Structure–Properties Relationships Involved in the Embrittlement of Epoxies
Abstract
:1. Introduction
2. Experimental Procedure
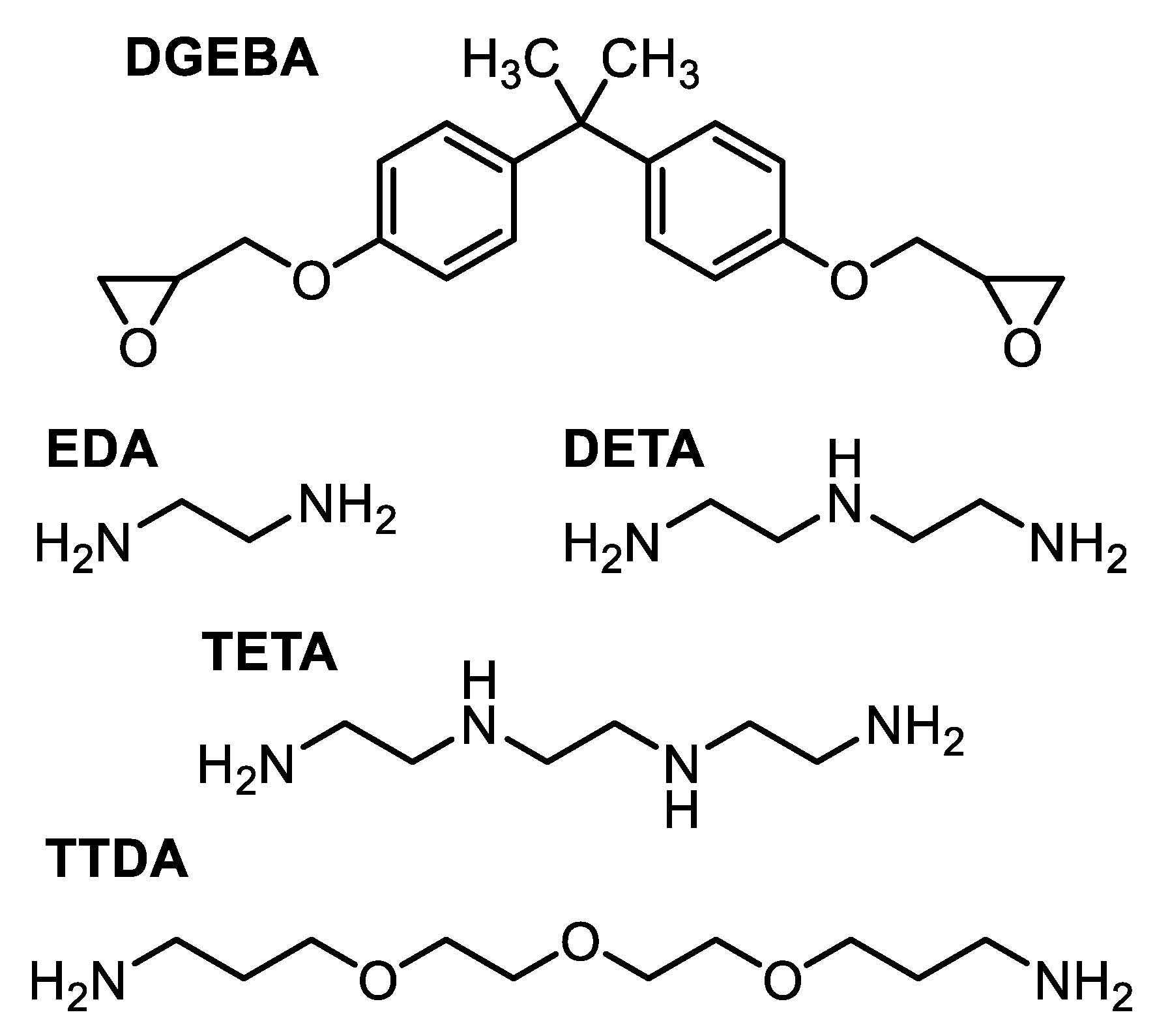
2.1. Materials
- -
- DGEBA-TTDA was cured for 1 h at 60 °C with a heating press and post-cured for 3 h in a vacuum at 80 °C.
