Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pilot-Scale UF Process Setup
2.3. BDOC Removal by UF
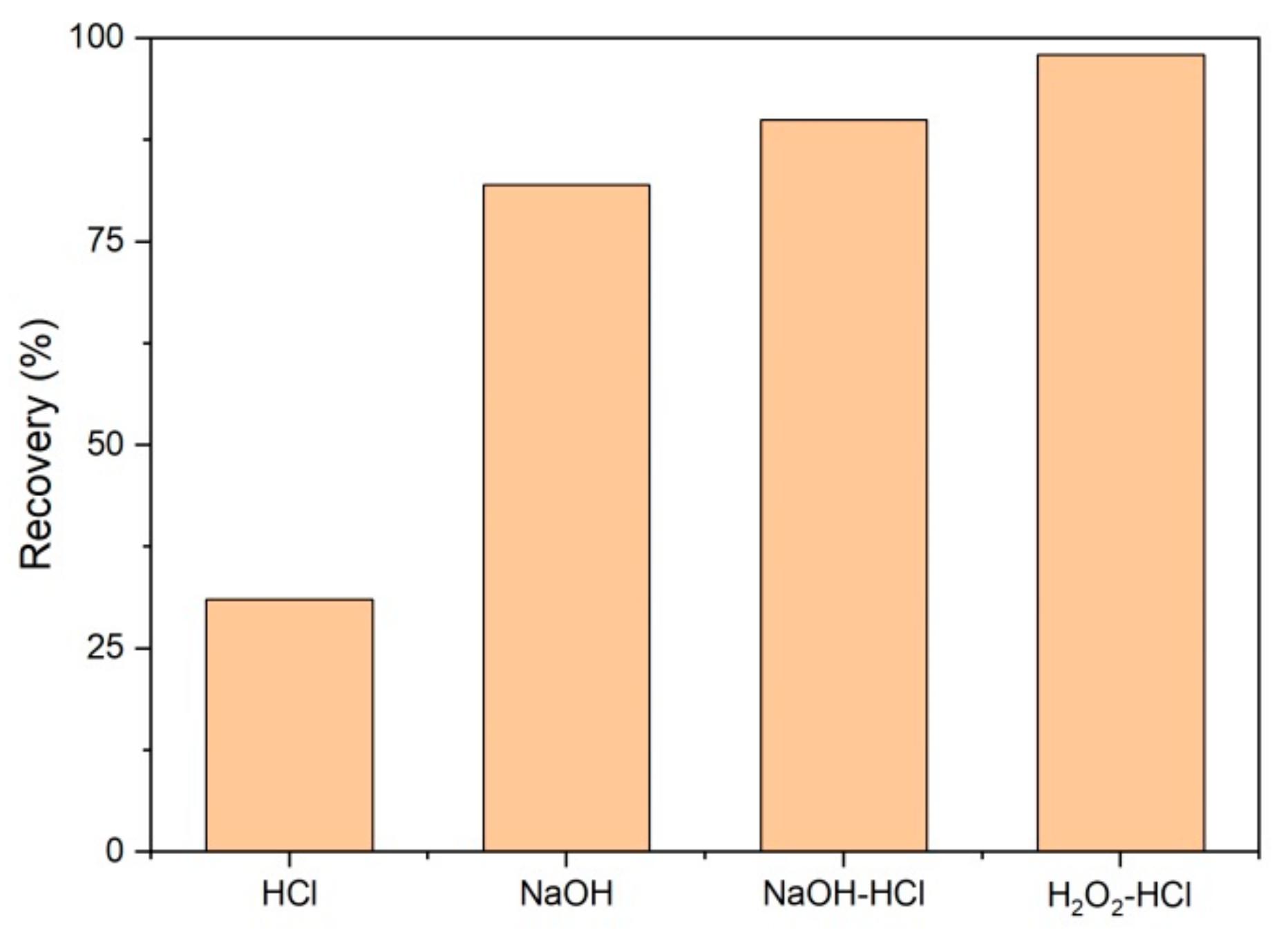
2.4. Cleaning and Recycling of UF Membranes
2.5. Analysis Methods
3. Results and Discussion
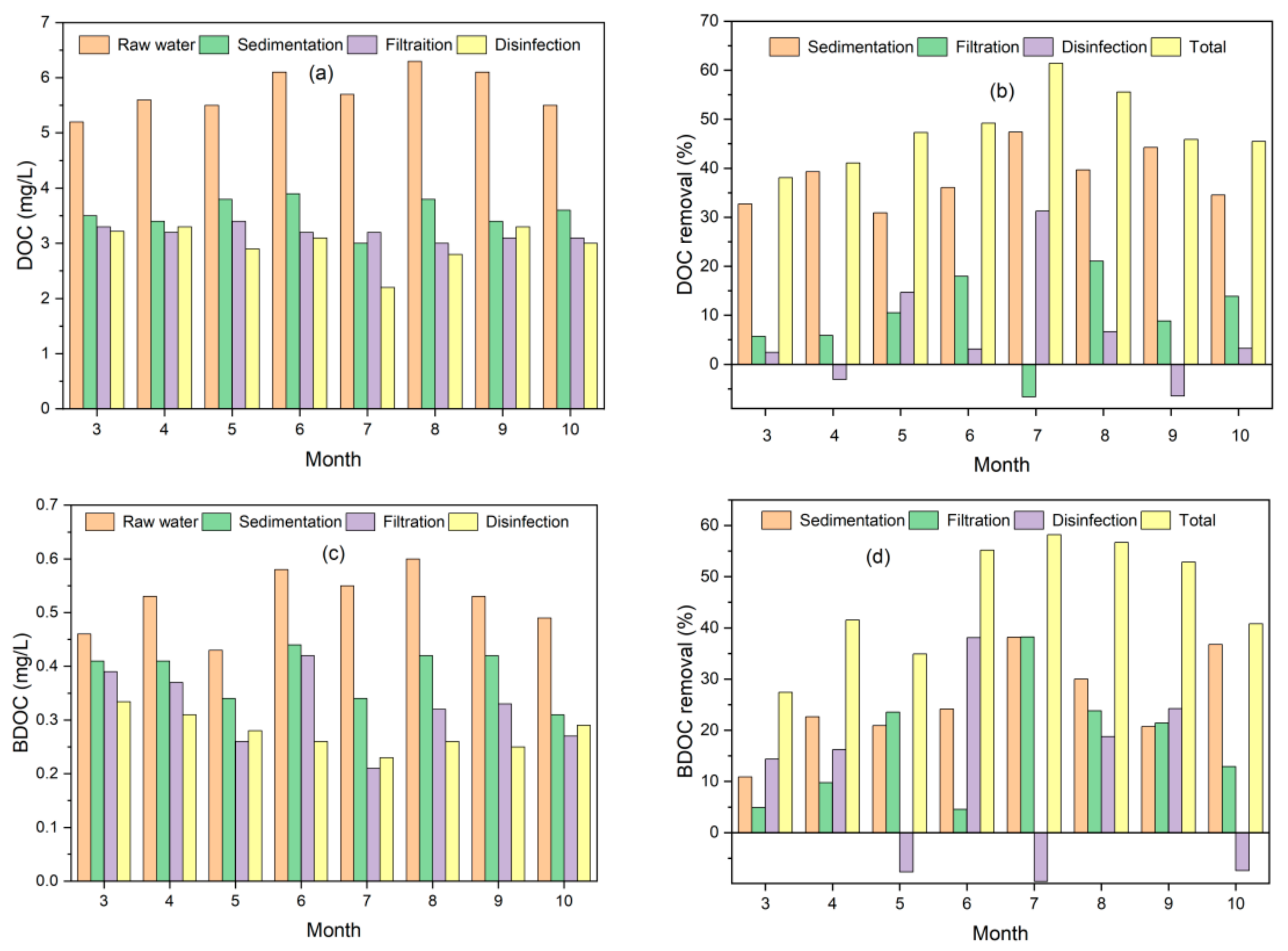
3.1. DOC and BDOC Removal by Conventional Treatment Processes
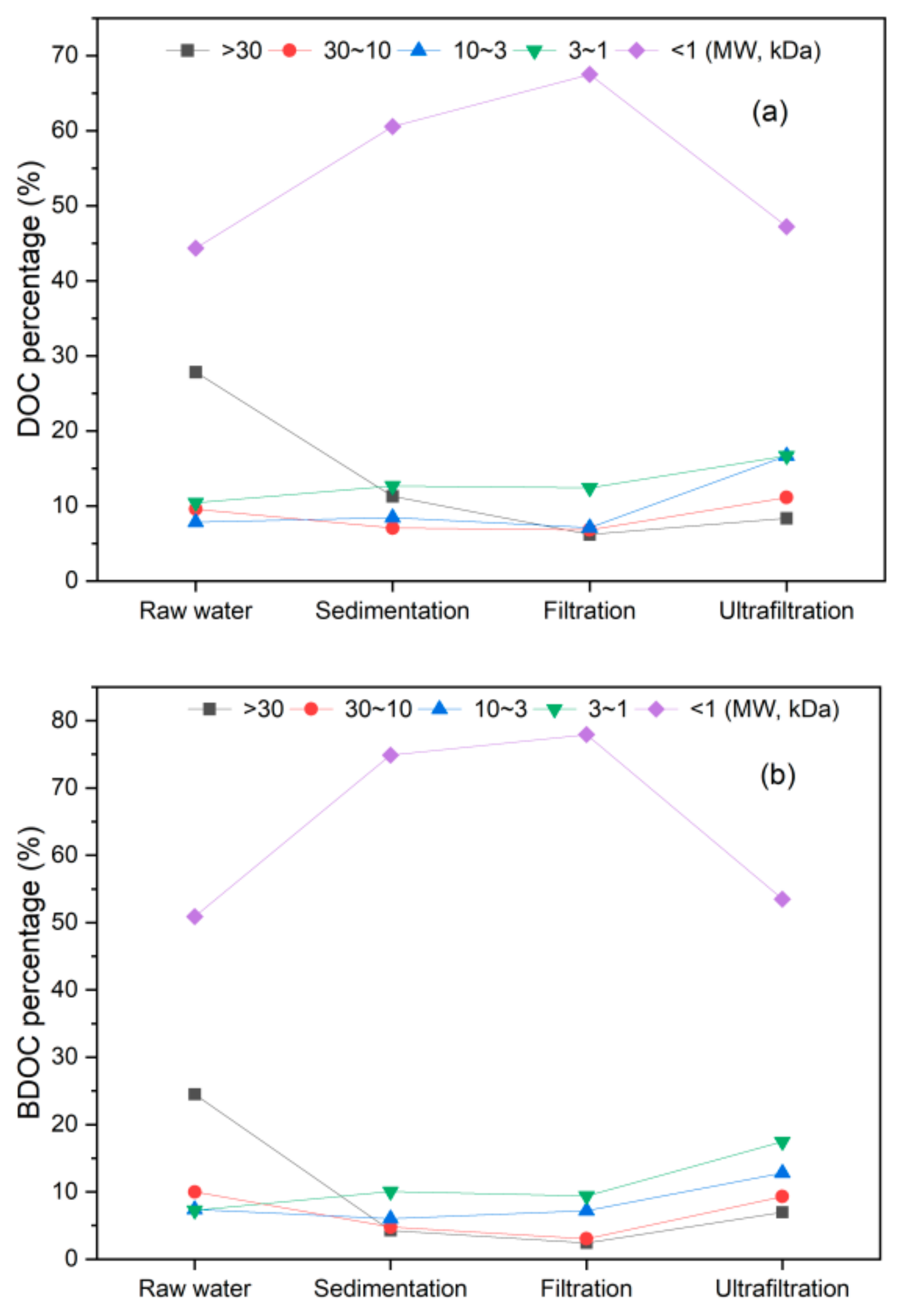
3.2. Micro-Polluted Water Treatment by UF
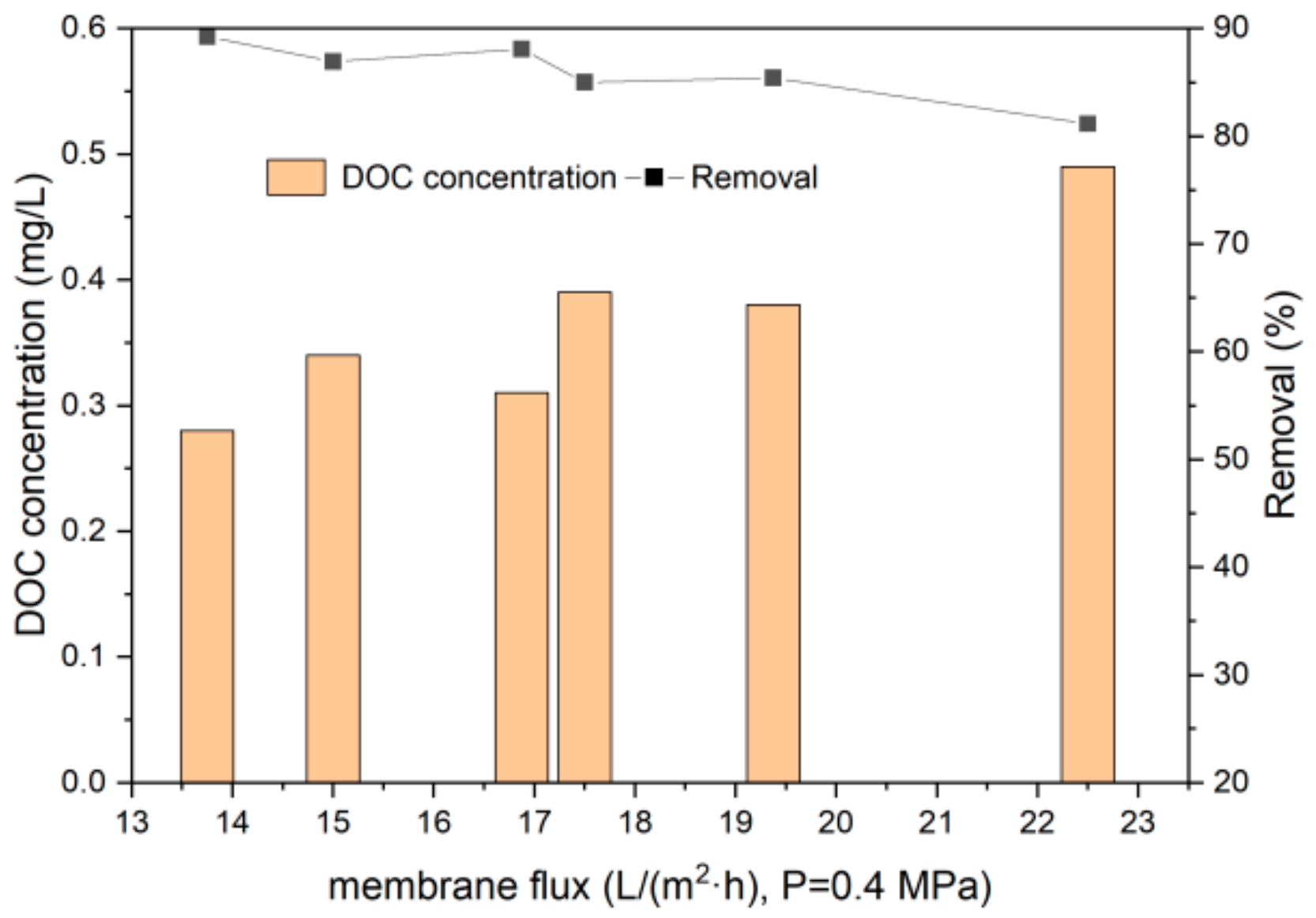
3.2.1. DOC Removal by UF
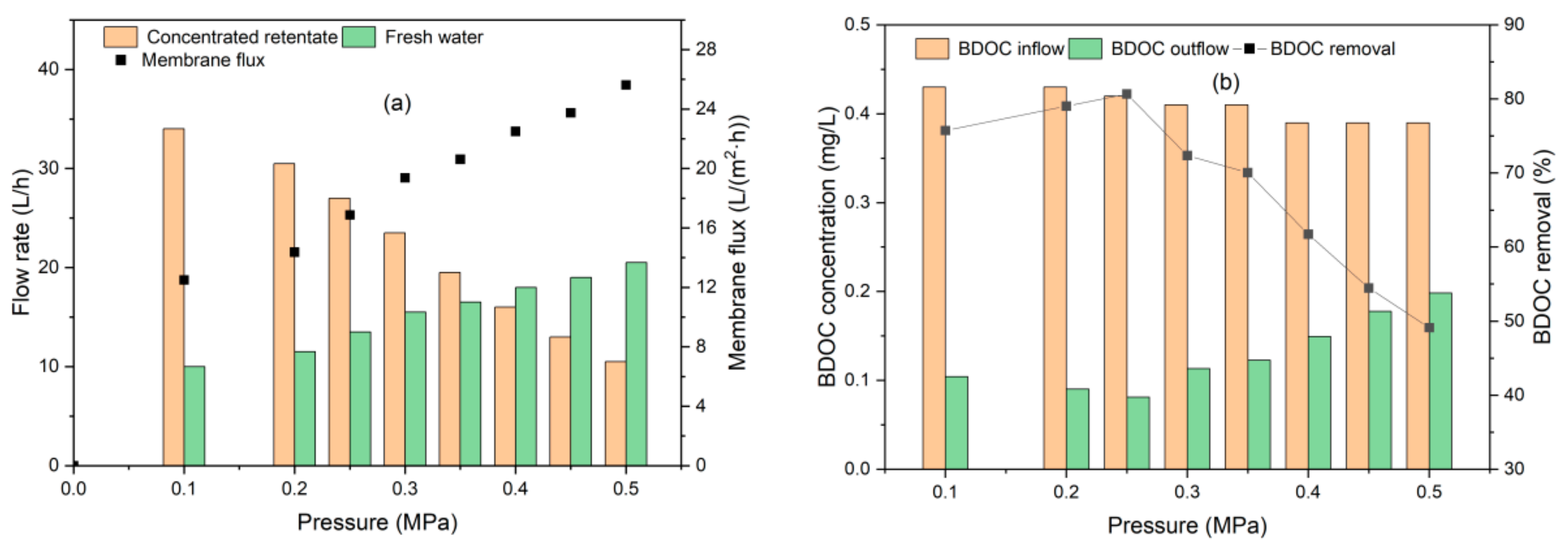
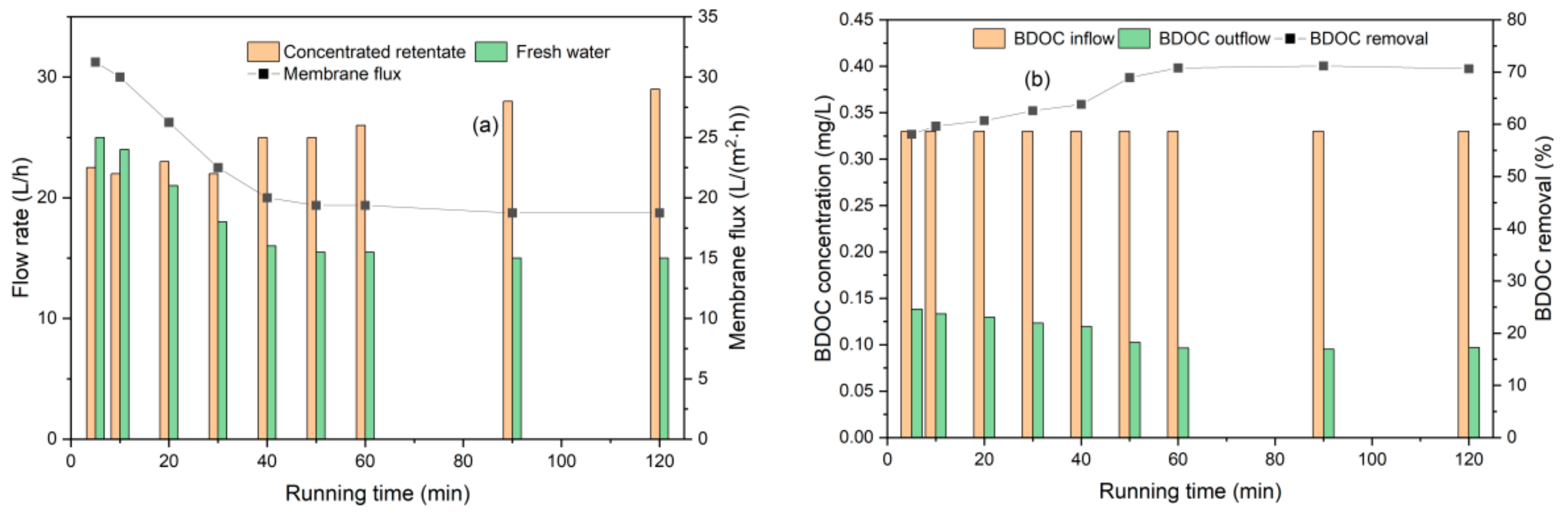
3.2.2. BDOC Removal by UF
3.3. UF Membrane Fouling and Cleaning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olivieri, V.P.; Snead, M.C.; Krusé, C.W.; Kawata, K. Stability and effectiveness of chlorine disinfectants in water distribution systems. Environ. Health Perspect. 1986, 69, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Volk, C.J.; LeChevallier, M.W. Assessing biodegradable organic matter. J.-Am. Water Work. Assoc. 2000, 92, 64–76. [Google Scholar] [CrossRef]
- Terry, L.G.; Summers, R.S. Biodegradable organic matter and rapid-rate biofilter performance: A review. Water Res. 2018, 128, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Rosinska, A.; Rakocz, K. Risk assessment and the effect of chlorination on the content of forms of biodegradable organic carbon in water intended for consumption. Desalin. Water Treat. 2020, 199, 371–379. [Google Scholar] [CrossRef]
