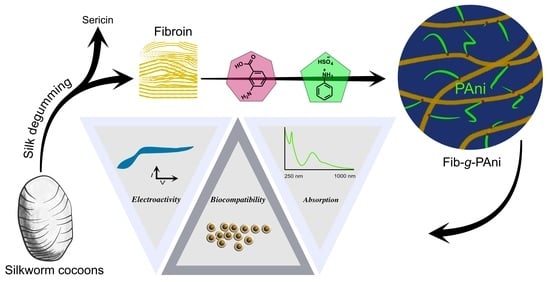
Silk Fibroin-g-Polyaniline Platform for the Design of Biocompatible-Electroactive Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silk Degumming
2.3. Synthesis of the Fibroin-g-Polyaniline Copolymer
2.3.1. Fischer Esterification
2.3.2. Oxidative Polymerization
2.4. PAni Polymerization
2.5. Cytotoxicity Assays on Fib-g-PAni
2.6. Characterization
3. Results and Discussion
3.1. Chemical Characterization
3.2. Optical Activity
3.3. Thermal Stability
3.4. Morphology
3.5. Electroactivity
3.6. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.; Sun, B.; Fang, J.; Zhang, K.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- AL-Oqla, F.M.; Sapuan, S.M.; Anwer, T.; Jawaid, M.; Hoque, M.E. Natural fiber reinforced conductive polymer composites as functional materials: A review. Synth. Met. 2015, 206, 42–54. [Google Scholar] [CrossRef]
- Shahadat, M.; Khan, M.Z.; Rupani, P.F.; Embrandiri, A.; Sultana, S.; Ahammad, S.Z.; Wazed Ali, S.; Sreekrishnan, T.R. A critical review on the prospect of polyaniline-grafted biodegradable nanocomposite. Adv. Colloid Interface Sci. 2017, 249, 2–16. [Google Scholar] [CrossRef]
- Ou, X.; Xu, X. A simple method to fabricate poly(aniline-co-pyrrole) with highly improved electrical conductivity via pre-polymerization. RSC Adv. 2016, 6, 13780–13785. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Xia, Z.; Gong, Q.; Liu, X.; Yang, Y.; Gao, Q. Facile preparation of polyaniline covalently grafted to isocyanate functionalized reduced graphene oxide nanocomposite for high performance flexible supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125172. [Google Scholar] [CrossRef]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Boonpavanitchakul, K.; Jarussophon, S.; Pimpha, N.; Kangwansupamonkon, W.; Magaraphan, R. Silk sericin as a bio-initiator for grafting from synthesis of polylactide via ring-opening polymerization. Eur. Polym. J. 2019, 121, 109265. [Google Scholar] [CrossRef]
- Nong, Y.; Zhou, Z.; Yuan, J.; Wang, P.; Yu, Y.; Wang, Q.; Fan, X. Bio-Inspired Coloring and Functionalization of Silk Fabric via Laccase-Catalyzed Graft Polymerization of Arylamines. Fibers Polym. 2020, 21, 1927–1937. [Google Scholar] [CrossRef]
- Zhou, B.; He, M.; Wang, P.; Fu, H.; Yu, Y.; Wang, Q.; Fan, X. Synthesis of silk fibroin-g-PAA composite using H2O2-HRP and characterization of the in situ biomimetic mineralization behavior. Mater. Sci. Eng. C 2017, 81, 291–302. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Xu, M.; Cai, H.; Liu, Z.; Chen, F.; Chen, L.; Chen, X.; Cheng, X.; Dai, F.; Li, Z. Breathable, Degradable Piezoresistive Skin Sensor Based on a Sandwich Structure for High-Performance Pressure Detection. Adv. Electron. Mater. 2021, 7, 2100368. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Liu, H.; Fan, Y. Double coating of graphene oxide–polypyrrole on silk fibroin scaffolds for neural tissue engineering. J. Bioact. Compat. Polym. 2020, 35, 216–227. [Google Scholar] [CrossRef]
- Gao, D.; Parida, K.; Lee, P.S. Emerging Soft Conductors for Bioelectronic Interfaces. Adv. Funct. Mater. 2020, 30, 1907184. [Google Scholar] [CrossRef]
- Ahmad, S.; Sultan, A.; Mohammad, F. Electrically Conductive Polyaniline/Silk Fibroin Composite for Ammonia and Acetaldehyde Sensing. Polym. Polym. Compos. 2018, 26, 177–187. [Google Scholar] [CrossRef]
- Hong, J.; Han, X.; Shi, H.; Jin, L.; Yao, J. Preparation of conductive silk fibroin yarns coated with polyaniline using an improved method based on in situ polymerization. Synth. Met. 2018, 235, 89–96. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, S.Y.; Wan, H.-Y.; Chen, Y.-T.; Yu, S.-K.; Wu, H.-C.; Yang, T.-I. Electrospun Hydrophobic Polyaniline/Silk Fibroin Electrochromic Nanofibers with Low Electrical Resistance. Polymers 2020, 12, 2102. [Google Scholar] [CrossRef]
