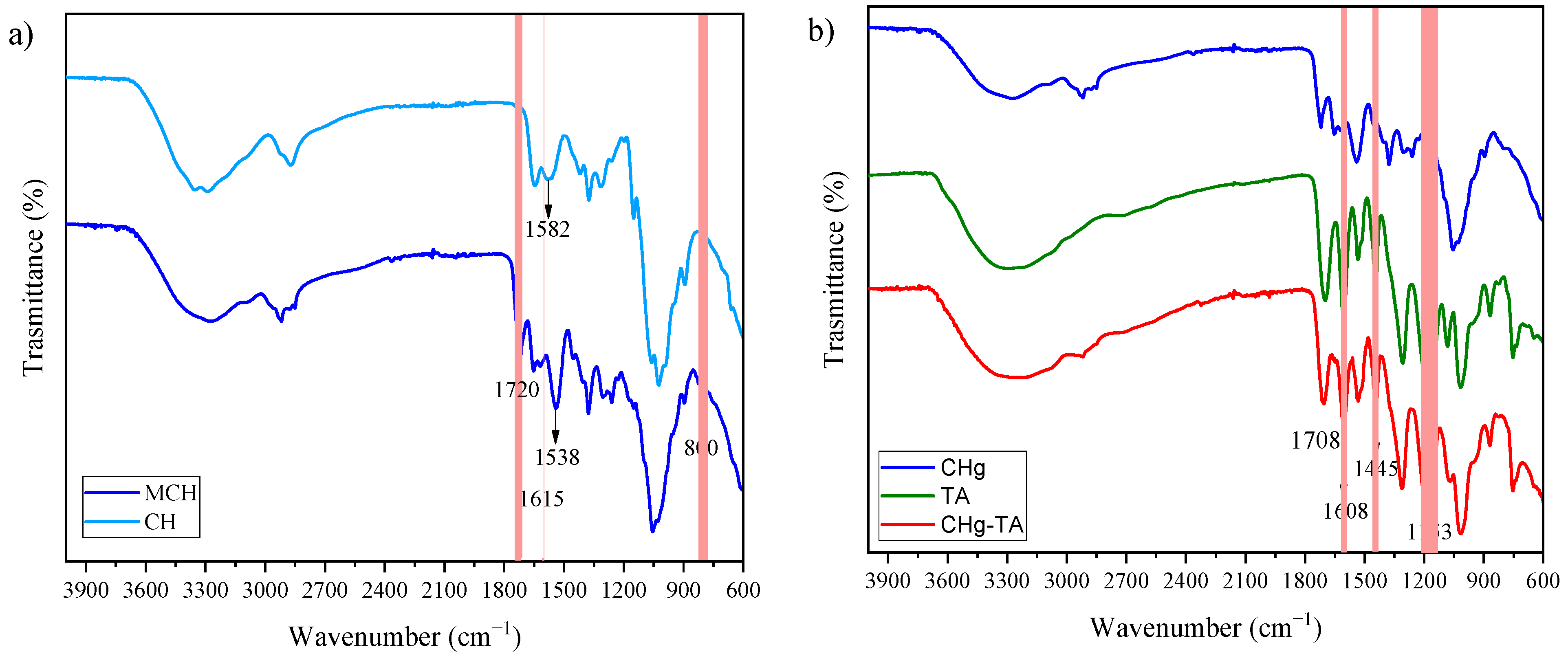
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
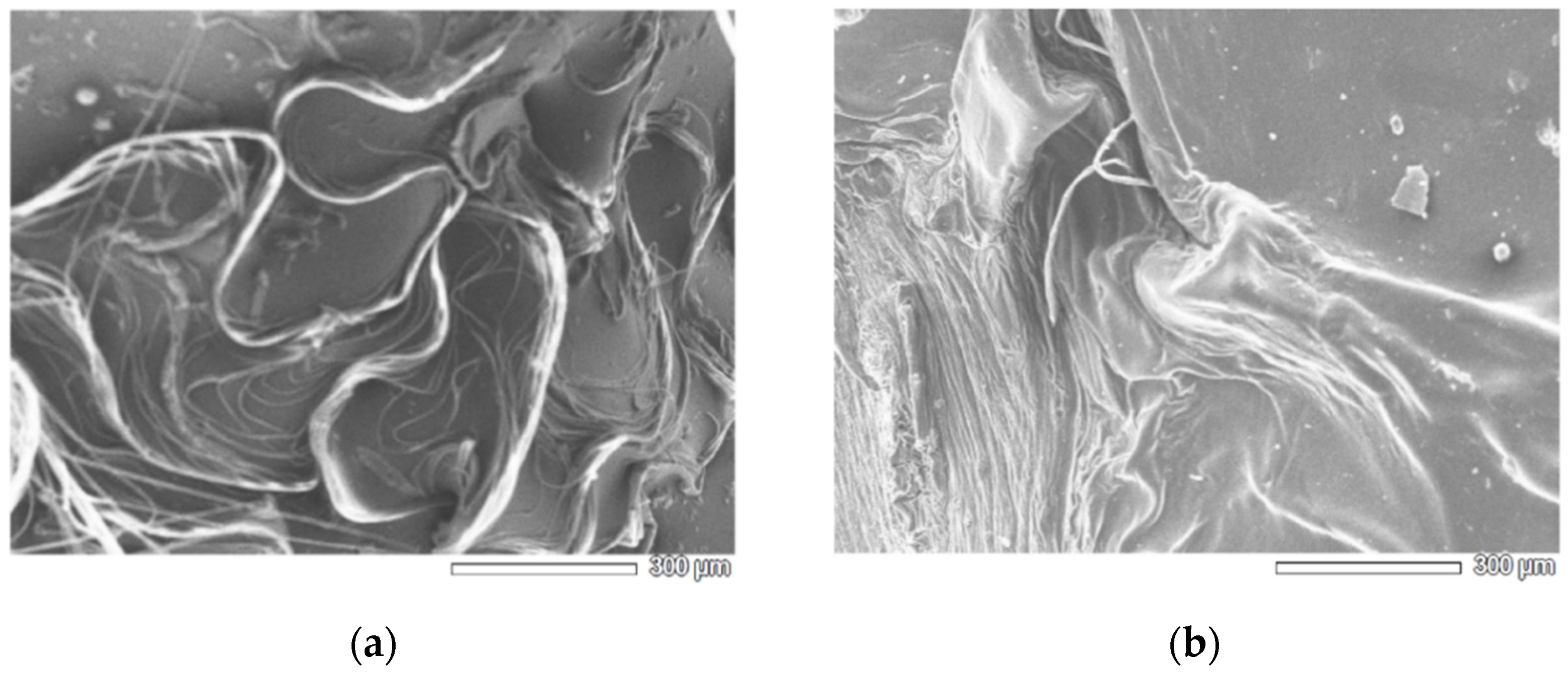
2.4. Surface Characterization with SEM
2.5. Folin–Ciocalteu Assay
2.6. Compression Test
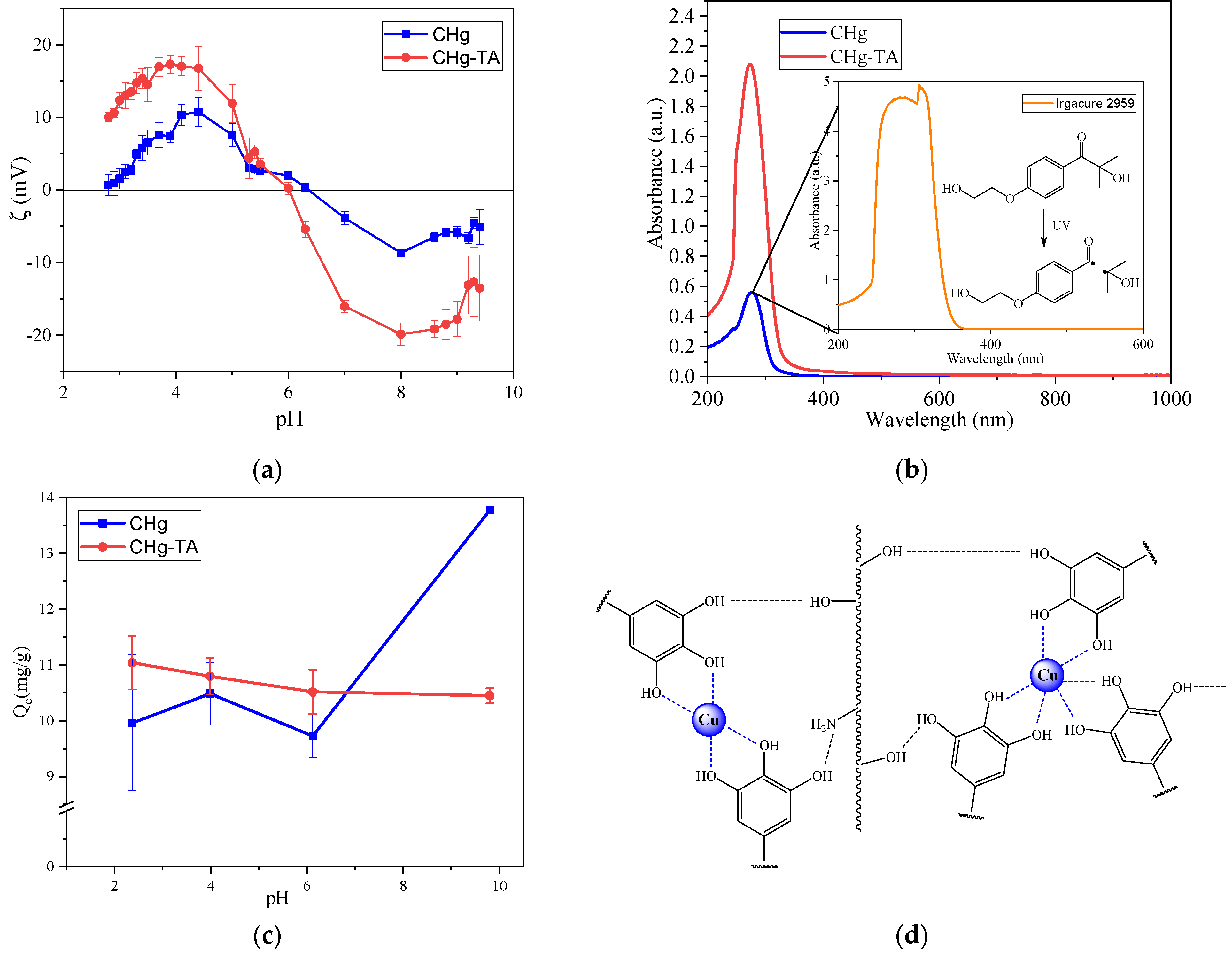
2.7. Zeta Potential
2.8. Adsorption Experiments
2.9. Recycling Experiments
3. Results and Discussion
3.1. Characterization of CHg and CHg-TA
3.2. Copper Ions Adsorption from Water
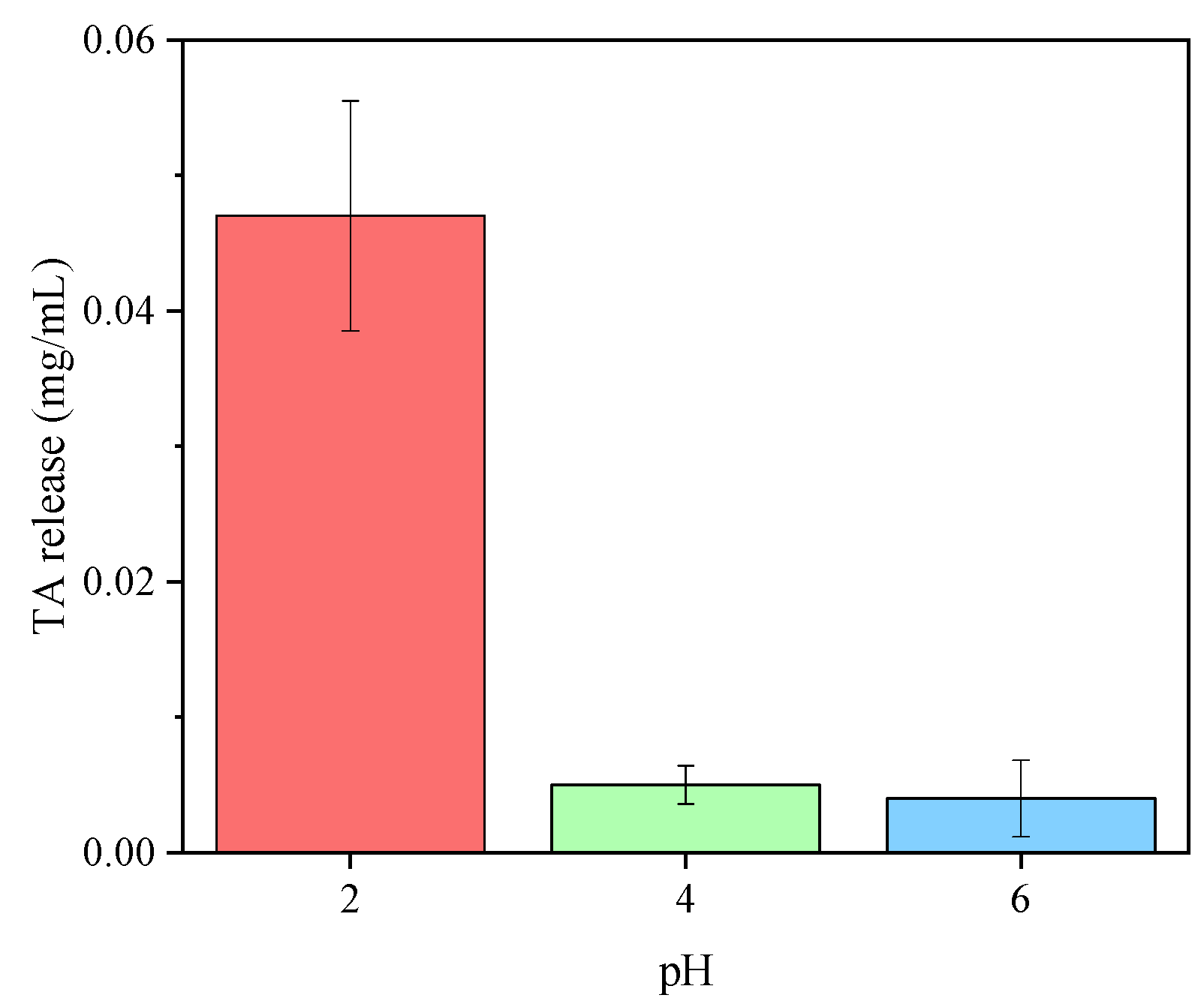
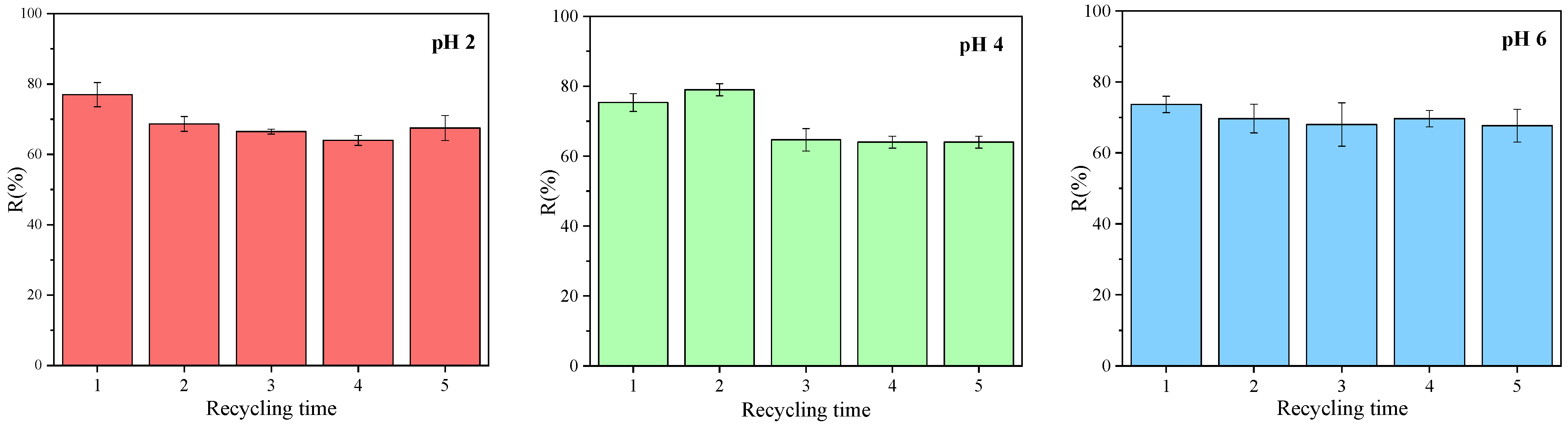
3.3. Regeneration Capability and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules 2022, 27, 4044. [Google Scholar] [CrossRef] [PubMed]
- Garmsiri, M.; Mortaheb, H. Enhancing performance of hybrid liquid membrane process supported by porous anionic exchange membranes for removal of cadmium from wastewater. Chem. Eng. J. 2015, 264, 241–250. [Google Scholar] [CrossRef]
- Prabhu, V.; Lee, S.; Clack, H. Electrostatic Precipitation of Powdered Activated Carbon and Implications for Secondary Mercury Adsorption within Electrostatic Precipitators. Energy Fuels 2011, 25, 1010–1016. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristovao, R.O.; Boaventura, R.A.R.; Vilar, V.J.P. As(III) and Cr(VI) oxyanion removal from water by advanced oxidation/reduction processes—A review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Nienhauser, A.B.; Fajardo, A.S.; Bansal, R.; Conrad, C.L.; Fortner, J.D.; Marcos-Hernandez, M.; Rogers, T.; Villagran, D.; Wong, M.S.; et al. Disparities between experimental and environmental conditions: Research steps toward making electrochemical water treatment a reality. Curr. Opin. Electrochem. