A Micro-Nano Structure Formed by SiC/Graphene Oxide Self-Assembly Improves the Wear Resistance and Corrosion Resistance of an Epoxy-Based Composite Coating
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials and Sample Preparation
2.1.1. Materials
2.1.2. Preparation of SiC-GO Composite Filler
2.1.3. Preparation of the Coating
2.2. Sample Characterization
2.3. Electrochemical Characterization and Long-Term Performance of the New Composite Coatings
2.3.1. EIS Test and Accelerated Durability Test
2.3.2. Evaluation of Corrosion Resistance in Short/Long Runs
2.4. Evaluation of Wear Behavior and Abrasion Resistance
3. Results and Discussion
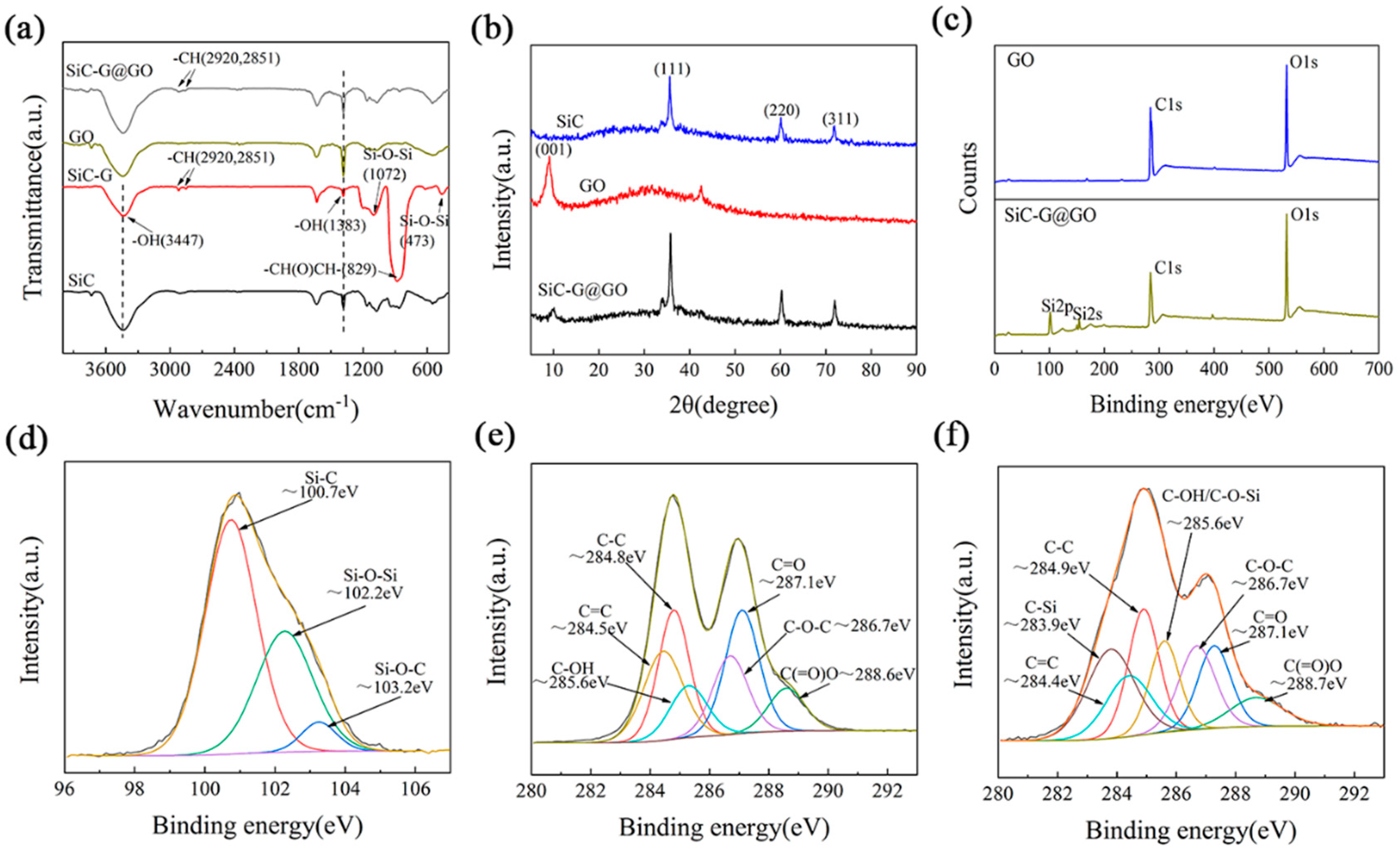
3.1. Characterization of SiC-G@GO
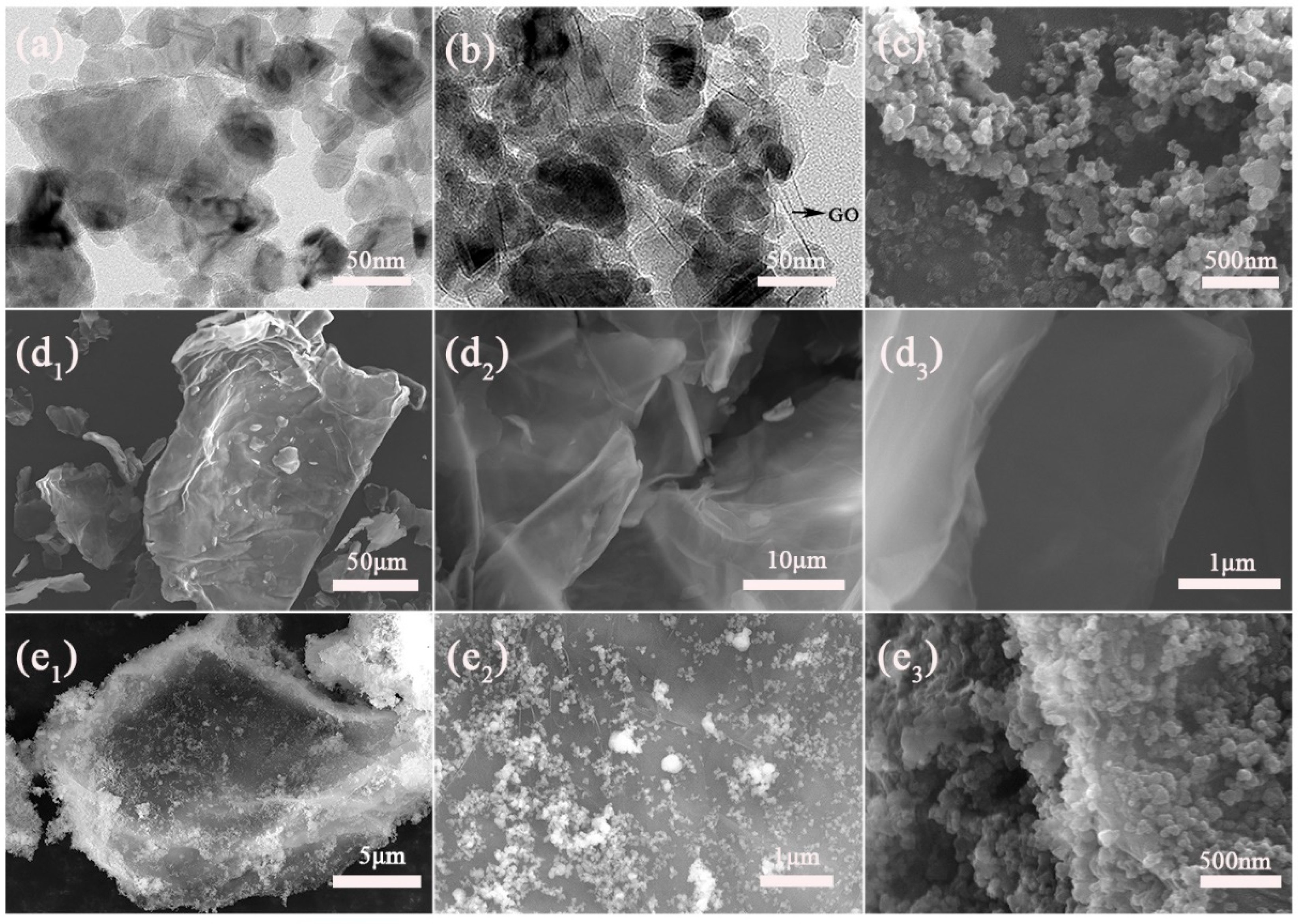
3.2. FE-SEM and TEM Analyses
3.3. Dispersion Experiment
3.4. Cure Kinetic Analysis
3.5. Anti-Corrosion Performance of Composite Coatings
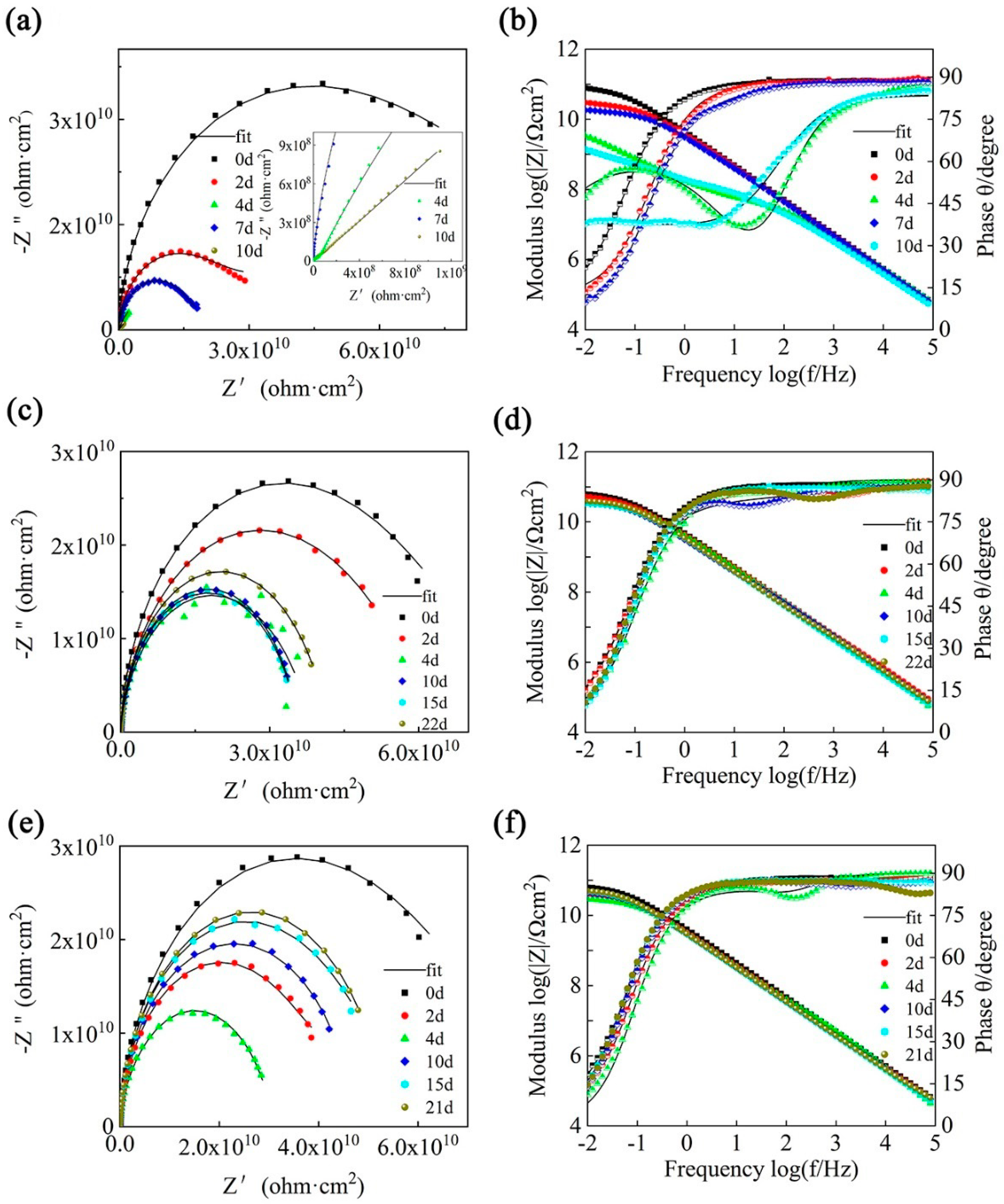
3.5.1. EIS Measurement of Composite Coatings
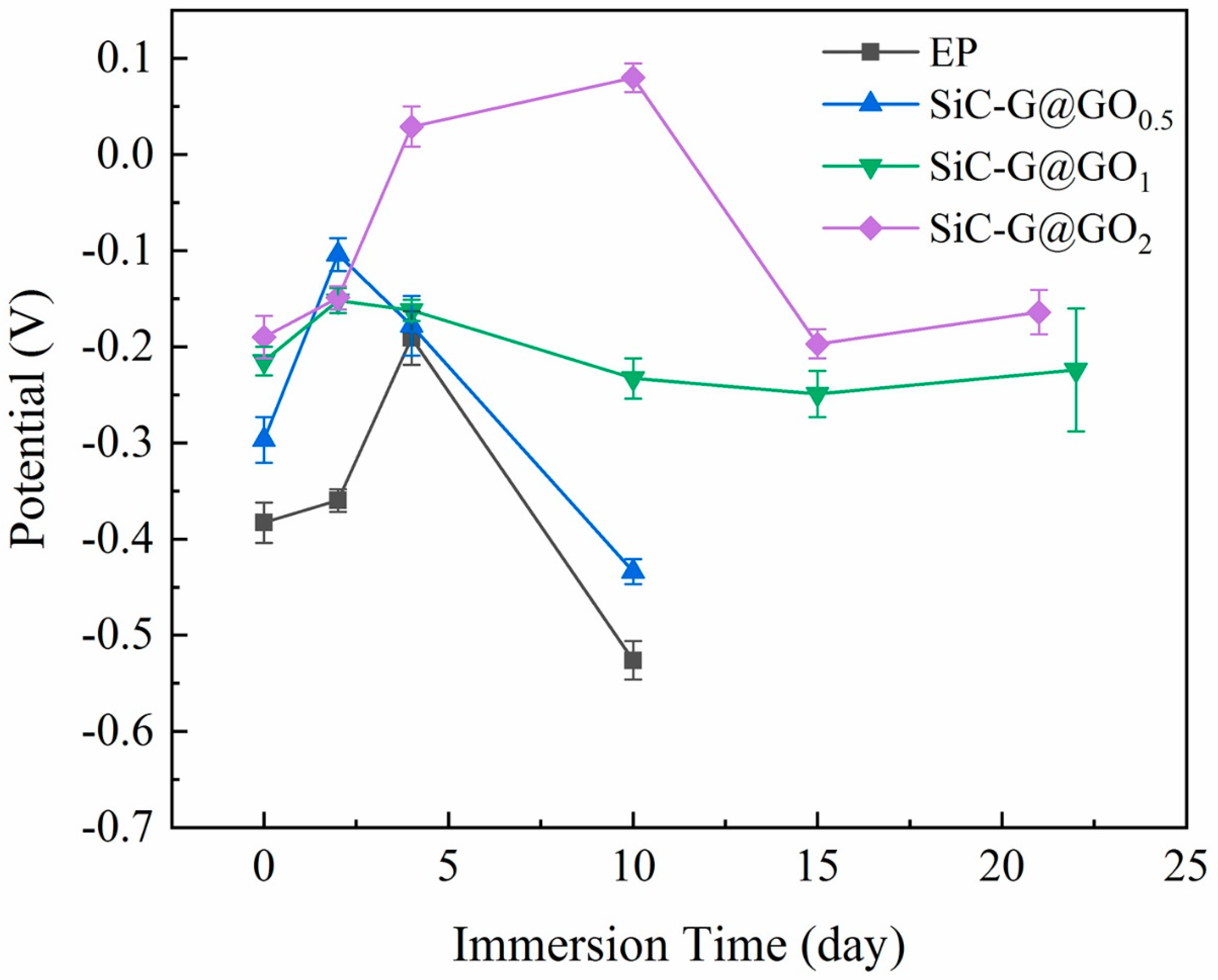
3.5.2. Open Circuit Potential Analysis
3.5.3. Potentiodynamic Polarization Measurements
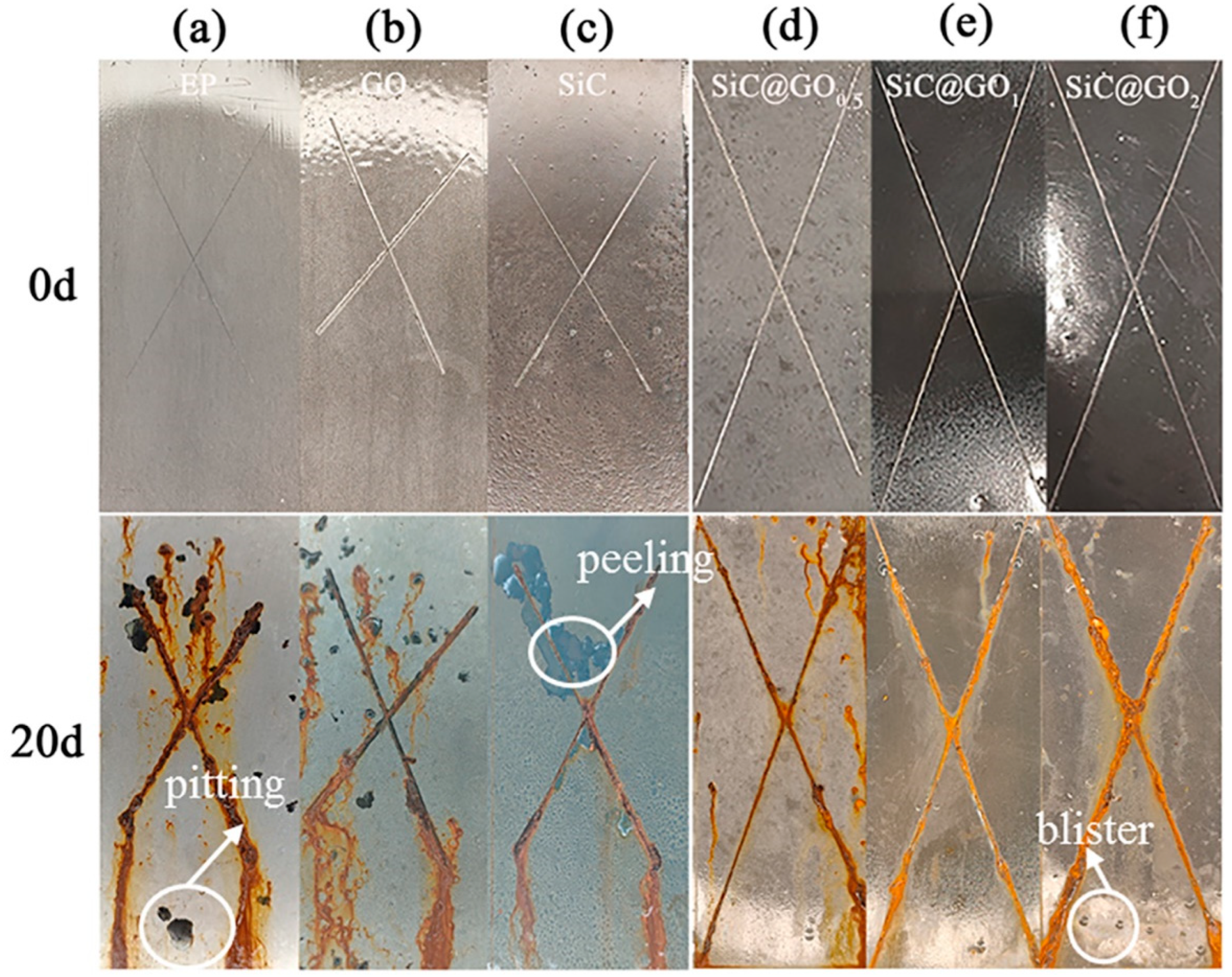
3.6. Analysis of the Neutral Salt Spray Test
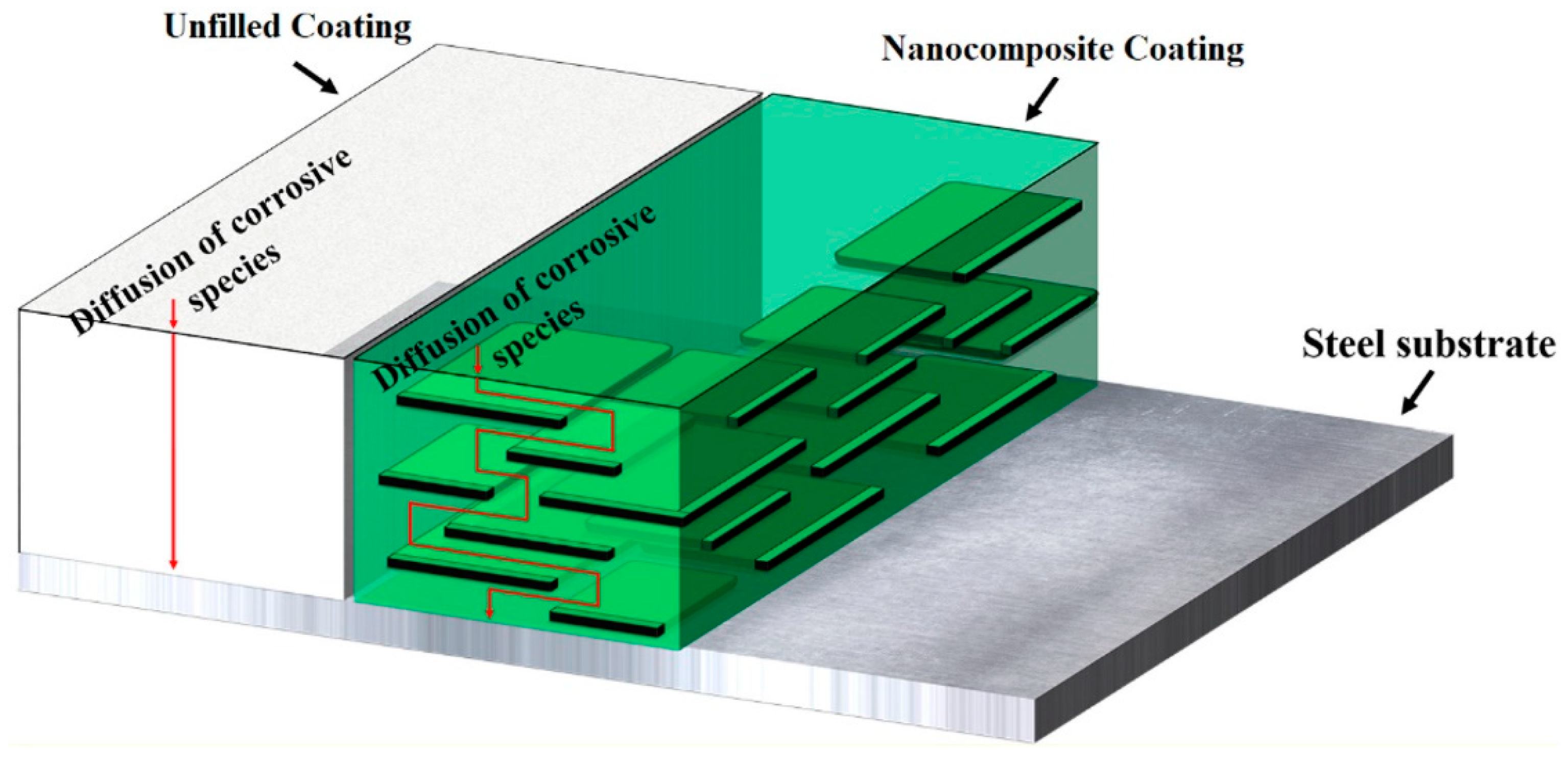
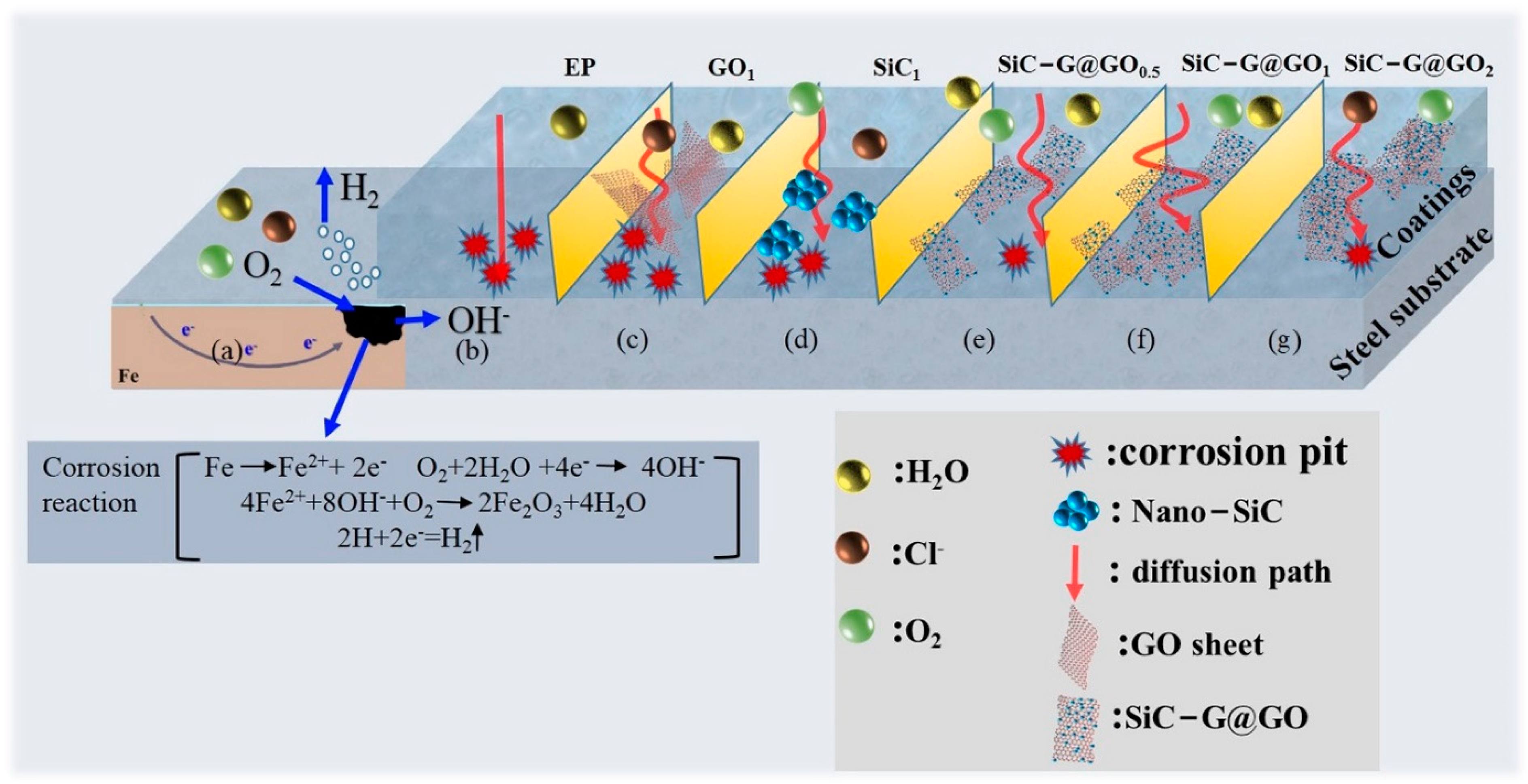
3.7. Schematic Diagram of the Mechanism
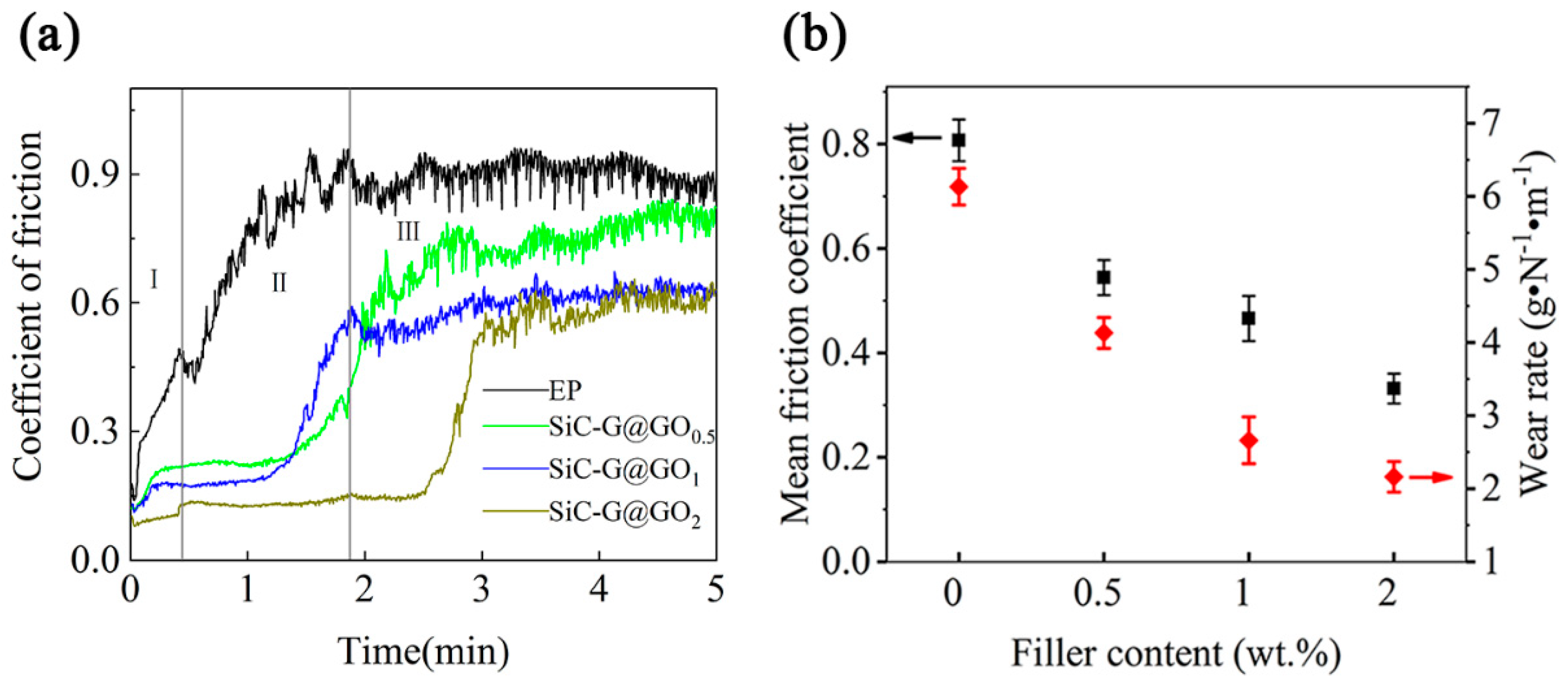
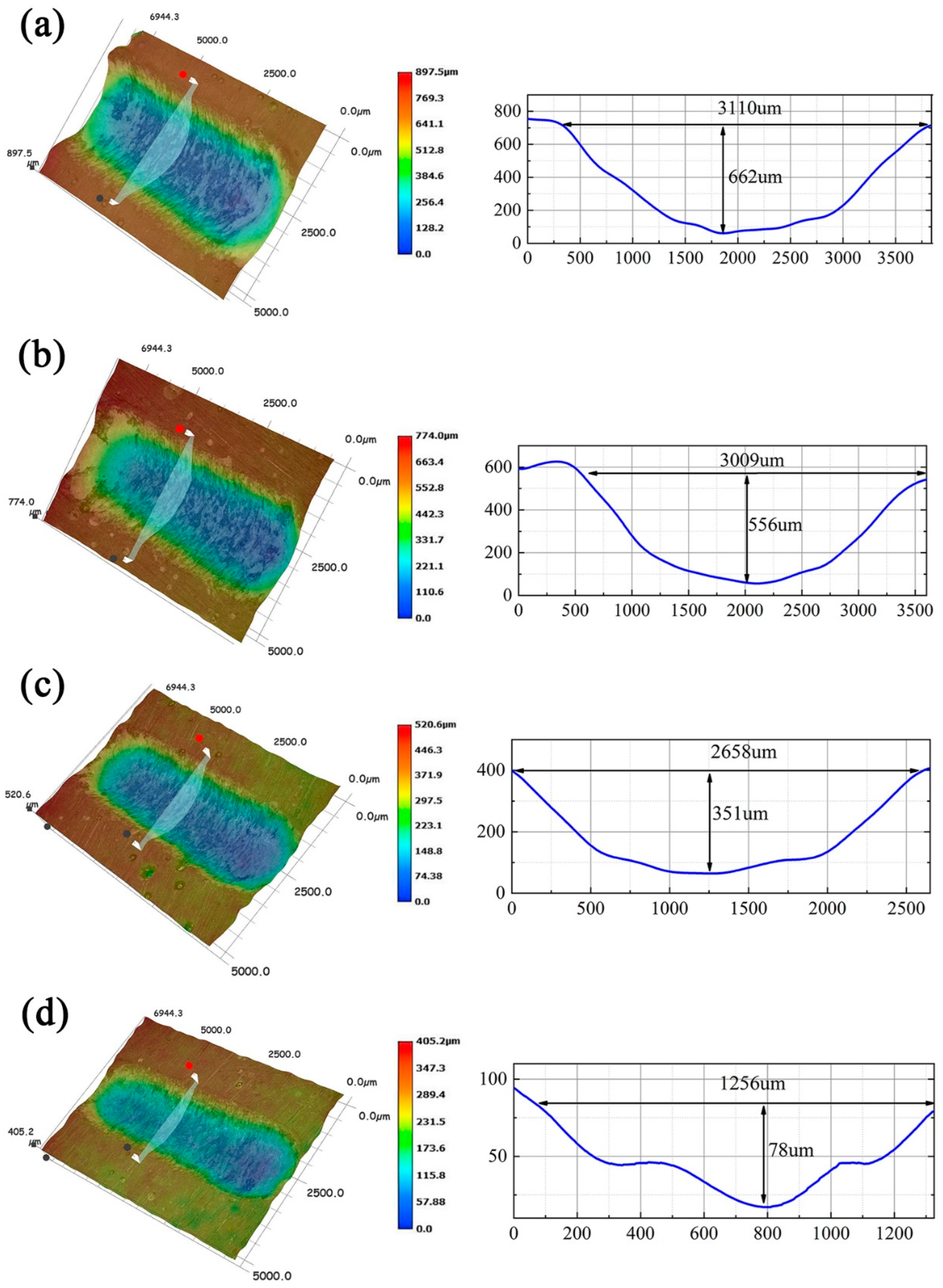
3.8. Tribological Properties of the Composite Coatings
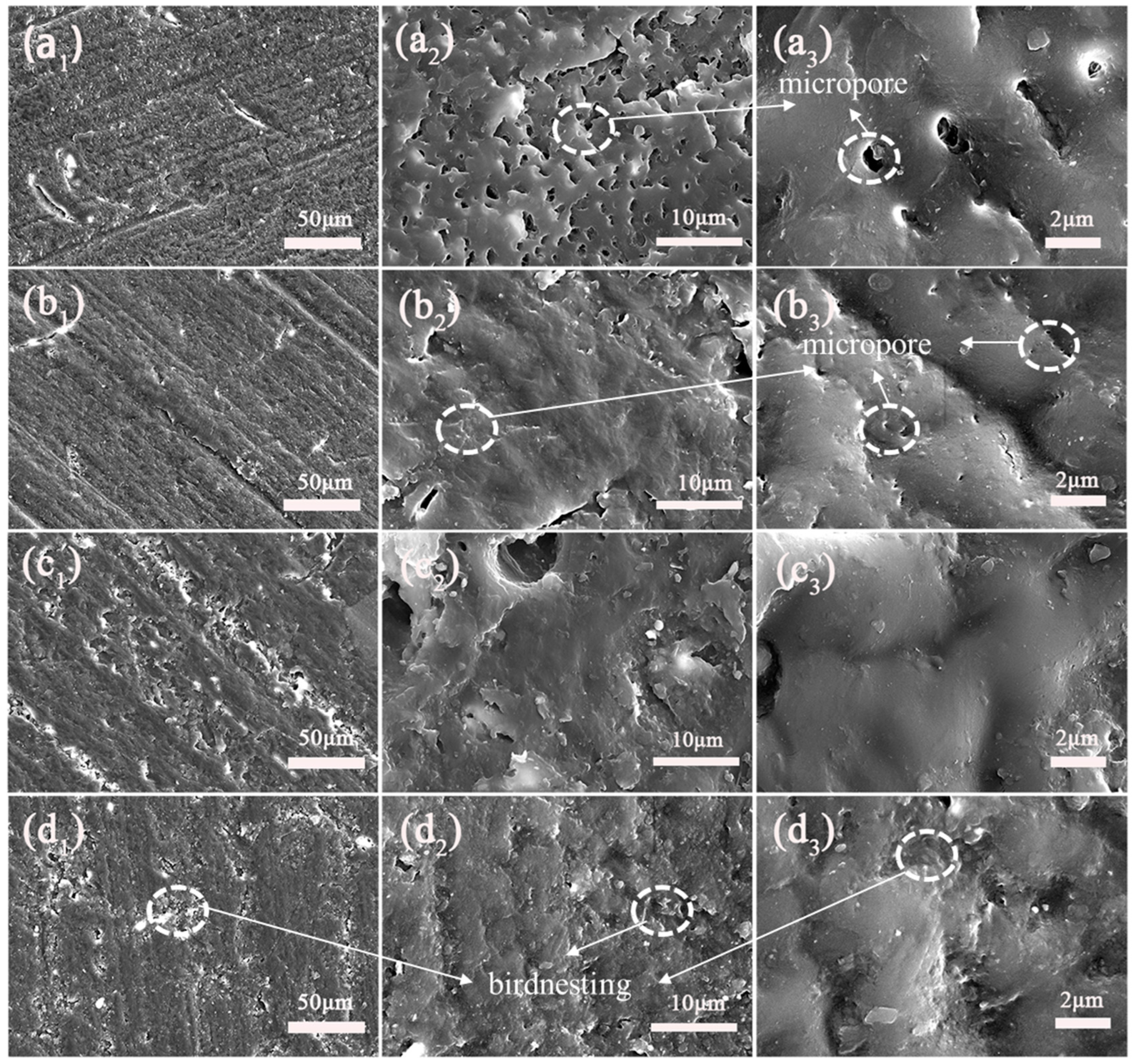
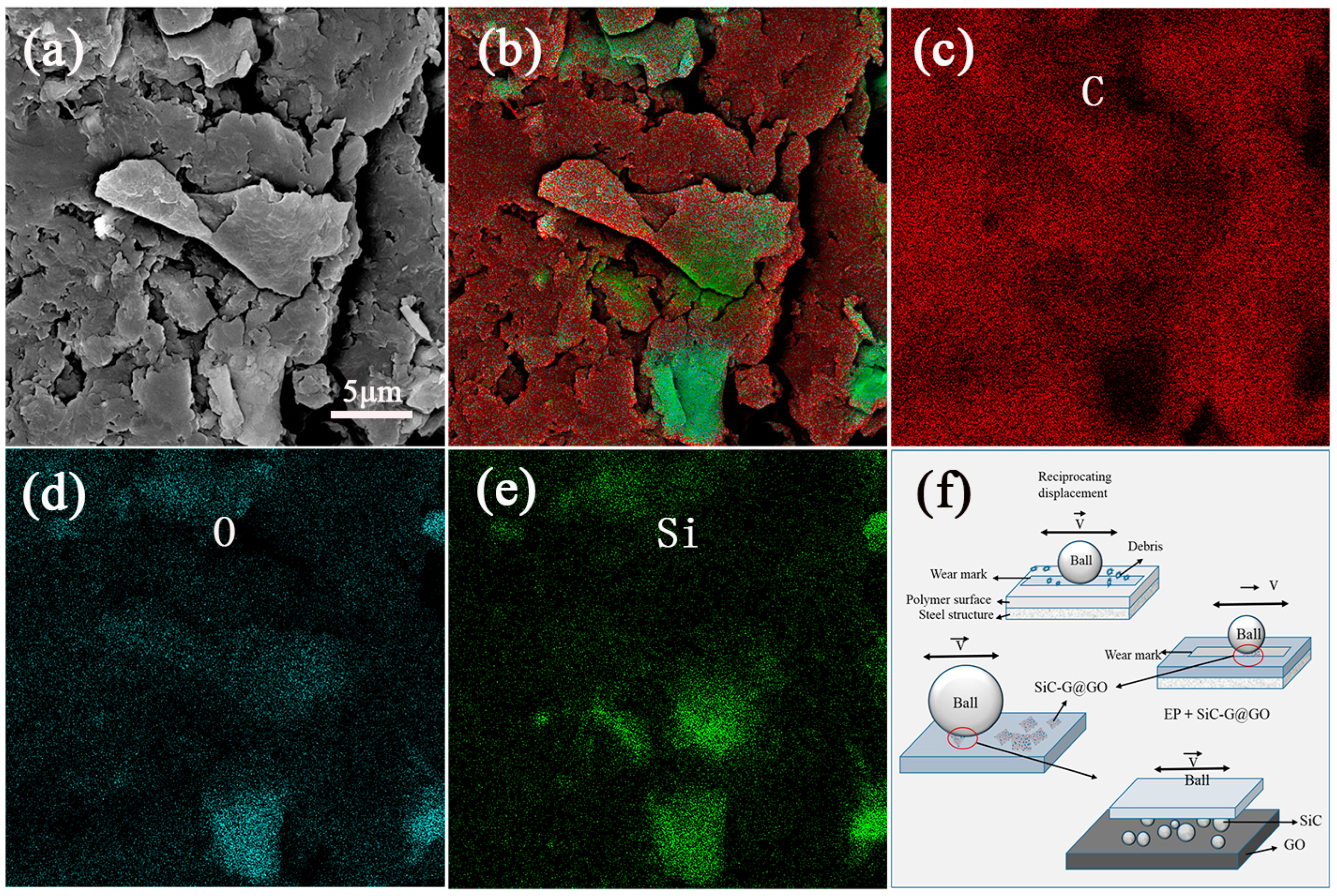
3.9. Research on the Mechanism of the Wear Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Honarvar Nazari, M.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Wan, P.; Zhao, N.; Qi, F.; Zhang, B.; Xiong, H.; Yuan, H.; Liao, B.; Ouyang, X. Synthesis of PDA-BN@f-Al2O3 hybrid for nanocomposite epoxy coating with superior corrosion protective properties. Prog. Org. Coat. 2020, 146, 105713. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Liu, W.; Pu, J.; Chen, H.; Zhao, H.; Li, X. Improvement of anticorrosion ability of epoxy matrix in simulate marine environment by filled with superhydrophobic POSS-GO nanosheets. J. Hazard. Mater. 