- -
- DGEBA-EDA and DGEBA-DETA were cured according to the following experimental procedure: After mixing the prepolymers in stoichiometric proportions (according to the equivalent weights previously listed), the blends were set to thicken for up to 3 h in closed vials at ambient room temperature (actual time being heavily dependent on the nature and volume of the mixture and atmospheric conditions). Once they were thick enough, the blends were pressed using a hydropress for 1 h at 50 °C and 1 h at 90 °C. They were then post-cured for 30 min at 170 °C under vacuum.
- -
- Commercial epoxy was cured at room temperature (48 h).
2.2. Exposure Conditions
3. Characterization
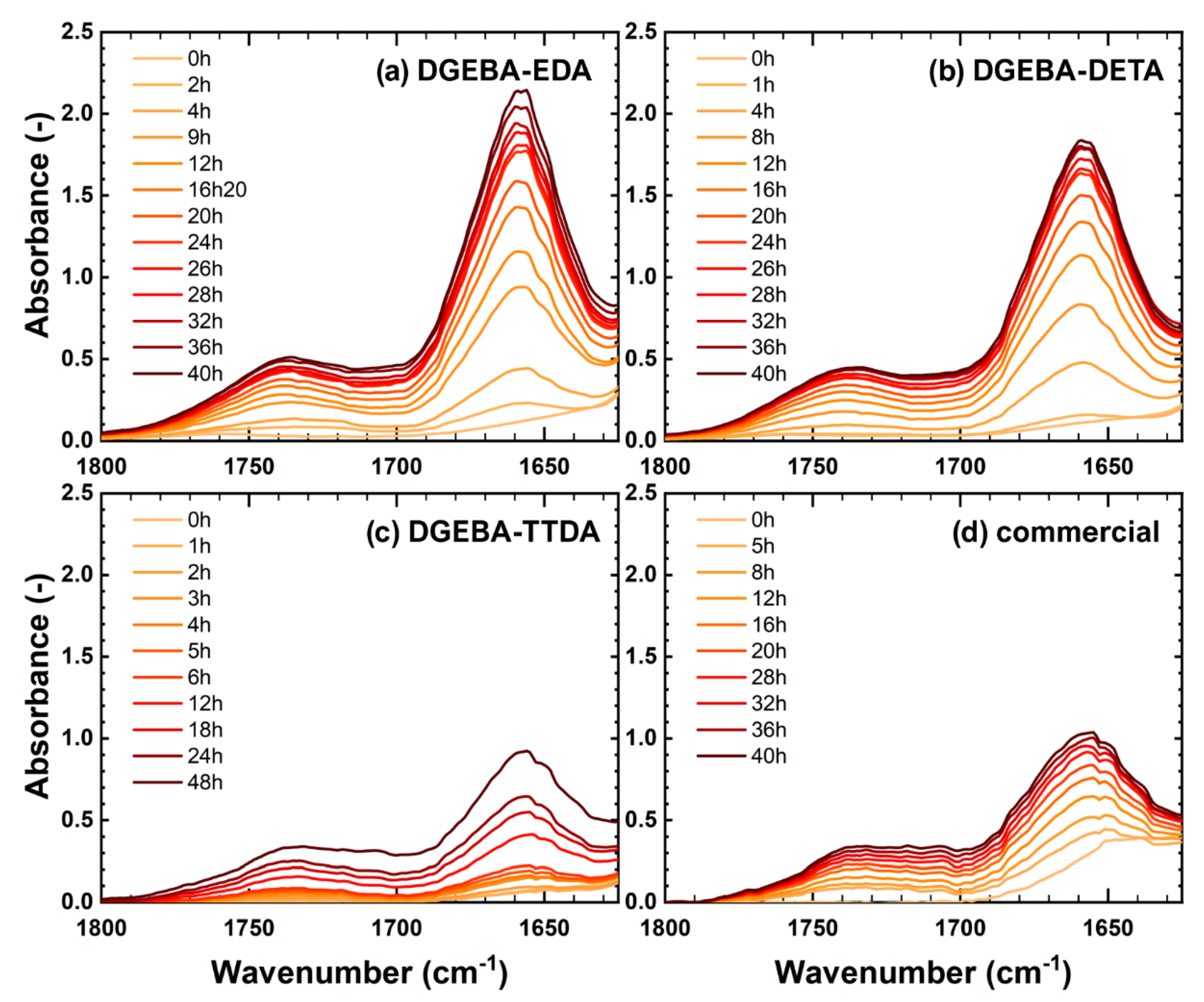
3.1. Fourier Transform InfraRed Spectroscopy
3.2. Differential Scanning Calorimetry
3.3. Mechanical Test
4. Results and Discussion
4.1. Accumulation of Stable Oxidation Products