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Zhang, J.; Wang, Y.; Wen, H. Role of chitosan-based hydrogels in pollutants adsorption and freshwater harvesting: A critical review. Int. J. Biol. Macromol. 2021, 189, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Agasti, N. Decontamination of heavy metal ions from water by composites prepared from waste. Curr. Res. Green Sustain. Chem. 2021, 4, 100088. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayaka, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Bakalár, T.; Pavolová, H.; Kyšeľa, K.; Hajduová, Z. Characterization of Cu(II) and Zn(II) Sorption onto Zeolite. Crystals 2022, 12, 908. [Google Scholar] [CrossRef]
- Sheng, G.; Yang, S.; Sheng, J.; Hu, J.; Tan, X.; Wang, X. Macroscopic and Microscopic Investigation of Ni(II) Sequestration on Diatomite by Batch, XPS, and EXAFS Techniques. Environ. Sci. Technol. 2011, 45, 7718–7726. [Google Scholar] [CrossRef]
- Fischer, C.; Oschatz, M.; Nickel, W.; Leistenschneider, D.; Kaskel, S.; Brunner, E. Bioinspired carbide-derived carbons with hierarchical pore structure for the adsorptive removal of mercury from aqueous solution. Chem. Commun. 2017, 53, 4845–4848. [Google Scholar] [CrossRef]
- McManamon, C.; Burke, A.M.; Holmes, J.D.; Morris, M.A. Amine-functionalised SBA-15 of tailored pore size for heavy metal adsorption. J. Colloid Interface Sci. 2012, 369, 330–337. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Noè, C.; Cosola, A.; Chiappone, A.; Hakkarainen, M.; Grützmacher, H.; Sangermano, M. From polysaccharides to UV-curable biorenewable organo/hydrogels for methylene blue removal. Polymer 2021, 235, 124257. [Google Scholar] [CrossRef]