2019, 364, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, G.; Wu, Q. Preparation of epoxy/ZrO2 composite coating on the Q235 surface by electrostatic spraying and its corrosion resistance in 3.5% NaCl solution. RSC Adv. 2022, 12, 10625–10633. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; LV, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Oliveira, J.D.; Rocha, R.C.; Galdino, A.G.D.S. Effect of Al2O3 particles on the adhesion, wear, and corrosion performance of epoxy coatings for protection of umbilical cables accessories for subsea oil and gas production systems. J. Mater. Res. Technol. 2019, 8, 1729–1736. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, Y.; Choi, S.-R.; Ko, S.-J.; Kim, K.; Kim, J.G. A photoelectrochemical coating for corrosion protection using Fe3O4@MoS2 core-shell and sunflower oil. Mater. Chem. Phys. 2022, 276, 125385. [Google Scholar] [CrossRef]
- Zhou, Z.; Pourhashem, S.; Wang, Z.; Duan, J.; Zhang, R.; Hou, B. Distinctive roles of graphene oxide, ZnO quantum dots, and their nanohybrids in anti-corrosion and anti-fouling performance of waterborne epoxy coatings. Chem. Eng. J. 2022, 439, 135765. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Ganash, A.A.; Alhebshi, N.A.; Alyoubi, N.H. Fabrication of a poly(o-toluidine-co-aniline)/SiO2 nanocomposite for an electrochemical supercapacitor application. J. Appl. Electrochem. 2020, 50, 1019–1035. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M.; Bordbar, S.; Karimi-Maleh, H. Effects of surface treatment of TiO2 nanoparticles on the adhesion and anticorrosion properties of the epoxy coating on mild steel using electrochemical technique. Prog. Org. Coat. 2018, 119, 99–108. [Google Scholar] [CrossRef]
- Wan, R.; Chen, S.; Tang, X.; Feng, Z.; Liu, J.; Li, Y. Effect mechanism of the Fe3O4 nanoparticles on mechanical properties and anticorrosion performances of epoxy coatings. Prog. Org. Coat. 2022, 173, 107181. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Chen, Y.; Yin, X.; Liu, Y. Smart coatings embedded with polydopamine-decorated layer-by-layer assembled SnO2 nanocontainers for the corrosion protection of 304 stainless steels. J. Colloid Interface Sci. 2020, 579, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Li, F.; Di, H.; Zhang, L.; Zhan, Y. h-BN decorated with Fe3O4 nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion performance of epoxy coatings. J. Alloys Compd. 