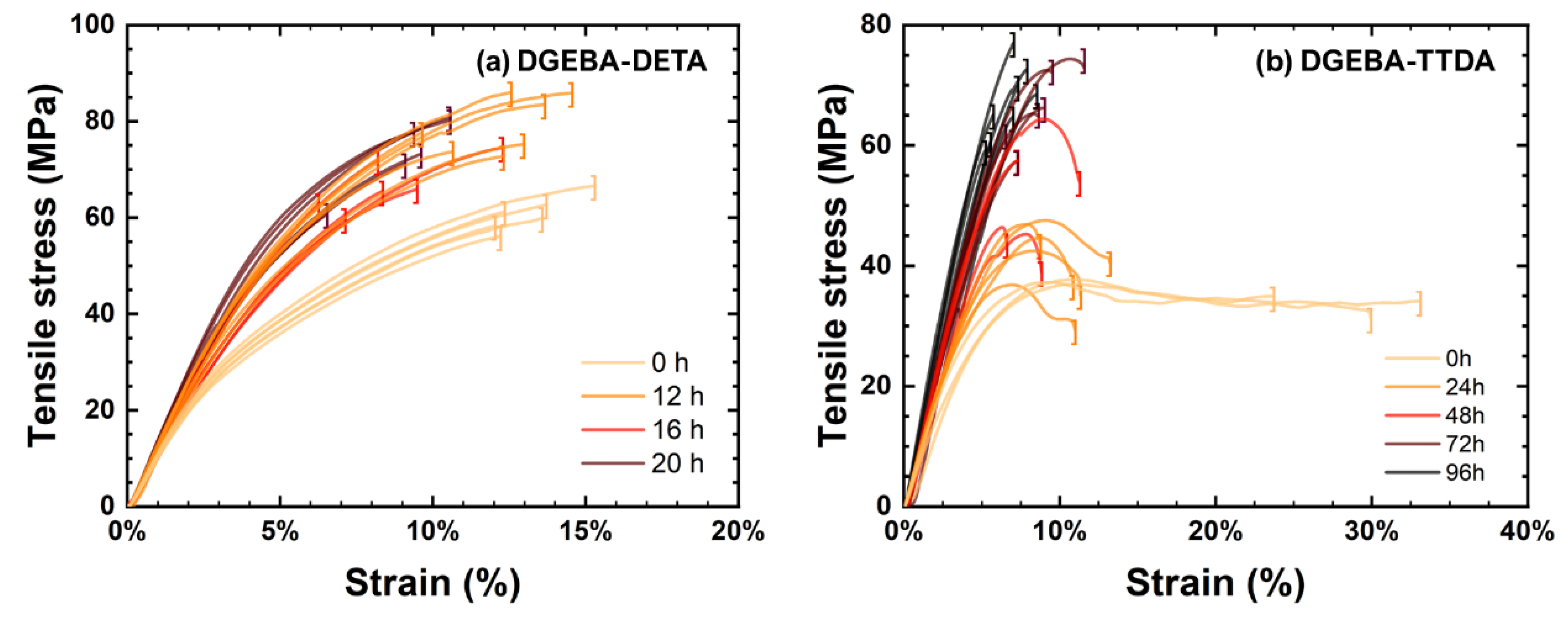
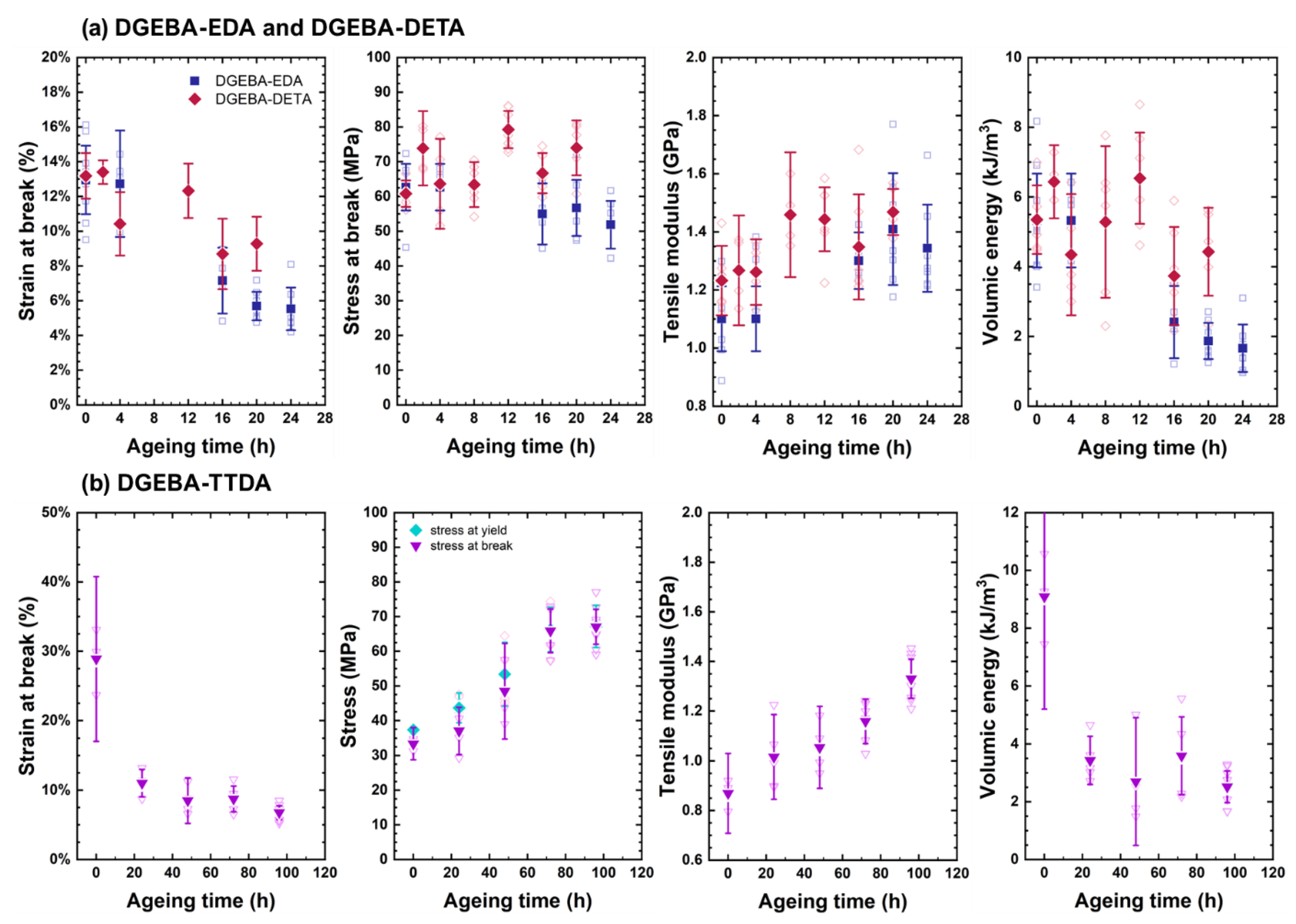
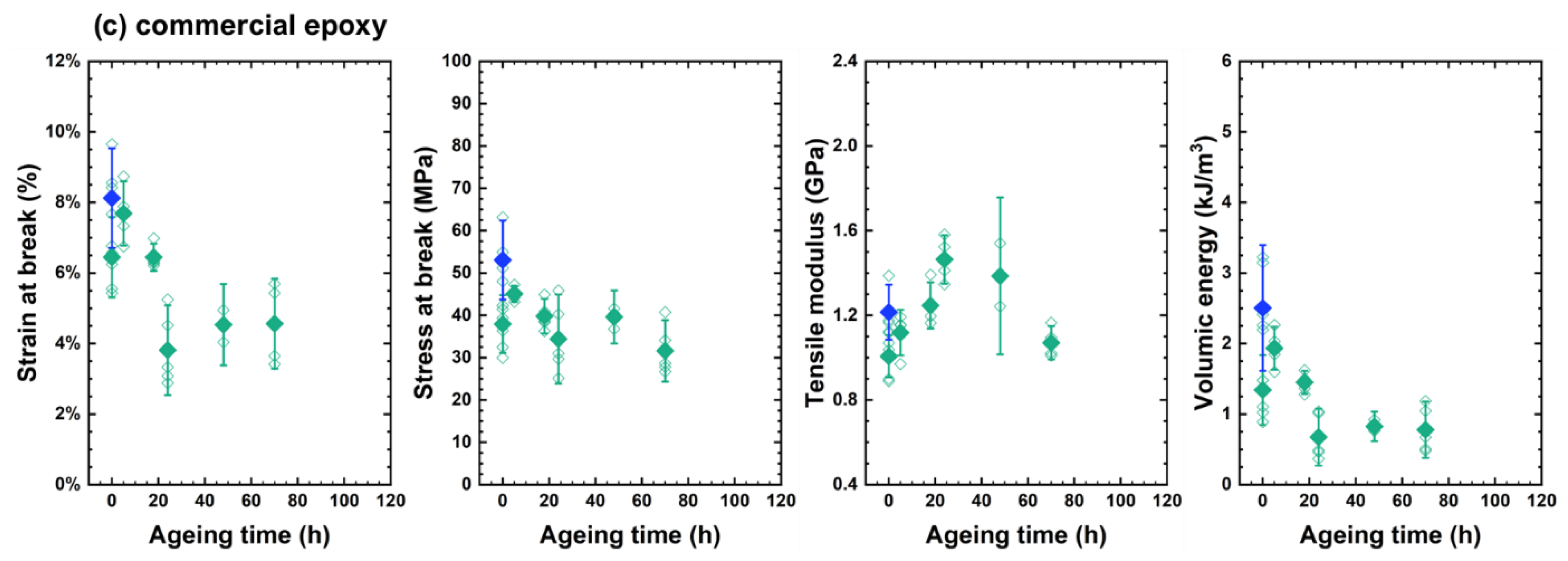
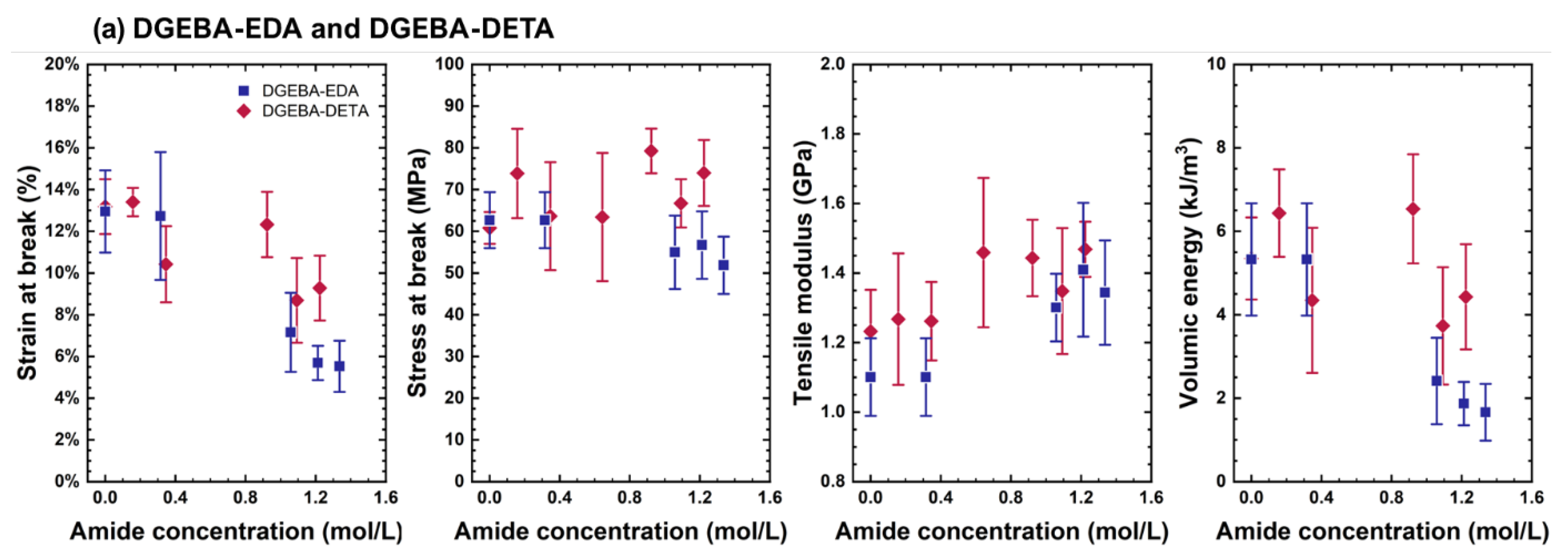
4.2. Changes in Mechanical Properties
- ➀
- The elastic modulus continuously increased.
- ➁
- Ultimate strain continuously decreased.