- Noè, C.; Tonda-Turo, C.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Light Processable Starch Hydrogels. Polymers 2020, 12, 1359. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Dodson, J.R.; Parker, H.L.; Muñoz Garcia, A.M.; Hicken, A.; Asemave, K.; Farmer, T.J.; He, H. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Adsorption of Heavy Metal Ions: Role of Chitosan and Cellulose for Water Treatment. Langmuir 2015, 79, 109–155. [Google Scholar]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Dhanesh, S.; Mishra, M.; Mishra, A.K.; Anjali, S. Removal of Lead from Waste Water Using Low Cost Adsorbent. Int. Res. J. Environ. Sci. 2013, 2, 23–26. [Google Scholar]
- Pontoni, L.; Fabbricino, M. Use of chitosan and chitosan-derivatives to remove arsenic from aqueous solutions—A mini review. Carbohydr. Res. 2012, 356, 86–92. [Google Scholar] [CrossRef]
- Modrzejewska, Z. Sorption mechanism of copper in chitosan hydrogel. React. Funct. Polym. 2013, 73, 719–729. [Google Scholar] [CrossRef]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate Sensing of Chitin and Chitosan. PLoS Pathog. 2013, 9, e1003080. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Hanifpour, F.; Taheri, R.; Javadian, H.; Ghasemi, M. Comparison of using formaldehyde and carboxy methyl chitosan in preparation of Fe3O4 superparamagnetic nanoparticles-chitosan hydrogel network: Sorption behavior toward bovine serum albumin. Process Saf. Environ. Prot. 2016, 102, 119–128. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.D.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Kalagasidis Krušić, M.T. Sorption of zinc by novel pH-sensitive hydrogels based on chitosan, itaconic acid and methacrylic acid. J. Hazard. Mater. 2011, 192, 846–854. [Google Scholar] [CrossRef]