2016, 685, 743–751. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Qin, S.; Xue, Q.; Zhao, H.; Wang, L. Processable poly(2-butylaniline)/hexagonal boron nitride nanohybrids for synergetic anticorrosive reinforcement of epoxy coating. Corros. Sci. 2018, 131, 187–198. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, G.; Dong, Y.; Zhang, Y. Anticorrosive epoxy coatings based on polydopamine modified molybdenum disulfide. Prog. Org. Coat. 2019, 133, 154–160. [Google Scholar] [CrossRef]
- Khalili Dermani, A.; Kowsari, E.; Ramezanzadeh, B.; Amini, R. Utilizing imidazole based ionic liquid as an environmentally friendly process for enhancement of the epoxy coating/graphene oxide composite corrosion resistance. J. Ind. Eng. Chem. 2019, 79, 353–363. [Google Scholar] [CrossRef]
- Uddin, A.; Khatoon, R.; Estevez, D.; Salem, M.; Ali, A.; Attique, S.; Lu, J.; Qin, F.X. Waste paper cellulose based-MoS2 hybrid composites: Towards sustainable green shielding. Mater. Today Commun. 2022, 31, 103858. [Google Scholar] [CrossRef]
- Xu, Y.L.; Uddin, A.; Estevez, D.; Luo, Y.; Peng, H.X.; Qin, F.X. Lightweight microwire/graphene/silicone rubber composites for efficient electromagnetic interference shielding and low microwave reflectivity. Compos. Sci. Technol. 2020, 189, 108022. [Google Scholar] [CrossRef]
- Uddin, A.; Estevez, D.; Qin, F.X. From functional units to material design: A review on recent advancement of programmable microwire metacomposites. Compos. Part A Appl. Sci. Manuf. 2022, 153, 106734. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and Anticorrosive Properties of Graphene/Epoxy Resin Composites Coating Prepared by in-Situ Method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Cai, M.; Li, W.; Fan, X.; Zhu, M. Amino-functionalized Ti3C2Tx with anti-corrosive/wear function for waterborne epoxy coating. J. Mater. Sci. Technol. 2020, 54, 144–159. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Wang, Y.; Luo, R.; Wang, J. Preparing SiC/diamond coatings via chemical vapor deposition of SiC on diamond-coated graphite and their frictional properties. Ceram. Int. 2016, 42, 4470–4476. [Google Scholar] [CrossRef]
