Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
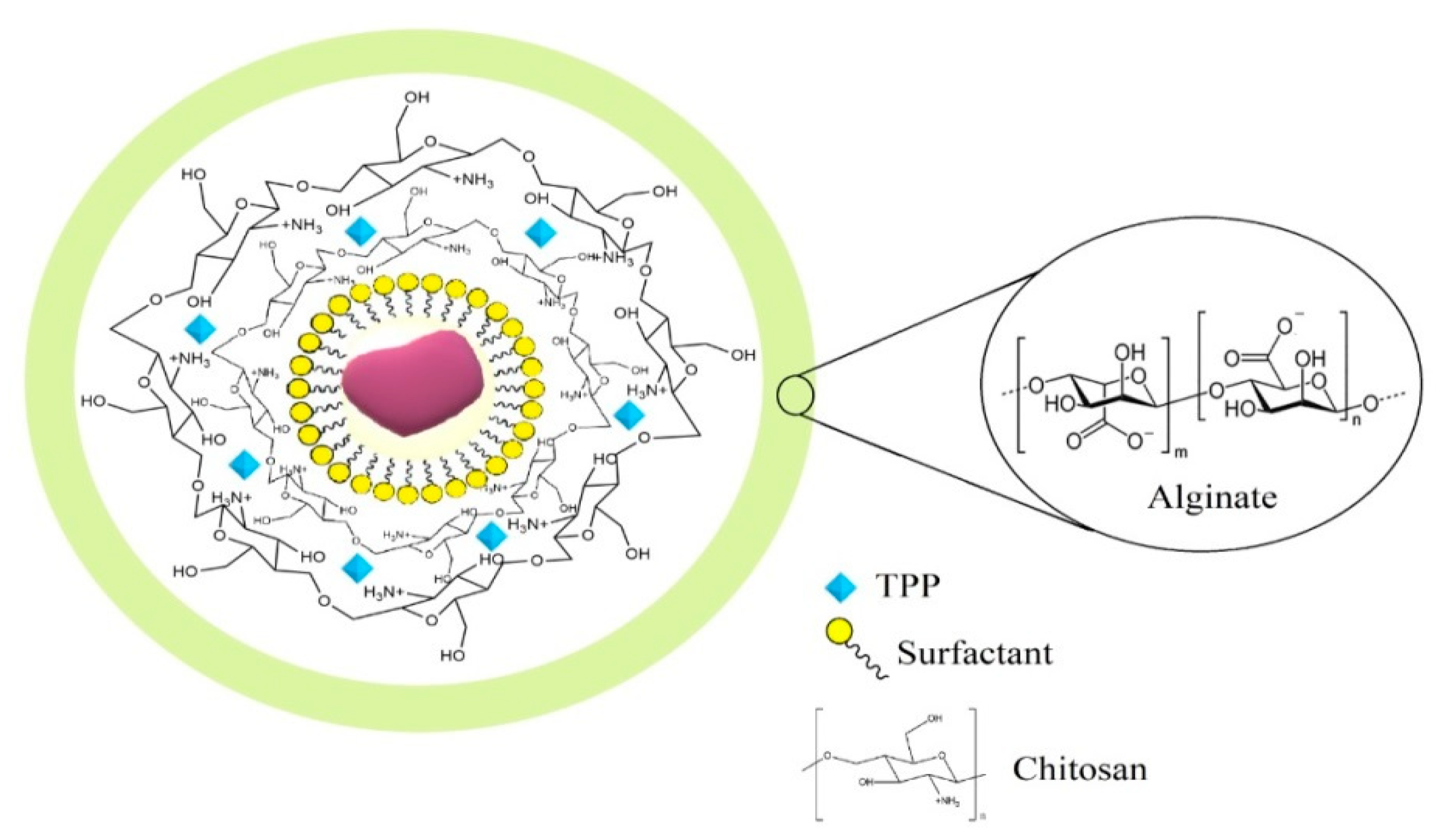
2.2. Nanoparticles Synthesis
2.3. Characterization of Cardanol-Loaded NPs
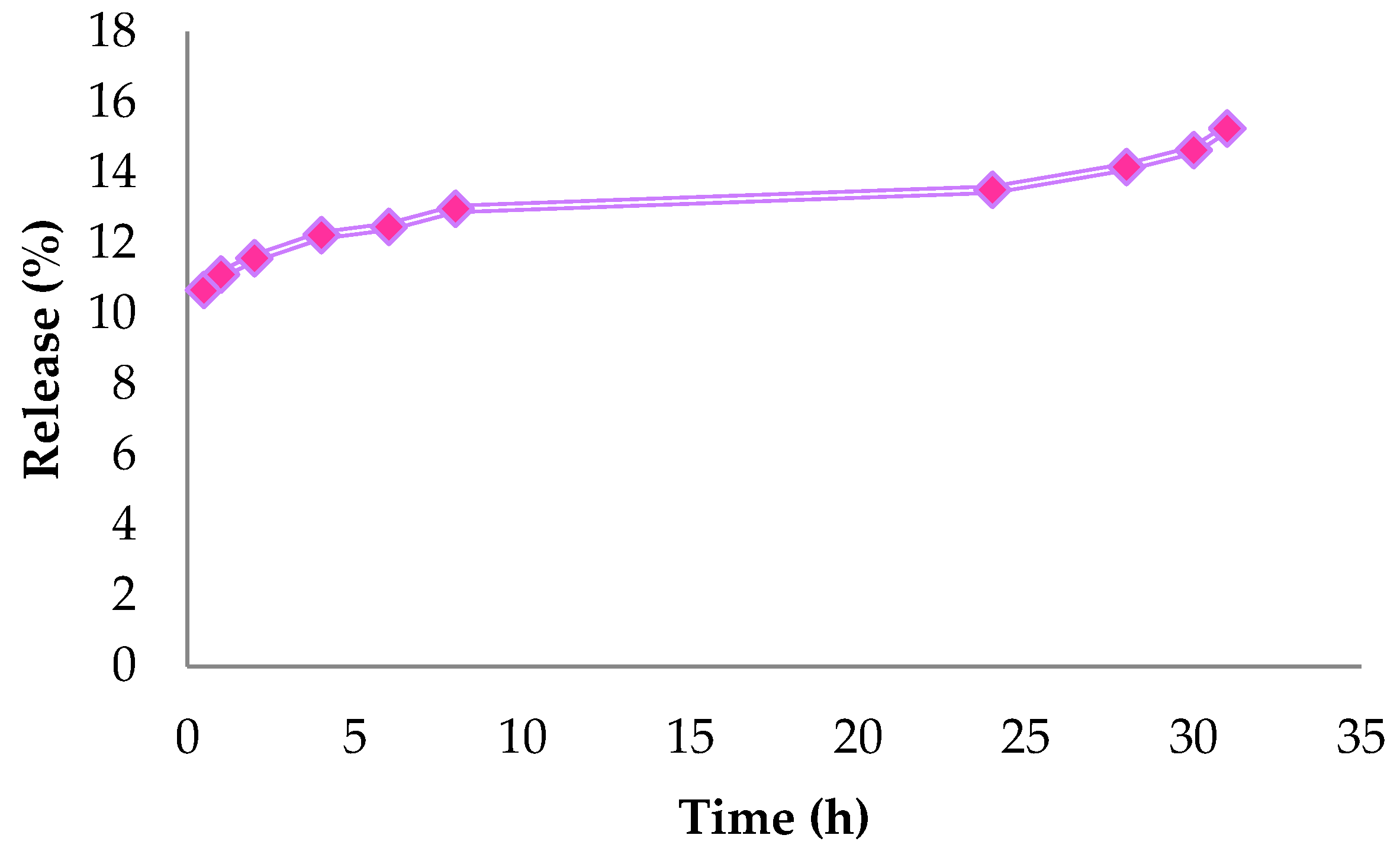
2.4. In Vitro Release
2.5. Determination of Antioxidant Activity
3. Results and Discussion
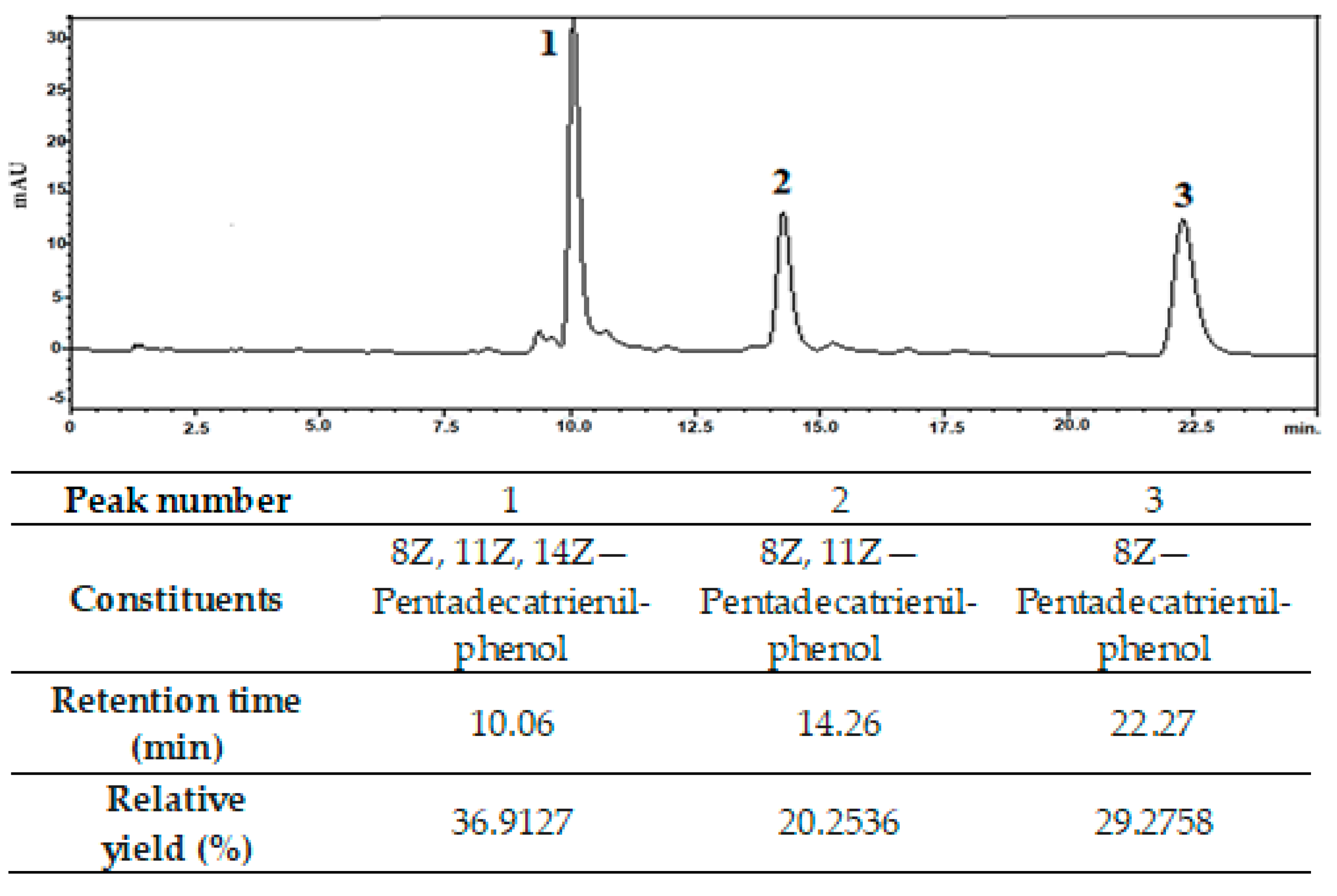
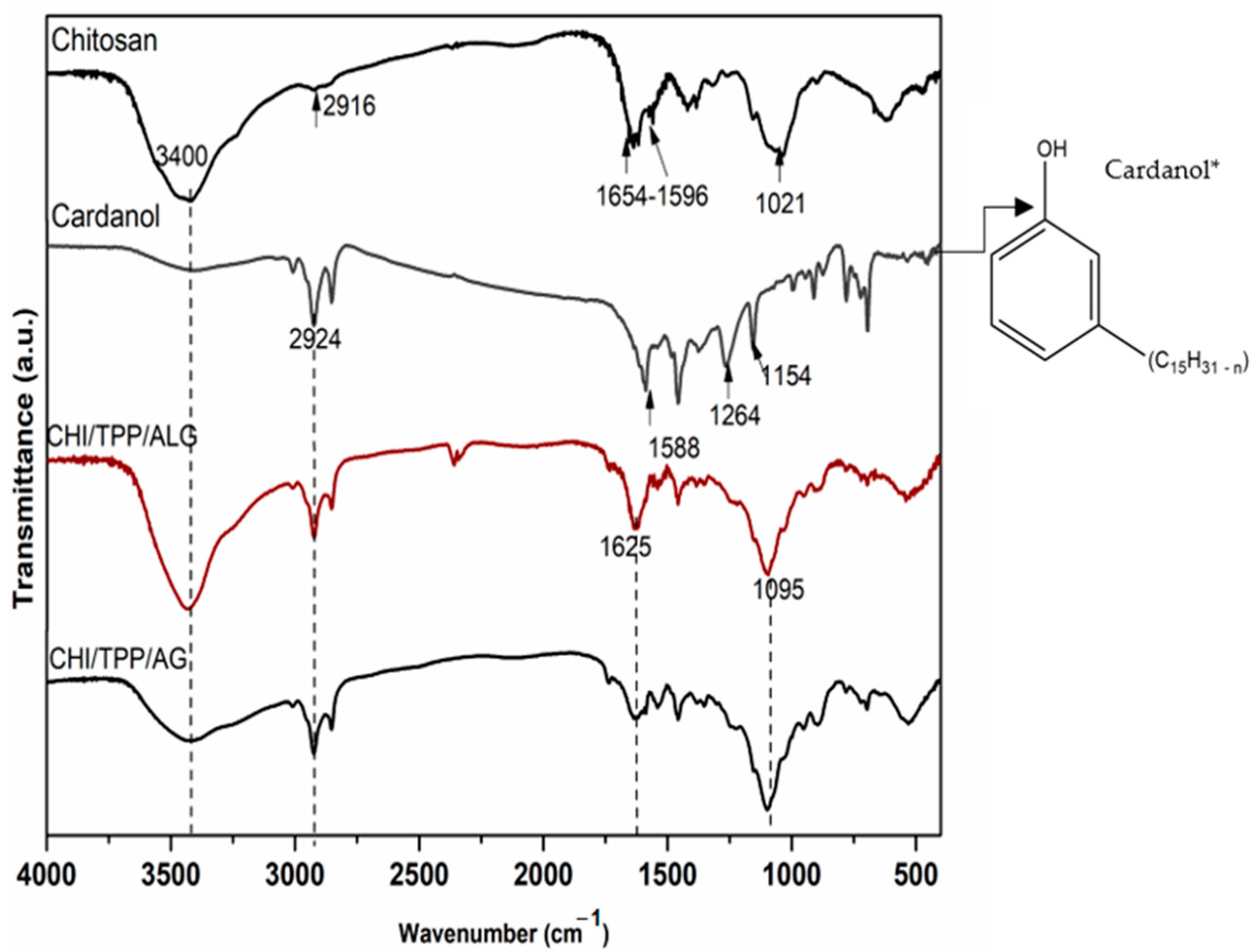
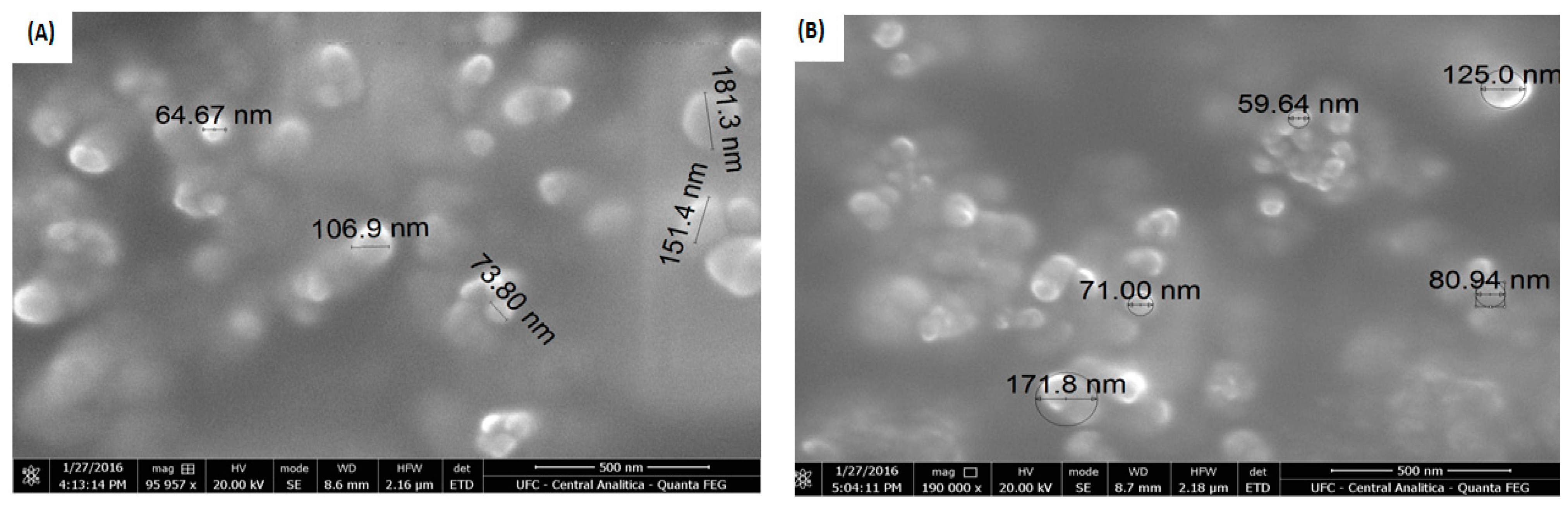
3.1. Structural and Morphological Characterization of NPs and Cardanol
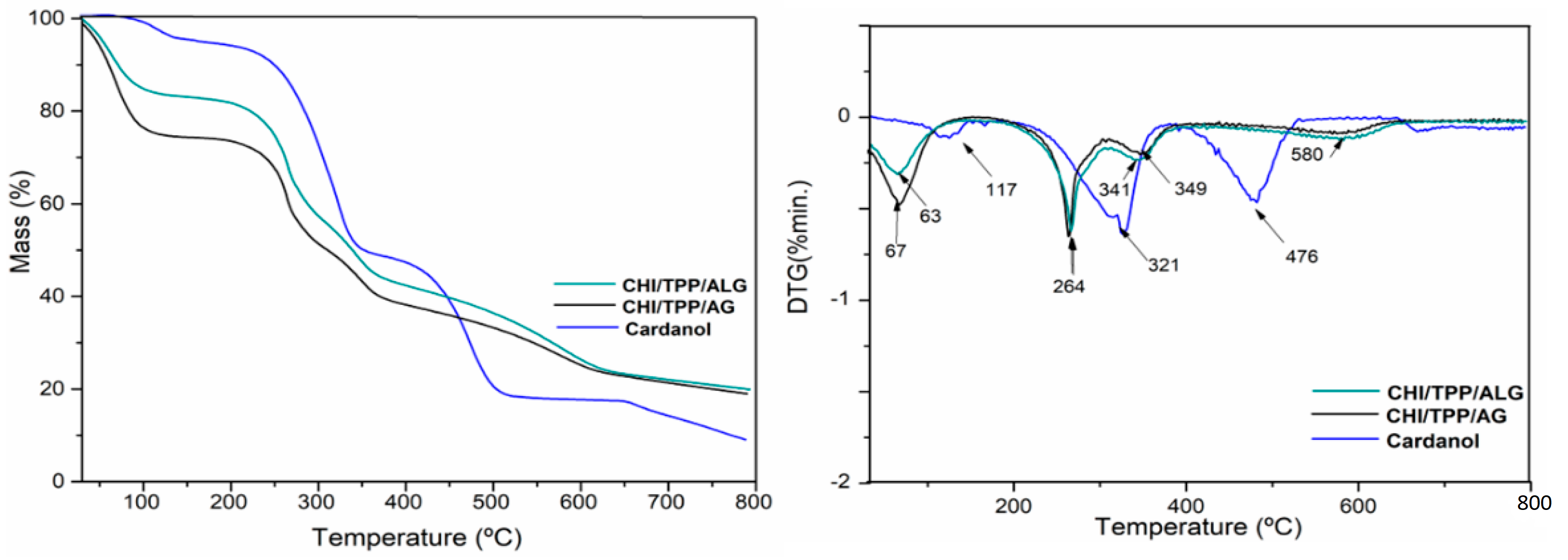
3.2. Thermal Analysis
3.3. Release Curve
3.4. Evaluation of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maia, F.J.N.; Ribeiro, F.W.P.; Rangel, J.H.G.; Lomonaco, D.; Luna, F.M.T.; de Lima-Neto, P.; Correia, A.N.; Mazzetto, S.E. Evaluation of antioxidant action by electrochemical and accelerated oxidation experiments of phenolic compounds derived from cashew nut shell liquid. Ind. Crops Prod. 2015, 67, 281–286. [Google Scholar] [CrossRef]