- ➂
- Stress at break first increased in link with the increase in the elastic modulus and decreased in link with the decrease in ultimate strain. Its changes were driven by two opposite trends: (i) the total loss of plasticity, so that the decrease in ultimate stress is linked to the decrease in ultimate strain, and (ii) the «antiplasticization», due to loss of plasticizer and loss of beta relaxation inducing the increase in ultimate stress. In DGEBA-TTDA, another phenomenon must be taken into account: the increase in yield stress due to crosslinking, which seems to have induced an increase in ultimate stress (stress at break). Lastly, the comparison of DGEBA-EDA, DGEBA-DETA and commercial epoxy suggests that plasticizer loss (if any) had a negligible influence. As a matter of fact, the stress-strain curves for commercial epoxy before and after evaporation under vacuum of plasticizer are depicted in Appendix A and do not show significant differences.
- ➃
- The volumic energy (determined as the “area under the stress-strain curve”) decreased with the decrease in ultimate strain (both phenomena being undoubtedly related).
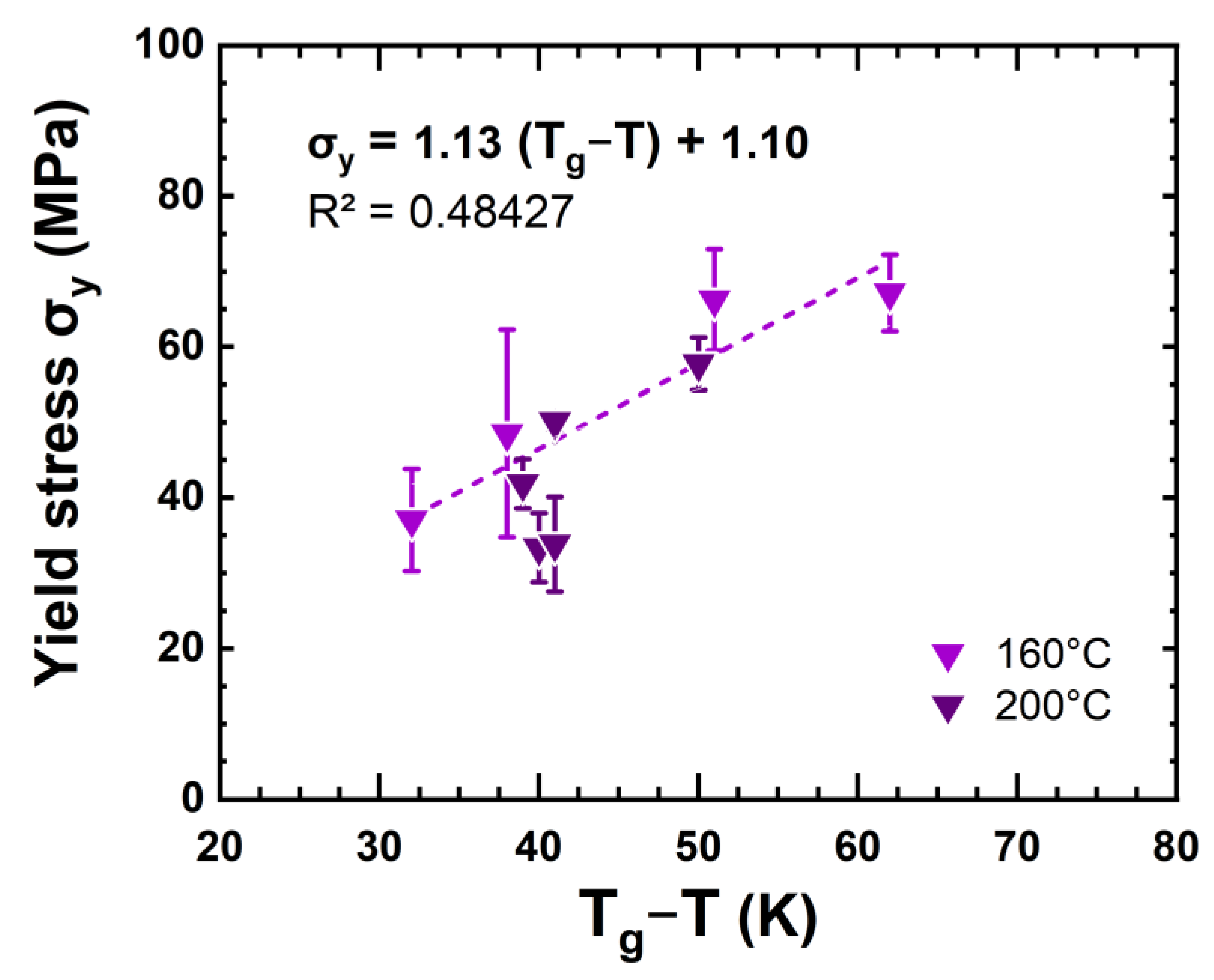
4.3. Correlation between Chemical and Mechanical Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Bellenger, V.; Verdu, J.; Morel, E. Effect of structure on glass transition temperature of amine crosslinked epoxies. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1219–1234. [Google Scholar] [CrossRef]
- Sindt, O.; Perez, J.; Gerard, J.F. Molecular architecture-mechanical behaviour relationships in epoxy networks. Polymer 1996, 37, 2989–2997. [Google Scholar] [CrossRef]
- Grillet, A.C.; Galy, J.; Gérard, J.-F.; Pascault, J.-P. Mechanical and viscoelastic properties of epoxy networks cured with aromatic diamines. Polymer 1991, 32, 1885–1891. [Google Scholar] [CrossRef]
- KalitaIhor, D.J.; Bret, T.; Chisholm, J.; Webster, D.C. Novel bio-based epoxy resins from eugenol as an alternative to BPA epoxy and high throughput screening of the cured coatings. Polymer 2021, 233, 124191. [Google Scholar] [CrossRef]
- Morsch, S.; Stuart, S.E.; Lyon, B.; Gibbon, S.R.; Irwin, M. The location of adsorbed water in pigmented epoxy-amine coatings. Prog. Org. Coat. 2022, 173, 107223. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, B.; Wei, K.; Wang, X.; Kang, X.; Zhang, H.; Shi, J. Effect of humid and thermal environments on the performance of an epoxy resin pavement filling joint material. Constr. Build. Mater. 2022, 357, 129158. [Google Scholar] [CrossRef]
- Delor-Jestin, F.; Drouin, D.; Cheval, P.-Y.; Lacoste, J. Thermal and photochemical ageing of epoxy resin–Influence of curing agents. Polym. Degrad. Stab. 2006, 91, 1247–1255. [Google Scholar] [CrossRef]