- Zanon, M.; Chiappone, A.; Garino, N.; Canta, M.; Frascella, F.; Hakkarainen, M.; Pirri, C.F.; Sangermano, M. Microwave-assisted methacrylation of chitosan for 3D printable hydrogels in tissue engineering. Mater. Adv. 2022, 3, 514–525. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Santos, S.; Bacelo, H.A.M.; Boaventura, R.A.R.; Botelho, C.M.S. Tannin-Adsorbents for Water Decontamination and for the Recovery of Critical Metals: Current State and Future Perspectives. Biotechnol. J. 2019, 14, 1900060. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.U.; Kandasubramanian, B. Tannins for wastewater treatment. SN Appl. Sci. 2020, 2, 1081. [Google Scholar] [CrossRef]
- Beltrán Heredia, J.; Sánchez Martín, J. Removing heavy metals from polluted surface water with a tannin-based flocculant agent. J. Hazard. Mater. 2009, 165, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M.; Şengil, I.A. Adsorption and desorption behavior of silver ions onto valonia tannin resin. Trans. Nonferrous Met. Soc. China 2012, 22, 2846–2854. [Google Scholar] [CrossRef]
- Issaoui, H.; Sallem, F.; Lafaille, J.; Grassl, B.; Charrier-El Bouhtoury, F. Biosorption of Heavy Metals from Water onto Phenolic Foams Based on Tannins and Lignin Alkaline Liquor. Int. J. Environ. Res. 2021, 15, 369–381. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.; Ma, C.; Li, H. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2021, 138, 49880. [Google Scholar] [CrossRef]
- Pei, Y.; Chu, S.; Chen, Y.; Li, Z.; Zhao, J.; Liu, S.; Wu, X.; Liu, J.; Zheng, X.; Tang, K. Tannin-immobilized cellulose hydrogel fabricated by a homogeneous reaction as a potential adsorbent for removing cationic organic dye from aqueous solution. Int. J. Biol. Macromol. 2017, 103, 254–260. [Google Scholar] [CrossRef]