- Chung, S.; Hwang, B.H. A microstructural study of the wear behaviour of SiCp/Al composites. Tribol. Int. 1994, 27, 307–314. [Google Scholar] [CrossRef]
- Li, Q.; Song, G.M.; Zhang, Y.Z.; Lei, T.C.; Chen, W.Z. Microstructure and dry sliding wear behavior of laser clad Ni-based alloy coating with the addition of SiC. Wear 2003, 254, 222–229. [Google Scholar] [CrossRef]
- Lin, L. Mechanisms of pigment dispersion. Pigment. Resin Technol. 2003, 32, 78–88. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of nanoparticles aggregation/agglomeration on the interfacial/interphase and tensile properties of nanocomposites. Compos. Part B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.-d.; Liu, J.; Wang, X.; Gui, T.-j.; Li, B.-j.; Tang, Y.-z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Qu, Q.; Su, Z.; Qin, T.; Da, Y.; Tian, X. Self-assembled three-dimensional structure with optimal ratio of GO and SiC particles effectively improving the thermal conductivity and reliability of epoxy composites. Compos. Commun. 2020, 22, 100448. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Zhang, J.; Chen, S. Preparation and mechanical properties researches of silane coupling reagent modified β-silicon carbide filled epoxy composites. Polym. Bull. 2009, 62, 689–697. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Ebrahimi, M. Role of a hybrid dye-clay nano-pigment (DCNP) on corrosion resistance of epoxy coatings. Prog. Org. Coat. 2018, 114, 223–232. [Google Scholar] [CrossRef]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Ollivier-Lamarque, L.; Lallart, M.; Mary, N.; Uchimoto, T.; Livi, S.; Marcelin, S.; Miki, H. Dielectric analysis of water uptake in polymer coating using spatially defined Fick’s law and mixing rule. Prog. Org. Coat. 2020, 148, 105846. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, F.; Jiang, G.; Wang, X.; Hu, R.; Wang, R.; Xi, X.; Wang, S.; Wang, T.; Chen, W. Superhydrophobic and high adhesive performance of functionalized graphene films. Powder Technol. 2012, 230, 247–251. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Yu, M.; Li, S.