- Zahra, Y.; Djouani, F.; Fayolle, B.; Kuntz, M.; Verdu, J. Thermo-oxidative aging of epoxy coating systems. Prog. Org. Coat. 2014, 77, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Musto, P.; Abbate, M.; Pannico, M.; Scarinzi, G.; Ragosta, G. Improving the photo-oxidative stability of epoxy resins by use of functional POSS additives: A spectroscopic, mechanical and morphological study. Polymer 2012, 53, 5016–5036. [Google Scholar] [CrossRef]
- Delozanne, J.; Desgardin, N.; Cuvillier, N.; Richaud, E. Thermal oxidation of aromatic epoxy-diamine networks. Polym. Degrad. Stab. 2019, 166, 174–187. [Google Scholar] [CrossRef]
- Colin, X.; Essatbi, F.; Delozanne, J.; Moreau, G. Towards a general kinetic model for the thermal oxidation of epoxy-diamine networks. Effect of the molecular mobility around the glass transition temperature. Polym. Degrad. Stab. 2020, 181, 109314. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Origin of epoxies embrittlement during oxidative ageing. Polym. Test. 2017, 63, 448–454. [Google Scholar] [CrossRef]
- Rasoldier, N.; Colin, X.; Verdu, J.; Bocquet, M.; Olivier, L.; Chocinski-Arnault, L.; Lafarie-Frenot, M.C. Model systems for thermo-oxidised epoxy composite matrices. Compos. Part A: Appl. Sci. Manuf. 2008, 39, 1522–1529. [Google Scholar] [CrossRef]
- Gilbert, D.G.; Ashby, M.F.; Beaumont, P.W.R. Modulus-maps for amorphous polymers. J. Mater. Sci. 1986, 21, 3194–3210. [Google Scholar] [CrossRef]
- Ernault, E.; Dirrenberger, J.; Richaud, E.; Fayolle, B. Prediction of stress induced by heterogeneous oxidation: Case of epoxy/amine networks. Polym. Degrad. Stab. 2019, 162, 112–121. [Google Scholar] [CrossRef] [Green Version]
- le Craz, S.; Pethrick, R.A. Solvent Effects on Cure 1-Benzyl Alcohol on Epoxy Cure. Int. J. Polym. Mater. Polym. Biomater. 2011, 60, 441–455. [Google Scholar] [CrossRef]
- Perdigão, N.F.; Castro, V.G.; Silva, G.G. Glass transition behavior in epoxy nanocomposites with oxidized and aminated carbon nanotubes, graphene oxide and nano-calcium carbonate. Thermochim. Acta 2022, 717, 179331. [Google Scholar] [CrossRef]
- Liu, W.; Huang, W.; Song, N.; Wu, Y.; Zhao, X.; Chen, K. Effect of stoichiometry on chemical structure, dielectric and mechanical properties of epoxy resin under gamma irradiation. Radiat. Phys. Chem. 2022, 202, 110551. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal oxidation of epoxies: Influence of diamine hardener. Polym. Degrad. Stab. 2016, 134, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Delannoy, R.; Tognetti, V.; Richaud, E. Experimental and theoretical insights on the thermal oxidation of epoxy-amine networks. Polym. Degrad. Stab. 2022, 110188. [Google Scholar] [CrossRef]