- Li, X.; Ming, J.; Ning, X. Wet-spun conductive silk fibroin–polyaniline filaments prepared from a formic acid–shell solution. J. Appl. Polym. Sci. 2018, 136, 47127. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Ohgo, K.; Bagusat, F.; Asakura, T.; Scheler, U. Investigation of Structural Transition of Regenerated Silk Fibroin Aqueous Solution by Rheo-NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 4182–4186. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanopart. Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Conejo-Dávila, A.S.; Hernández-Escobar, C.A.; Vega-Rios, A.; Rodríguez-Sánchez, I.; Estrada-Monje, A.; de León-Gómez, R.E.D.; Zaragoza-Contreras, E.A. Selective polymerization of a new bifunctional monomer via free radical polymerization and oxidative route. Synth. Met. 2020, 259, 116258. [Google Scholar] [CrossRef]
- Shaw, A.A.; Wainschel, L.A.; Shetlar, M.D. The Photochemistry of p -Aminobenzoic Acid. Photochem. Photobiol. 1992, 55, 647–656. [Google Scholar] [CrossRef]
- Conejo-Dávila, A.S.; Moya-Quevedo, M.A.; Chávez-Flores, D.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate. Polymers 2021, 13, 2349. [Google Scholar] [CrossRef] [PubMed]
- Manseki, K.; Yu, Y.; Yanagida, S. A phenyl-capped aniline tetramer for Z907/tert-butylpyridine-based dye-sensitized solar cells and molecular modelling of the device. Chem. Commun. 2013, 49, 1416. [Google Scholar] [CrossRef] [Green Version]
- Ayub, Z.H.; Arai, M.; Hirabayashi, K. Quantitative structural analysis and physical properties of silk fibroin hydrogels. Polymer 1994, 35, 2197–2200. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Hudecki, A.; Kamińska, I.; Cieślak, M. Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier. Materials 2021, 14, 6919. [Google Scholar] [CrossRef] [PubMed]
- Borah, R.; Kumar, A.; Das, M.K.; Ramteke, A. Surface functionalization-induced enhancement in surface properties and biocompatibility of polyaniline nanofibers. RSC Adv. 2015, 5, 48971–48982. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Duan, X.; Liu, D.; Guo, J.; Liu, P. Crosslinked polyaniline nanorods with improved electrochemical performance as electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 12323–12329. [Google Scholar] [CrossRef]
- Alves, W.F.; Venancio, E.C.; Leite, F.L.; Kanda, D.H.F.; Malmonge, L.F.; Malmonge, J.A.; Mattoso, L.H.C. Thermo-analyses of polyaniline and its derivatives. Thermochim. Acta. 2010, 502, 43–46. [Google Scholar] [CrossRef]
- Chen, C.H. Thermal studies of polyaniline doped with dodecyl benzene sulfonic acid directly prepared via aqueous dispersions. J. Polym. Res. 2002, 9, 195–200. [Google Scholar] [CrossRef]
- Rice, W.L.; Firdous, S.; Gupta, S.; Hunter, M.; Foo, C.W.P.; Wang, Y.; Kim, H.J.; Kaplan, D.L.; Georgakoudi, I. Non-invasive characterization of structure and morphology of silk fibroin biomaterials using non-linear microscopy. Biomaterials 2008, 29, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Yavarinasab, A.; Abedini, M.; Tahmooressi, H.; Janfaza, S.; Tasnim, N.; Hoorfar, M. Potentiodynamic Electrochemical Impedance Spectroscopy of Polyaniline-Modified Pencil Graphite Electrodes for Selective Detection of Biochemical Trace Elements. Polymers 2021, 14, 31. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Conejo-Dávila, A.S.; Casas-Soto, C.R.; Aparicio-Martínez, E.P.; Chávez-Flores, D.; Ramos-Sánchez, V.H.; Dominguez, R.B.; Osuna, V.C.; Estrada-Monje, A.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature. Int. J. Mol. Sci. 2022, 23, 8085. [Google Scholar] [CrossRef]