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloys Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. Surface modification of Cr2O3 nanoparticles with 3-amino propyl trimethoxy silane (APTMS). Part 1: Studying the mechanical properties of polyurethane/Cr2O3 nanocomposites. Prog. Org. Coat. 2014, 77, 1663–1673. [Google Scholar] [CrossRef]
- Amrollahi, S.; Ramezanzadeh, B.; Yari, H.; Ramezanzadeh, M.; Mahdavian, M. In-situ growth of ceria nanoparticles on graphene oxide nanoplatelets to be used as a multifunctional (UV shield/radical scavenger/anticorrosive) hybrid compound for exterior coatings. Prog. Org. Coat. 2019, 136, 105241. [Google Scholar] [CrossRef]
- Nechanicky, M.A.; Chew, K.W.; Sellinger, A.; Laine, R.M. α-Silicon carbide/β-silicon carbide particulate composites via polymer infiltration and pyrolysis (PIP) processing using polymethylsilane. J. Eur. Ceram. Soc. 2000, 20, 441–451. [Google Scholar] [CrossRef]
- Gao, J.; Tang, L.; Shen, Z.; Dong, Y.; Wang, Z.; Lyu, J.; Li, J.; Yu, H.-Q. Coupling of SiC and CeO2 nanosheets to enhance solar energy utilization and optimize catalytic ozonation. Appl. Catal. B Environ. 2022. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Puglia, D.; Saeb, M.R. Protocol for nonisothermal cure analysis of thermoset composites. Prog. Org. Coat. 2019, 131, 333–339. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Bushy-surface hybrid nanoparticles for developing epoxy superadhesives. Appl. Surf. Sci. 2019, 479, 1148–1160. [Google Scholar] [CrossRef]
- Lendvay-Győrik, G.; Mészáros, L.; Mészáros, G.; Lengyel, B. Some interesting peculiarities in the high frequency part of the impedance diagram obtained in the case of iron dissolution in dilute H2SO4 solution. Corros. Sci. 2000, 42, 79–90. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Bierwagen, G.P. Influence of the composition of working fluids on flow-accelerated organic coating degradation: Deionized water versus electrolyte solution. Corros. Sci. 2012, 55, 97–106. [Google Scholar] [CrossRef]