- Zheng, L.; Shi, J.; Chi, Y. Tannic Acid Physically Cross-Linked Responsive Hydrogel. Macromol. Chem. Phys. 2018, 219, 1800234. [Google Scholar] [CrossRef]
- Sun, H.; Xia, N.; Liu, Z.; Kong, F.; Wang, S. Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere 2019, 236, 124370. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Qu, D.; Gao, H.; Yan, L.; Chen, Y.; Crittenden, J. Tannic acid-metal complex modified MXene membrane for contaminants removal from water. J. Membr. Sci. 2021, 622, 119042. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; Von Elverfeldt, D.; Hagemeyer, C.E. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of Lead Adsorption on Chitosan/PVA Hydrogel Beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Yan, W.L.; Bai, R. Adsorption of lead and humic acid on chitosan hydrogel beads. Water Res. 2005, 39, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Höll, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Y.; Huang, D.; Wang, A. Cu2+ removal from aqueous solution by modified chitosan hydrogels. J. Chem. Technol. Biotechnol. 2012, 87, 1010–1016. [Google Scholar] [CrossRef]
- Noè, C.; Zanon, M.; Arencibia, A.; López-Muñoz, M.-J.; Fernández de Paz, N.; Calza, P.; Sangermano, M. UV-Cured Chitosan and Gelatin Hydrogels for the Removal of As(V) and Pb(II) from Water. Polymers 2022, 14, 1268. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Ma, Y.; Li, K.; Li, M.; Yu, Y.; Wang, L.; Qiu, H. Collagen/cellulose hydrogel beads reconstituted from ionic liquid solution for Cu(II) adsorption. Carbohydr. Polym. 2013, 98, 736–743. [Google Scholar] [CrossRef]