- Armando Zaragoza-Contreras, E.; Stockton-Leal, M.; Hernández-Escobar, C.A.; Hoshina, Y.; Guzmán-Lozano, J.F.; Kobayashi, T. Synthesis of core–shell composites using an inverse surfmer. J. Colloid Interface Sci. 2012, 377, 231–236. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk Fibroin-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [Green Version]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Igarashi, Y.; Takasu, Y.; Saito, H.; Tsubouchi, K. Identification of fibroin-derived peptides enhancing the proliferation of cultured human skin fibroblasts. Biomaterials 2004, 25, 467–472. [Google Scholar] [CrossRef]
- Kucekova, Z.; Humpolicek, P.; Kasparkova, V.; Perecko, T.; Lehocký, M.; Hauerlandová, I.; Sáha, P.; Stejskal, J. Colloidal polyaniline dispersions: Antibacterial activity, cytotoxicity and neutrophil oxidative burst. Colloids Surf. B Biointerfaces 2014, 116, 411–417. [Google Scholar] [CrossRef]
- Li, Y.-S.; Chen, B.-F.; Li, X.-J.; Zhang, W.K.; Tang, H.-B. Cytotoxicity of Polyaniline Nanomaterial on Rat Celiac Macrophages In Vitro. PLoS ONE 2014, 9, e107361. [Google Scholar] [CrossRef] [Green Version]
- Kaba, S.; Egorova, E. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Lu, X.; Zhu, H. Natural silk fibroin/polyaniline (core/shell) coaxial fiber: Fabrication and application for cell proliferation. Compos. Sci. Technol. 2013, 77, 37–41. [Google Scholar] [CrossRef]








| Sample | Fib (g) | 3-Aminobenzoic Acid/g (mmol) | Anilinium Sulfate/g (mmol) | APS/g (mmol) | Yield/% |
|---|---|---|---|---|---|
| Fib-g-Pani 1:0.1 | 1 | 0.15 (1.1) | 0.10 (0.523) | 0.149 (0.654) | 15% |
| Fib-g-Pani 1:0.25 | 1 | 0.15 (1.1) | 0.25 (1.307) | 0.373 (1.634) | 45% |
| Fib-g-Pani 1:0.5 | 1 | 0.15 (1.1) | 0.50 (2.615) | 0.746 (3.269) | 87% |
| Fib-g-Pani 1:1 | 1 | 0.15 (1.1) | 1.00 (5.230) | 1.492 (6.537) | 95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Vela, E.V.; Conejo-Dávila, A.S.; Hernández-Escobar, C.A.; Dominguez, R.B.; Chávez-Flores, D.; Tapia-Lopez, L.V.; Piñon-Balderrama, C.; Estrada-Monje, A.; Luna-Velasco, M.A.; Osuna, V.C.; et al. Silk Fibroin-g-Polyaniline Platform for the Design of Biocompatible-Electroactive Substrate. Polymers 2022, 14, 4653. https://doi.org/10.3390/polym14214653
Flores-Vela EV, Conejo-Dávila AS, Hernández-Escobar CA, Dominguez RB, Chávez-Flores D, Tapia-Lopez LV, Piñon-Balderrama C, Estrada-Monje A, Luna-Velasco MA, Osuna VC, et al. Silk Fibroin-g-Polyaniline Platform for the Design of Biocompatible-Electroactive Substrate. Polymers. 2022; 14(21):4653. https://doi.org/10.3390/polym14214653
Chicago/Turabian StyleFlores-Vela, Elsa Veronica, Alain Salvador Conejo-Dávila, Claudia Alejandra Hernández-Escobar, Rocio Berenice Dominguez, David Chávez-Flores, Lillian V. Tapia-Lopez, Claudia Piñon-Balderrama, Anayansi Estrada-Monje, María Antonia Luna-Velasco, Velia Carolina Osuna, and et al. 2022. "Silk Fibroin-g-Polyaniline Platform for the Design of Biocompatible-Electroactive Substrate" Polymers 14, no. 21: 4653. https://doi.org/10.3390/polym14214653
APA StyleFlores-Vela, E. V., Conejo-Dávila, A. S., Hernández-Escobar, C. A., Dominguez, R. B., Chávez-Flores, D., Tapia-Lopez, L. V., Piñon-Balderrama, C., Estrada-Monje, A., Luna-Velasco, M. A., Osuna, V. C., & Zaragoza-Contreras, E. A. (2022). Silk Fibroin-g-Polyaniline Platform for the Design of Biocompatible-Electroactive Substrate. Polymers, 14(21), 4653. https://doi.org/10.3390/polym14214653