- Liu, W.; Wu, H.; Wang, Z.; Ong, S.L.; Hu, J.Y.; Ng, W.J. Investigation of assimilable organic carbon (AOC) and bacterial regrowth in drinking water distribution system. Water Res. 2002, 36, 891–898. [Google Scholar] [CrossRef]
- Skovhus, T.L.; Soborg, D.A.; Braga, F.S.; Hojris, B.; Kristensen, K.B.; Hansen, K.L. Effects of early biofilm formation on water quality during commissioning of new polyethylene pipes. Environ. Sci.-Water Res. Technol. 2022, 8, 1992–2005. [Google Scholar] [CrossRef]
- Silhan, J.; Corfitzen, C.B.; Albrechtsen, H.J. Effect of temperature and pipe material on biofilm formation and survival of Escherichia coli in used drinking water pipes: A laboratory-based study. Water Sci. Technol. 2006, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Guan, Y.T.; Zhu, W.P. Effect of biofilm on cast iron pipe corrosion in drinking water distribution system: Corrosion scales characterization and microbial community structure investigation. Corros. Sci. 2008, 50, 2816–2823. [Google Scholar] [CrossRef]
- Schaule, G.; Moschnitschka, D.; Schulte, S.; Tamachkiarow, A.; Flemming, H.C. Biofilm growth in response to various concentrations of biodegradable material in drinking water. Water Sci. Technol. 2007, 55, 191–195. [Google Scholar] [CrossRef][Green Version]
- Van der Kooij, D.; Martijn, B.; Schaap, P.G.; Hoogenboezem, W.; Veenendaal, H.R.; van der Wielen, P. Improved biostability assessment of drinking water with a suite of test methods at a water supply treating eutrophic lake water. Water Res. 2015, 87, 347–355. [Google Scholar] [CrossRef]