- Peng, Z.; Zhong, H. Synthesis and Properties of Tannic Acid-Based Hydrogels. Part B Phys. 2014, 53, 233–242. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liao, X.; Shi, B. Adsorptive removal of Cu(II) from aqueous solutions using collagen-tannin resin. J. Hazard. Mater. 2011, 186, 1058–1063. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Liang, S.; Hu, K.; Hua, D.; Liu, S.; Sun, J.; Sun, M.; Wang, T.; Okoro, O.V.; et al. Polyphenol rich green tea waste hydrogel for removal of copper and chromium ions from aqueous solution. Clean. Eng. Technol. 2021, 4, 100167. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Tang, K.; Zhang, K.; Zou, Z.; Gao, X. High-Strength Chitin Based Hydrogels Reinforced by Tannic Acid Functionalized Graphene for Congo Red Adsorption. J. Polym. Environ. 2020, 28, 984–994. [Google Scholar] [CrossRef]
- Feng, Z.; Hakkarainen, M.; Grützmacher, H.; Chiappone, A.; Sangermano, M. Photocrosslinked Chitosan Hydrogels Reinforced with Chitosan-Derived Nano-Graphene Oxide. Macromol. Chem. Phys. 2019, 220, 1900174. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z.; Ciardelli, G.; Hakkarainen, M.; Sangermano, M. Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Zhang, X.; Ferraris, S.; Prenesti, E.; Verné, E. Surface functionalization of bioactive glasses with natural molecules of biological significance, Part I: Gallic acid as model molecule. Appl. Surf. Sci. 2013, 287, 329–340. [Google Scholar] [CrossRef]
- Ferraris, S.; Zhang, X.; Prenesti, E.; Corazzari, I.; Turci, F.; Tomatis, M.; Vernè, E. Gallic acid grafting to a ferrimagnetic bioactive glass-ceramic. J. Non-Cryst. Solids 2016, 432, 167–175. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Wahyono, T.; Astuti, D.A.; Wiryawan, I.K.G.