- Rouillon, C.; Bussiere, P.-O.; Desnoux, E.; Collin, S.; Vial, C.; Therias, S.; Gardette, J.-L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation. Polym. Degrad. Stab. 2016, 128, 200–208. [Google Scholar] [CrossRef]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Delannoy, R.; Richaud, E.; Tognetti, V.; Quélennec, B.; Delbreilh, L.; Delpouve, N. Macromolecular mobility changes induced by thermal ageing of epoxy-amine polymer networks. Special Issue: Times of Polymers and Composites (TOP), October 2022. Macromol. Symp. 2022, 405, 2100228. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal-oxidation of epoxy/amine followed by glass transition temperature changes. Polym. Degrad. Stab. 2017, 138, 82–90. [Google Scholar] [CrossRef]
- Quelennec, B.; Duan, Z.; Delannoy, R.; Gay, N.; Agostini, F.; Skoczylas, F.; Briffaut, M.; Tognetti, V.; Delpouve, N.; Delbreilh, L.; et al. Effect of physical and chemical ageing on barrier properties of epoxy coatings. Constr. Build. Mater. 2022; submitted. [Google Scholar]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 2002. [Google Scholar]
- Kambour, R.P. Correlations of the dry crazing resistance of glassy polymers with other physical properties. Polym. Commun. 1983, 24, 292–296. [Google Scholar]
- Cook, W.D.; Mayr, A.E.; Edward, G.H. Yielding behaviour in model epoxy thermosets—II. Temperature dependence. Polymer 1998, 39, 3725–3733. [Google Scholar] [CrossRef]
- Urbaniak, M. Modele plastycznoœci Eyringa i Robertsona dotycz¹ce tworzywa epoksydowego “EPY®”. Polim. Wars. 2008, 53, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Fayolle, B.; Audouin, L.; Verdu, J. Oxidation induced embrittlement in polypropylene—A tensile testing study. Polym. Degrad. Stab. 2000, 70, 333–340. [Google Scholar] [CrossRef]
- Fayolle, B.; Colin, X.; Audouin, L.; Verdu, J. Mechanism of degradation induced embrittlement in polyethylene. Polym. Degrad. Stab. 2007, 92, 231–238. [Google Scholar] [CrossRef]



| System | (°C) | (°C) | ΔH (J g−1) | (°C) |
|---|---|---|---|---|
| DGEBA-TTDA | 82 | 114 | 355 | 63 |
| DGEBA-EDA | 76 | 103 | 440 | 145 |
| DGEBA-DETA | 70 | 100 | 501 | 147 |
| Commercial epoxy | 66 | 112 | 182 | 71 |
| System | MUCR (g mol−1) | Number of Hydroxypropylether Groups/UCR | c0 (mol L−1) |
|---|---|---|---|
| DGEBA-TTDA | 904 | 4 | ~5.3 |
| DGEBA-EDA | 744 | 4 | ~6.8 |
| DGEBA-DETA | 904 | 5 | ~6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delannoy, R.; Tognetti, V.; Richaud, E. Structure–Properties Relationships Involved in the Embrittlement of Epoxies. Polymers 2022, 14, 4685. https://doi.org/10.3390/polym14214685
Delannoy R, Tognetti V, Richaud E. Structure–Properties Relationships Involved in the Embrittlement of Epoxies. Polymers. 2022; 14(21):4685. https://doi.org/10.3390/polym14214685
Chicago/Turabian StyleDelannoy, Romain, Vincent Tognetti, and Emmanuel Richaud. 2022. "Structure–Properties Relationships Involved in the Embrittlement of Epoxies" Polymers 14, no. 21: 4685. https://doi.org/10.3390/polym14214685