- Zhang, K.J.; Pan, R.J.; Zhang, T.Q.; Xu, J.; Zhou, X.Y.; Yang, Y.L. A novel method: Using an adenosine triphosphate (ATP) luminescence-based assay to rapidly assess the biological stability of drinking water. Appl. Microbiol. Biotechnol. 2019, 103, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Dukan, S.; Levi, Y.; Piriou, P.; Guyon, F.; Villon, P. Dynamic modelling of bacterial growth in drinking water networks. Water Res. 1996, 30, 1991–2002. [Google Scholar] [CrossRef]
- Nissinen, T.K.; Miettinen, I.T.; Martikainen, P.J.; Vartiainen, T. Molecular size distribution of natural organic matter in raw and drinking waters. Chemosphere 2001, 45, 865–873. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T. Application of cross-linked stearic acid nanoparticles with dialysis membranes for methylene blue recovery. Sep. Purif. Technol. 2018, 204, 21–29. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T.; Wu, Y.; Cao, X.; Hankins, N.P. Inorganic anion removal using micellar enhanced ultrafiltration (MEUF), modeling anion distribution and suggested improvements of MEUF: A review. Chem. Eng. J. 2020, 398, 125413. [Google Scholar] [CrossRef]
- Bray, R.T.; Jankowska, K.; Kulbat, E.; Łuczkiewicz, A.; Sokołowska, A. Ultrafiltration process in disinfection and advanced treatment of tertiary treated wastewater. Membranes 2021, 11, 221. [Google Scholar] [CrossRef]
- Gray, H.E.; Powell, T.; Choi, S.Y.; Smith, D.S.; Parker, W.J. Organic phosphorus removal using an integrated advanced oxidation-ultrafiltration process. Water Res. 2020, 182, 115968. [Google Scholar] [CrossRef] [PubMed]