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in The Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Liu, W.; Sun, S.; Cao, Z.; Zhang, X.; Yao, K.; Lu, W.W.; Luk, K.D.K. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials 2005, 26, 2705–2711. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Yang, J.; Du, H.; Chai, N.; Sha, Z.; Geng, M.; Zhou, X.; He, C. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly for Particle Engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Zhu, C.; Fan, D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate wound repair. Colloids Surf. B Biointerfaces 2021, 205, 111869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, F.; Li, L.; Cao, M.; Zuo, D.; Liu, H. Removal of anionic dyes from aqueous solutions by adsorption of chitosan-based semi-IPN hydrogel composites. Compos. Part B 2012, 43, 1570–1578. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Copper Adsorption on chitosan-cellulose hydrogel beades: Beahaviours and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Huang, X.; Liao, X.; Shi, B. Hg(II) removal from aqueous solution by bayberry tannin-immobilized collagen fiber. J. Hazard. Mater. 2009, 170, 1141–1148. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Han, X.; Yao, Y.; Zhao, W.; Han, R.; Li, S.; Zhang, Y.; Zheng, C. Insights into the adsorption mechanism of tannic acid by a green synthesized nano-hydroxyapatite and its effect on aqueous Cu(II) removal. Sci. Total Environ. 2021, 778, 146189. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wei, J.; Zhu, X.; Qiu, S.; Zhao, H. Copper Tannic Acid-Coordinated Metal−Organic Nanosheets for Synergistic Antimicrobial and Antifouling Coatings. ACS Appl. Mater. Interfaces 2021, 13, 10446–10456. [Google Scholar] [CrossRef]