- Mišković-stanković, V.B.; Dražić, D.M.; Teodorović, M.J. Electrolyte penetration through epoxy coatings electrodeposited on steel. Corros. Sci. 1995, 37, 241–252. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. Corrosion protection properties of novel epoxy nanocomposite coatings containing silane functionalized graphene quantum dots. J. Alloys Compd. 2018, 731, 1112–1118. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam-Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination of seawater-immersed anticorrosive coatings: Mapping of parameters affecting the rate. Prog. Org. Coat. 2010, 68, 283–292. [Google Scholar] [CrossRef]
- Mahdavi, F.; Forsyth, M.; Tan, M.Y.J. Techniques for testing and monitoring the cathodic disbondment of organic coatings: An overview of major obstacles and innovations. Prog. Org. Coat. 2017, 105, 163–175. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Guo, M.; Cui, Y.; Gao, J.; Tang, Z. Microstructures and high-temperature self-lubricating wear-resistance mechanisms of graphene-modified WC-12Co coatings. Friction 2021, 9, 315–331. [Google Scholar] [CrossRef]
- Lang, H.; Xu, Y.; Zhu, P.; Peng, Y.; Zou, K.; Yu, K.; Huang, Y. Superior lubrication and electrical stability of graphene as highly effective solid lubricant at sliding electrical contact interface. Carbon 2021, 183, 53–61. [Google Scholar] [CrossRef]








| Samples | β (°C/min) | ΔT (°C) | ΔH | ΔT* | ΔH* | CI |
|---|---|---|---|---|---|---|
| EP | 10 | 61.66 | 0.043 | 1.00 | 1.00 | 1.00 |
| SiC-G@GO0.5 | 10 | 59.03 | 0.049 | 0.95 | 1.14 | 1.08 |
| SiC-G@GO1 | 10 | 51.28 | 0.052 | 0.83 | 1.21 | 1.01 |
| SiC-G@GO2 | 10 | 59.02 | 0.049 | 0.95 | 1.14 | 1.08 |
| Samples | Time | CPE1 | R1(ohmcm2) | CPE2 | R2(ohmcm2) | CPE3 | R3(ohmcm2) | Circuit Diagram | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0(ohm−1cm−2sn) | n | Y0(ohm−1cm−2sn) | n | Y0(ohm−1cm−2sn) | n | ||||||
| EP | 0 d | 5.391 × 10−11 | 0.989 | 9.364 × 109 | 1.965 × 10−10 | 0.881 | 6.387 × 1010 | - | - | - | 2 |
| 2 d | 6.452 × 10−11 | 0.976 | 1.295 × 109 | 1.073 × 10−9 | 0.471 | 6.573 × 108 | - | - | - | 2 | |
| 4 d | 6.83 × 10−11 | 0.982 | 5.718 × 108 | 9.533 × 10−11 | 0.671 | 6.158 × 108 | - | - | - | 2 | |
| 10 d | 1.634 × 10−10 | 0.8 | 1.379 × 107 | 1.632 × 10−7 | 0.8 | 5.637 × 105 | - | - | - | 4 | |
| GO1 | 0 d | 8.437 × 10−11 | 0.976 | 1.347 × 1011 | - | - | - | 1 | |||