- Paula, R.S.F.; Vieira, R.S.; Luna, F.M.T.; Cavalcante, C.L.; Figueredo, I.M.; Candido, J.R.; Silva, L.P.; Marinho, E.S.; de Lima-Neto, P.; Lomonaco, D.; et al. A potential bio-antioxidant for mineral oil from cashew nutshell liquid: An experimental and theoretical approach. Braz. J. Chem. Eng. 2020, 37, 369–381. [Google Scholar] [CrossRef]
- Trevisan, M.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006, 44, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Song, F.; Li, Q.; Xia, H.; Li, M.; Shu, X.; Zhou, Y. Recent development of cardanol based polymer materials-a review. J. Renew. Mater. 2019, 7, 601–619. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.; Williams, J.A.; Kučerka, N.; Atkinson, J.; Wassall, S.R.; Katsaras, J.; Harroun, T.A. Tocopherol activity correlates with its location in a membrane: A new perspective on the antioxidant vitamine. J. Am. Chem. Soc. 2013, 135, 7523–7533. [Google Scholar] [CrossRef]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Rios, M.A.S.; Santos, F.F.P.; Maia, F.J.N.; Mazzetto, S.E. Evaluation of antioxidants on the thermo-oxidative stability of soybean biodiesel. J. Therm. Anal. Calorim. 2013, 112, 921–927. [Google Scholar] [CrossRef]
- Lopes, A.A.S.; Carneiro, E.A.; Rios, M.A.S.; Filho, J.J.H.; Carioca, J.O.B.; Barros, G.G.; Mazzetto, S.E. Study of antioxidant property of a thiophosphorated compound derived from cashew nut shell liquid in hydrogenated naphthenics oils. Braz. J. Chem. Eng. 2008, 25, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Kleinberg, M.N.; Rios, M.A.S.; Buarque, H.L.B.; Parente, M.M.V.; Cavalcante, C.L.; Luna, F.M.T. Influence of Synthetic and Natural Antioxidants on the Oxidation Stability of Beef Tallow Before Biodiesel Production. Waste Biomass Valorization 2019, 10, 797–803. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; Moreira, K.d.S.; de Oliveira, A.L.B.; Mota, G.F.; Souza, J.E.D.S.; Falcão, I.R.D.A.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase a from Candida antarctica onto Chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 64018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, F.O.M.D.S.; Silva, N.; Sipauba, M.D.S.; Pires, T.F.M.; Bomfim, T.A.; Junior, O.A.D.C.M.; Forte, M.M.D.C. Chitosan and Arabic gum nanoparticles for heavy metal adsorption. Polimeros 2018, 28, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Clemente-Napimoga, J.T.; Moreira, J.A.; Grillo, R.; de Melo, N.F.; Fraceto, L.F.; Napimoga, M.H. 15d-PGJ2-loaded in nanocapsules enhance the antinociceptive properties into rat temporomandibular hypernociception. Life Sci. 2012, 90, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Abreu, F.O.M.; Cavalcante, L.G.; Doudement, P.V.; Castro, A.M.; Nascimento, A.P.; Matos, J.E.X. Development of new method to obtain chitosan from the exoskeleton of Crabs using microwave radiation | Properties and Characteristics of Chitosan Obtained from the Uçá Crab Exoskeleton Using Microwave Radiation. Polimeros 2013, 23, 630–635. [Google Scholar]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) Nut shell liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef]
- Valério, R.B.R.; Silva, N.; Junior, J.R.P.; Ferreira, V.M.S.; Chaves, A.V.; Forte, M.M.D.C.; Morais, S.M.; Abreu, F.O.M.D.S. Influence of reaction conditions on the production of nanoparticles on the degree of encapsulation of Cardanol. Braz. J. Dev. 2020, 6, 52770–52786. [Google Scholar] [CrossRef]
- Yepez, B.; Espinosa, M.; López, S.; Bolaños, G. Producing antioxidant fractions from herbaceous matrices by supercritical fluid extraction. Fluid Phase Equilib. 2002, 194, 879–884. [Google Scholar] [CrossRef]
- Tyman, J.H.; Kiong, L.S. Long chain phenols: Part XI. Composition of natural cashew nutshell liquid (Anacardium occidentale) from various sources. Lipids 1978, 13, 525. Available online: http://link.springer.com/article/10.1007/BF02533591 (accessed on 2 September 2022). [CrossRef] [PubMed]
- Kumar, P.P.; Paramashivappa, R.; Vithayathil, P.J.; Rao, P.V.S.; Rao, A.S. Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) Nut shell liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, D.; Santiago, G.M.P.; Ferreira, Y.S.; Arriaga, M.C.; Mazzetto, S.E.; Mele, G.; Vasapollo, G. Study of technical CNSL and its main components as new green larvicides. Green Chem. 2009, 11, 31–33. [Google Scholar] [CrossRef]
- Attanasi, O.A.; Filippone, P. Cardanolo: Una Preziosa Materia Prima Rinnovabile. 2003, pp. 11–12. Available online: https://ora.uniurb.it/handle/11576/1887057 (accessed on 4 July 2022).