- Aelenei, N.; Popa, M.I.; Novac, O.; Lisa, G.; Balaita, L. Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J. Mater. Sci. Mater. Med. 2009, 20, 1095–1102. [Google Scholar] [CrossRef]
- Ky, I.; Le Floch, A.; Zeng, L.; Pechamat, L.; Jourdes, M.; Teissedre, P.-L. Tannins. Encycl. Food Health 2016, 247–255. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689. [Google Scholar] [CrossRef] [PubMed]
- Tóth, I.Y.; Szekeres, M.; Turcu, R.; Sáringer, S.; Illés, E.; Nesztor, D.; Tombácz, E. Mechanism of in Situ Surface Polymerization of Gallic Acid in an Environmental-Inspired Preparation of Carboxylated Core−Shell Magnetite Nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Lin, S.; Xiao, H.; Yang, Q.; Chen, S.; Yan, B.; Gu, Y. Environmentally friendly nanocomposites based on cellulose nanocrystals and polydopamine for rapid removal of organic dyes in aqueous solution. Cellulose 2020, 27, 2085–2097. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesia, R.; Ferraris, S.; Sangermano, M.; Spriano, S. UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymers 2022, 14, 4645. https://doi.org/10.3390/polym14214645
Sesia R, Ferraris S, Sangermano M, Spriano S. UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymers. 2022; 14(21):4645. https://doi.org/10.3390/polym14214645
Chicago/Turabian StyleSesia, Rossella, Sara Ferraris, Marco Sangermano, and Silvia Spriano. 2022. "UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water" Polymers 14, no. 21: 4645. https://doi.org/10.3390/polym14214645
APA StyleSesia, R., Ferraris, S., Sangermano, M., & Spriano, S. (2022). UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymers, 14(21), 4645. https://doi.org/10.3390/polym14214645