- Nassar, L.; Hegab, H.M.; Khalil, H.; Wadi, V.S.; Naddeo, V.; Banat, F.; Hasan, S.W. Development of green polylactic acid asymmetric ultrafiltration membranes for nutrient removal. Sci. Total Environ. 2022, 824, 153869. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T. Anion Exchange on Cationic Surfactant Micelles, and a Speciation Model for Estimating Anion Removal on Micelles during Ultrafiltration of Water. Langmuir 2017, 33, 6540–6549. [Google Scholar] [CrossRef]
- Chen, M.; Shafer-Peltier, K.; Randtke, S.J.; Peltier, E. Modeling arsenic (V) removal from water by micellar enhanced ultrafiltration in the presence of competing anions. Chemosphere 2018, 213, 285–294. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.; Kenner, C.; Albertson, E.; Chen, M.; Randtke, S.; Peltier, E. Removing scale-forming cations from produced waters. Environ. Sci. Water Res. Technol. 2020, 6, 132–143. [Google Scholar] [CrossRef]
- Hosch, J.; Staude, E. Preparation and investigation of chemically modified porous polyamide ultrafiltration membranes. J. Membr. Sci. 1996, 121, 71–82. [Google Scholar] [CrossRef]
- Eken, G.A.; Acar, M.H. Polysulfone-based amphiphilic copolymers: Effect of hydrophilic content on morphology and performance of ultrafiltration membranes. J. Appl. Polym. Sci. 2020, 137, 48306. [Google Scholar] [CrossRef]
- Huang, Y.H.; Shang, C.; Li, L. Novel N-doped graphene enhanced ultrafiltration nano-porous polyvinylidene fluoride membrane with high permeability and stability for water treatment. Sep. Purif. Technol. 2021, 267, 118622. [Google Scholar] [CrossRef]