- Neto, C.; Dantas, T.; Fonseca, J.; Pereira, M. Permeability studies in chitosan membranes. Effects of crosslinking and poly(ethylene oxide) addition. Carbohydr. Res. 2005, 340, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Pitombeira, N.A.; Neto, J.G.V.; Silva, D.A.; Feitosa, J.P.; Paula, H.C.; de Paula, R.C. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and Arabic gum polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Filho, J.C.P.; De Morais, S.M.; Sobrinho, A.C.N.; Cavalcante, G.S.; Silva, N.; Abreu, F. Design of chitosan-alginate core-shell nanoparticules loaded with anacardic acid and cardol for drug delivery. Polimeros 2019, 29, e2019060. [Google Scholar] [CrossRef]
- Dantas, F.M.L. Production Process of Nanoparticles Containing Active Substances and Their Pharmaceutical Compositions. BR n. PI 0802233-0. 17 July 2012. Available online: https://www.innocorepharma.com/nl/Technologies/Dosage%20forms/Nanospheres?gclid=EAIaIQobChMIy6Ta7Ov_-gIVAvd3Ch3UrQiCEAAYASAAEgIcAPD_BwE (accessed on 4 July 2022).
- Zheng, H.; Du, Y.; Yu, J.; Huang, R.; Zhang, L. Preparation and characterization of chitosan/poly(vinyl alcohol) blend fibers. J. Appl. Polym. Sci. 2001, 80, 2558–2565. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Khan, S.; Masood, S.; Siddiqui, K.; Alam, M.; Zafar, F.; Haque, Q.M.R.; Nishat, N. Utilization of renewable waste material for the sustainable development of thermally stable and biologically active aliphatic amine modified Cardanol (phenolic lipid)—Formaldehyde free standing films. J. Clean. Prod. 2018, 196, 1644–1656. [Google Scholar] [CrossRef]
- Rao, B.; Palanisamy, A. Synthesis of bio based low temperature curable liquid epoxy, benzoxazine monomer system from cardanol: Thermal and viscoelastic properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Aydın, H.; Yerlikaya, Ç.; Uzan, S. Equilibrium and kinetic studies of copper (II) ion uptake by modified wheat shells. Desalin. Water Treat. 2012, 44, 296–305. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Karim, M.R. Fabrication and characterization of poly(vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, M.D.P. Invertase stability in alginate beads. Effect of trehalose and chitosan inclusion and of drying methods. Food Res. Int. 2012, 47, 321–330. [Google Scholar] [CrossRef]
- Paula, H.C.; Sombra, F.M.; Cavalcante, R.D.F.; Abreu, F.O.; de Paula, R.C. Preparation and characterization of chitosan/cashew gum beads loaded with Lippia sidoides essential oil. Mater. Sci. Eng. C 2011, 31, 173–178. [Google Scholar] [CrossRef]
- Liu, M.; Meng, Q.; Niu, C.; Wang, Y.; Zhou, G.; Xu, C.; Liu, Y. Preparation and characterization of modified dual network dust suppression gel based on sodium alginate and soluble starch. Environ. Sci. Pollut. Res. 2022, 29, 69771–69784. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- André, W.; Junior, J.P.; Cavalcante, G.; Ribeiro, W.; Filho, J.A.; Cavalcanti, B.; De Morais, S.; De Oliveira, L.; Bevilaqua, C.; Abreu, F. Chitosan Nanoparticles Loaded with Carvacrol and Carvacryl Acetate for Improved Anthelmintic Activity. J. Braz. Chem. Soc. 2020, 31, 1614–1622. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mater. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Almeida Rodrigues, F.H. Ação Antioxidante de Derivados do Líquido da Castanha de Caju (LCC) Sobre a Degradação Termooxidativa do Poli (1,4-Cis-isopreno); Universidade Federal do Ceará: Fortaleza, Brazil, 2006. [Google Scholar]
- De Souza, J.R. Estudo da Desacetilação da Quitosana e Obtenção de Suas Nanopartículas para Aplicação em Engenharia de Tecidos, Diss.—Univ. São Paulo. 2017. Available online: http://www.teses.usp.br/teses/disponiveis/3/3137/tde-24102017-113542/ (accessed on 4 July 2022).