| 2 d | 6.928 × 10−9 | 0.708 | 4.286 × 107 | 3.3 × 10−7 | 0.513 | 1.601 × 107 | - | - | - | 2 | |
| 4 d | 1.36 × 10−8 | 0.669 | 3.366 × 105 | 3.463 × 10−7 | 0.427 | 3.436 × 106 | 4.238 × 10−7 | 1 | 5.294 × 107 | 3 | |
| 7 d | 9.904 × 10−10 | 0.763 | 2.179 × 107 | 1.046 × 10−9 | 0.999 | 5.043 × 108 | - | - | 4 | ||
| 10 d | 3.818 × 10−8 | 0.563 | 4.133 × 105 | 1.297 × 10−6 | 0.450 | 3.829 × 108 | - | - | - | 4 | |
| SiC1 | 0 d | 5.734 × 10−10 | 0.977 | 3.109 × 1011 | - | - | - | - | - | - | 1 |
| 2 d | 6.867 × 10−11 | 0.974 | 1.731 × 1010 | 5.024 × 10−11 | 0.565 | 1.195 × 1011 | - | - | - | 2 | |
| 4 d | 1.302 × 10−10 | 0.928 | 8.872 × 106 | 7.839 × 10−10 | 0.853 | 4.989 × 109 | - | - | - | 2 | |
| 7 d | 2.246 × 10−10 | 0.895 | 4.454 × 107 | 2.519 × 10−10 | 0.756 | 5.754 × 109 | - | - | - | 2 | |
| 10 d | 1.05 × 10−10 | 0.934 | 7.931 × 105 | 3.501 × 10−7 | 0.615 | 1.16 × 107 | - | - | - | 4 | |
| Samples | Time | CPE1 | R1(ohmcm2) | CPE2 | R2(ohmcm2) | CPE3 | R3(ohmcm2) | Circuit diagram | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0(ohm−1cm−2sn) | n | Y0(ohm−1cm−2sn) | n | Y0(ohm−1cm−2sn) | n | ||||||
| SiC-G@GO0.5 | 0 d | 3.716 × 10−11 | 0.973 | 8.643 × 1011 | - | - | - | - | - | - | 1 |
| 2 d | 2.582 × 10−11 | 0.335 | 1000 | 3.727 × 10−11 | 0.982 | 4.153 × 1010 | - | - | - | 2 | |
| 4 d | 6.835 × 10−11 | 0.927 | 4.036 × 107 | 1.712 × 10−9 | 0.667 | 1.691 × 1011 | - | - | - | 2 | |
| 7 d | 4.015 × 10−11 | 0.8 | 6.55 × 108 | 3.097 × 10−11 | 0.8 | 1.905 × 1010 | 3 | ||||
| 10 d | 7.97 × 10−11 | 0.938 | 2.777 × 107 | 9.176 × 10−11 | 0.884 | 4.002 × 107 | 2.513 × 10−7 | 0.473 | 1.365 × 1010 | 4 | |
| SiC-G@GO1 | 0 d | 3.528 × 10−11 | 0.969 | 6.776 × 1010 | - | - | - | - | - | - | 1 |
| 2 d | 4.186 × 10−10 | 0.946 | 4.786 × 1010 | - | - | - | - | - | - | 1 | |
| 4 d | 3.945 × 10−10 | 0.970 | 3.282 × 1010 | - | - | - | - | - | - | 1 | |
| 10 d | 3.819 × 10−11 | 0.8 | 6.779 × 109 | 2.123 × 10−11 | 0.8 | 3.084 × 1010 | - | - | - | 2 | |
| 15 d | 4.793 × 10−11 | 0.965 | 2.128 × 1010 | 6.974 × 10−11 | 0.699 | 1.452 × 1010 | - | - | - | 2 | |
| 22 d | 4.996 × 10−11 | 0.947 | 2.669 × 109 | 9.706 × 10−12 | 0.8 | 5.363 × 1010 | - | - | - | 2 | |
| SiC-G@GO2 | 0 d | 4.114 × 10−11 | 0.970 | 7.564 × 1011 | - | - | - | - | - | - | 1 |