- Peters, C.D.; Rantissi, T.; Gitis, V.; Hankins, N.P. Retention of natural organic matter by ultrafiltration and the mitigation of membrane fouling through pre-treatment, membrane enhancement, and cleaning—A review. J. Water Process Eng. 2021, 44, 102374. [Google Scholar] [CrossRef]
- Gibert, O.; Vera, M.; Cruz, S.; Boleda, M.R.; Paraira, M.; Martin-Alonso, J.; Casas, S.; Bernat, X. Characterisation of organic foulants on full-scale UF membranes during filtration, backwash and chemical cleaning episodes. Desalin. Water Treat. 2017, 89, 17–28. [Google Scholar] [CrossRef][Green Version]
- Yu, H.K.; Chang, H.Q.; Li, X.; Zhou, Z.W.; Song, W.C.; Ji, H.J.; Liang, H. Long-term fouling evolution of polyvinyl chloride ultrafiltration membranes in a hybrid short-length sedimentation/ultrafiltration process for drinking water production. J. Membr. Sci. 2021, 630, 119320. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Escobar, I.C.; Randall, A.A. Assimilable organic carbon (AOC) and biodegradable dissolved organic carbon (BDOC): Complementary measurements. Water Res. 2001, 35, 4444–4454. [Google Scholar] [CrossRef]
- You, X.N.; Li, X.Y.; Sillanpaa, M.; Wang, R.; Wu, C.Y.; Xu, Q.Q. Export of Dissolved Organic Carbon from the Source Region of Yangtze River in the Tibetan Plateau. Sustainability 2022, 14, 42441. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Zhang, L.; Zhu, G.W.; Chen, W.M. Spectral absorption and fluorescence of chromophoric dissolved organic matter in shallow lakes in the middle and lower reaches of the Yangtze River. J. Freshw. Ecol. 2005, 20, 451–459. [Google Scholar] [CrossRef][Green Version]