- Riosfacanha, M.; Mazzetto, S.; Beserracarioca, J.; Debarros, G. Evaluation of antioxidant properties of a phosphorated cardanol compound on mineral oils (NH10 and NH20). Fuel 2007, 86, 2416–2421. [Google Scholar] [CrossRef]
- Cardoso, J.; Ricci-Júnior, E.; Gentili, D.; Spinelli, L.; Lucas, E. Influence of Cardanol Encapsulated on the Properties of Poly(Lactic Acid) Microparticles. Quím. Nova 2018, 41, 273–283. [Google Scholar] [CrossRef]
- Luiza Horst, B. Universidade Federal De Santa Catarina, Programa de Pós-Graduação em Química, Microencapsulação do Corante Natural Antocianina em Matriz Polimérica de Quitosana e Quitosana/Alginato Através das Técnicas de Impregnação, Coacervação e Spray Drying. Master’s Thesis, Universidade Federal de Santa Catarina, Centro de Ciências Físicas e Matemáticas, Florianópolis, Brazil, 2009. [Google Scholar]
- Dalponte, I.; Mathias, L.; Jorge, R.M.M.; Weinschutz, R. Photocatalytic degradation of tartrazine with TiO2 immobilized on alginate beads. Quim. Nova 2016, 39, 1165–1169. [Google Scholar] [CrossRef]
- Maciel, Á.D.N. Licenciatura Em Ciências Naturais, Influência do íon Ca2+ no Desenvolvimento de Micropartículas de Alginato de Sódio; Faculdade Unb Planaltina: Brasília, Brazil, 2013. [Google Scholar]
- Rahman, M.S. Encapsulation, Stabilization, and Controlled Release of Food Ingredients and Bioactives; T.F. Group: Deerfield Beach, FL, USA, 2007; pp. 509–568. [Google Scholar]
- Rasool, B.K.A.; Fahmy, S.A.; Galeel, O.W.A. Impact of Chitosan as a disintegrant on the bioavailability of furosemide tablets: In vitro evaluation and in vivo simulation of novel formulations. Pak. J. Pharm. Sci. 2012, 25, 815–822. [Google Scholar]
| Particle Size (nm) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|
| NPs | PEAK 1 | PEAK 2 | |||
| CHI/TPP/ALG | 171.1 | 23.1% | 34.9 | 76.9% | −39.7 ± 4.9 |
| CHI/TPP/AG | 312.2 | 52.2% | 70.5 | 47.8% | −29.8 ± 4.0 |
| Code NPs | Temperature Intervals (°C) | T(Max) (°C) | Weight Loss (%) | Residue at 700 °C |
|---|---|---|---|---|
| Cardanol | 30–150 | 117.0 | 5.4 | 8.6 |
| 150–375 | 321.3 | 46.8 | ||
| 375–800 | 475.8 | 39.8 | ||
| CHI/TPP/ALG | 30–150 | 62.7 | 17.6 | 19.2 |
| 150–307 | 264.3 | 27 | ||
| 307–450 | 341.0 | 16.5 | ||
| 450–800 | 580.0 | 19.7 | ||
| CHI/TPP/AG | 30–150 | 66.7 | 25.6 | 19.0 |
| 150–307 | 263.0 | 24 | ||
| 307–450 | 349.0 | 14.6 | ||
| 450–800 | 580 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valério, R.B.R.; da Silva, N.A.; Junior, J.R.P.; Chaves, A.V.; de Oliveira, B.P.; Souza, N.F.; de Morais, S.M.; Santos, J.C.S.d.; Abreu, F.O.M.d.S. Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System. Polymers 2022, 14, 4695. https://doi.org/10.3390/polym14214695
Valério RBR, da Silva NA, Junior JRP, Chaves AV, de Oliveira BP, Souza NF, de Morais SM, Santos JCSd, Abreu FOMdS. Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System. Polymers. 2022; 14(21):4695. https://doi.org/10.3390/polym14214695
Chicago/Turabian StyleValério, Roberta Bussons Rodrigues, Nilvan Alves da Silva, José Ribamar Paiva Junior, Anderson Valério Chaves, Bruno Peixoto de Oliveira, Nágila Freitas Souza, Selene Maia de Morais, José Cleiton Sousa dos Santos, and Flávia Oliveira Monteiro da Silva Abreu. 2022. "Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System" Polymers 14, no. 21: 4695. https://doi.org/10.3390/polym14214695
APA StyleValério, R. B. R., da Silva, N. A., Junior, J. R. P., Chaves, A. V., de Oliveira, B. P., Souza, N. F., de Morais, S. M., Santos, J. C. S. d., & Abreu, F. O. M. d. S. (2022). Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System. Polymers, 14(21), 4695. https://doi.org/10.3390/polym14214695