| 2 d | 5.398 × 10−11 | 0.958 | 4.014 × 1010 | - | - | - | - | - | - | 1 | |
| 4 d | 5.457 × 10−10 | 0.955 | 2.765 × 1010 | - | - | - | - | - | - | 1 | |
| 10 d | 5.924 × 10−11 | 0.959 | 3.626 × 1010 | 2.539 × 10−10 | 0.788 | 1.093 × 1010 | - | - | - | 2 | |
| 15 d | 5.684 × 10−11 | 0.969 | 4.146 × 1010 | 1.405 × 10−10 | 0.829 | 2.11 × 1010 | - | - | - | 2 | |
| 21 d | 4.018 × 10−11 | 0.978 | 4.956 × 105 | 1.652 × 10−11 | 0.929 | 3.529 × 1010 | 7.325 × 10−11 | 0.695 | 1.861 × 1010 | 3 | |
| Samples | Soaking Time | Ecorr (Vvs.SCE) | icorr (A/cm2) | β a (V/dec) | −β c (V/dec) | CR (cm Year−1) | EPE% | Coating Thickness (μm) |
|---|---|---|---|---|---|---|---|---|
| Iron substrate | 10 min | −0.487 | 3.46 × 10−6 | 0.104 | −0.439 | 2.68 × 10−2 | - | 0 |
| EP | 10 d | −0.674 | 1.412 × 10−7 | 0.125 | 0.112 | 1.1 × 10−3 | - | 101.3 |
| GO1 | 10 d | −0.655 | 1.862 × 10−7 | 0.095 | 0.199 | 1.4 × 10−3 | −31 | 98.2 |
| SiC1 | 10 d | −0.659 | 8.884 × 10−8 | 0.142 | 0.276 | 6.65 × 10−4 | 37 | 103.5 |
| SiC-G@GO0.5 | 10 d | −0.466 | 6.95 × 10−8 | 0.259 | 0.167 | 5.38 × 10−4 | 50 | 97.3 |
| SiC-G@GO1 | 25 d | −0.311 | 1.51 × 10−11 | 0.268 | 0.081 | 1.17 × 10−7 | 99 | 96.6 |
| SiC-G@GO2 | 25 d | −0.404 | 1.64 × 10−9 | 0.037 | 0.251 | 1.27 × 10−5 | 98 | 99.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Tan, Y.; Qi, F.; Zhang, B.; Zhou, A.; Zhao, N.; Ouyang, X. A Micro-Nano Structure Formed by SiC/Graphene Oxide Self-Assembly Improves the Wear Resistance and Corrosion Resistance of an Epoxy-Based Composite Coating. Polymers 2022, 14, 4704. https://doi.org/10.3390/polym14214704
Tang J, Tan Y, Qi F, Zhang B, Zhou A, Zhao N, Ouyang X. A Micro-Nano Structure Formed by SiC/Graphene Oxide Self-Assembly Improves the Wear Resistance and Corrosion Resistance of an Epoxy-Based Composite Coating. Polymers. 2022; 14(21):4704. https://doi.org/10.3390/polym14214704
Chicago/Turabian StyleTang, Jun, Yali Tan, Fugang Qi, Biao Zhang, Ao Zhou, Nie Zhao, and Xiaoping Ouyang. 2022. "A Micro-Nano Structure Formed by SiC/Graphene Oxide Self-Assembly Improves the Wear Resistance and Corrosion Resistance of an Epoxy-Based Composite Coating" Polymers 14, no. 21: 4704. https://doi.org/10.3390/polym14214704