- Yan, C.X.; Yang, Y.; Zhou, J.L.; Liu, M.; Nie, M.H.; Shi, H.; Gu, L.J. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.; Hynds, P.; Flynn, R. An overview of dissolved organic carbon in groundwater and implications for drinking water safety. Hydrogeol. J. 2017, 25, 959–967. [Google Scholar] [CrossRef]
- Marais, S.S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. Comparison of natural organic matter removal by ultrafiltration, granular activated carbon filtration and full scale conventional water treatment. J. Environ. Chem. Eng. 2018, 6, 6282–6289. [Google Scholar] [CrossRef]
- Brooks, E.; Freeman, C.; Gough, R.; Holliman, P.J. Tracing dissolved organic carbon and trihalomethane formation potential between source water and finished drinking water at a lowland and an upland UK catchment. Sci. Total Environ. 2015, 537, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Knocke, W.R.; West, S.; Hoehn, R.C. Effects of low temperature on the removal of trihalomethane precursors by coagulation. J.-Am. Water Work. Assoc. 1986, 78, 189–195. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Ni, J.; Qu, J.; Ni, W.; Van Leeuwen, J. Natural organic matter (NOM) removal in a typical North-China water plant by enhanced coagulation: Targets and techniques. Sep. Purif. Technol. 2009, 68, 320–327. [Google Scholar] [CrossRef]
- Haberkamp, J.; Ruhl, A.S.; Ernst, M.; Jekel, M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration. Water Res. 2007, 41, 3794–3802. [Google Scholar] [CrossRef]
- Domany, Z.; Galambos, I.; Vatai, G.; Bekassy-Molnar, E. Humic substances removal from drinking water by membrane filtration. Desalination 2002, 145, 333–337. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, B.; Sun, M.; Cho, J. Characterizations of NOM included in NF and UF membrane permeates. Desalination 2005, 173, 131–142. [Google Scholar] [CrossRef]
- Ates, N.; Yilmaz, L.; Kitis, M.; Yetis, U. Removal of disinfection by-product precursors by UF and NF membranes in low-SUVA waters. J. Membr. Sci. 2009, 328, 104–112. [Google Scholar] [CrossRef]
- Lafi, R.; Gzara, L.; Lajimi, R.H.; Hafiane, A. Treatment of textile wastewater by a hybrid ultrafiltration/electrodialysis process. Chem. Eng. Process.—Process Intensif. 2018, 132, 105–113. [Google Scholar] [CrossRef]
- Fane, A.G.; Fell, C.J.D.; Waters, A.G. The relationship between membrane surface pore characteristics and flux for ultrafiltration membranes. J. Membr. Sci. 1981, 9, 245–262. [Google Scholar] [CrossRef]
- Choi, K.Y.J.; Dempsey, B.A. Bench-scale evaluation of critical flux and TMP in low-pressure membrane filtration. J.-Am. Water Work. Assoc. 2005, 97, 134–143. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Servais, P.; Billen, G.; Laurent, P.; Levi, Y.; Randon, G. Studies of BDOC and bacterial dynamics in the drinking water distribution system of the Northern Parisian suburbs. Rev. Des Sci. L’eau/J. Water Sci. 1992, 5, 69–89. [Google Scholar] [CrossRef]
- Sayed Razavi, S.K.; Harris, J.L.; Sherkat, F. Fouling and cleaning of membranes in the ultrafiltration of the aqueous extract of soy flour. J. Membr. Sci. 1996, 114, 93–104. [Google Scholar] [CrossRef]
- Lee, H.; Amy, G.; Cho, J.; Yoon, Y.; Moon, S.-H.; Kim, I.S. Cleaning strategies for flux recovery of an ultrafiltration membrane fouled by natural organic matter. Water Res. 2001, 35, 3301–3308. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef]
- Mosqueda-Jimenez, D.B.; Huck, P.M.; Basu, O.D. Fouling characteristics of an ultrafiltration membrane used in drinking water treatment. Desalination 2008, 230, 79–91. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Shen, S.; Zhang, F.; Zhang, C.; Xiong, J. Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control. Polymers 2022, 14, 4689. https://doi.org/10.3390/polym14214689
Chen M, Shen S, Zhang F, Zhang C, Xiong J. Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control. Polymers. 2022; 14(21):4689. https://doi.org/10.3390/polym14214689
Chicago/Turabian StyleChen, Ming, Shuhuai Shen, Fan Zhang, Cong Zhang, and Jianglei Xiong. 2022. "Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control" Polymers 14, no. 21: 4689. https://doi.org/10.3390/polym14214689
APA StyleChen, M., Shen, S., Zhang, F., Zhang, C., & Xiong, J. (2022). Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control. Polymers, 14(21), 4689. https://doi.org/10.3390/polym14214689






