3D-Printed Pectin/Carboxymethyl Cellulose/ZnO Bio-Inks: Comparative Analysis with the Solution Casting Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zinc Oxide Nanoparticles
2.3. Preparation of Pectin/CMC/ZnO Bio-Inks and Films
2.4. Morphology and Structural Analysis
2.5. Optical Analysis and Surface Color
2.6. Water Vapor Permeability (WVP) and Water Contact Angle (WCA)
2.7. Thickness and Mechanical Properties
2.8. Antimicrobial Activity
2.9. Cytotoxicity
2.10. Statistical Analysis
3. Results and Discussion
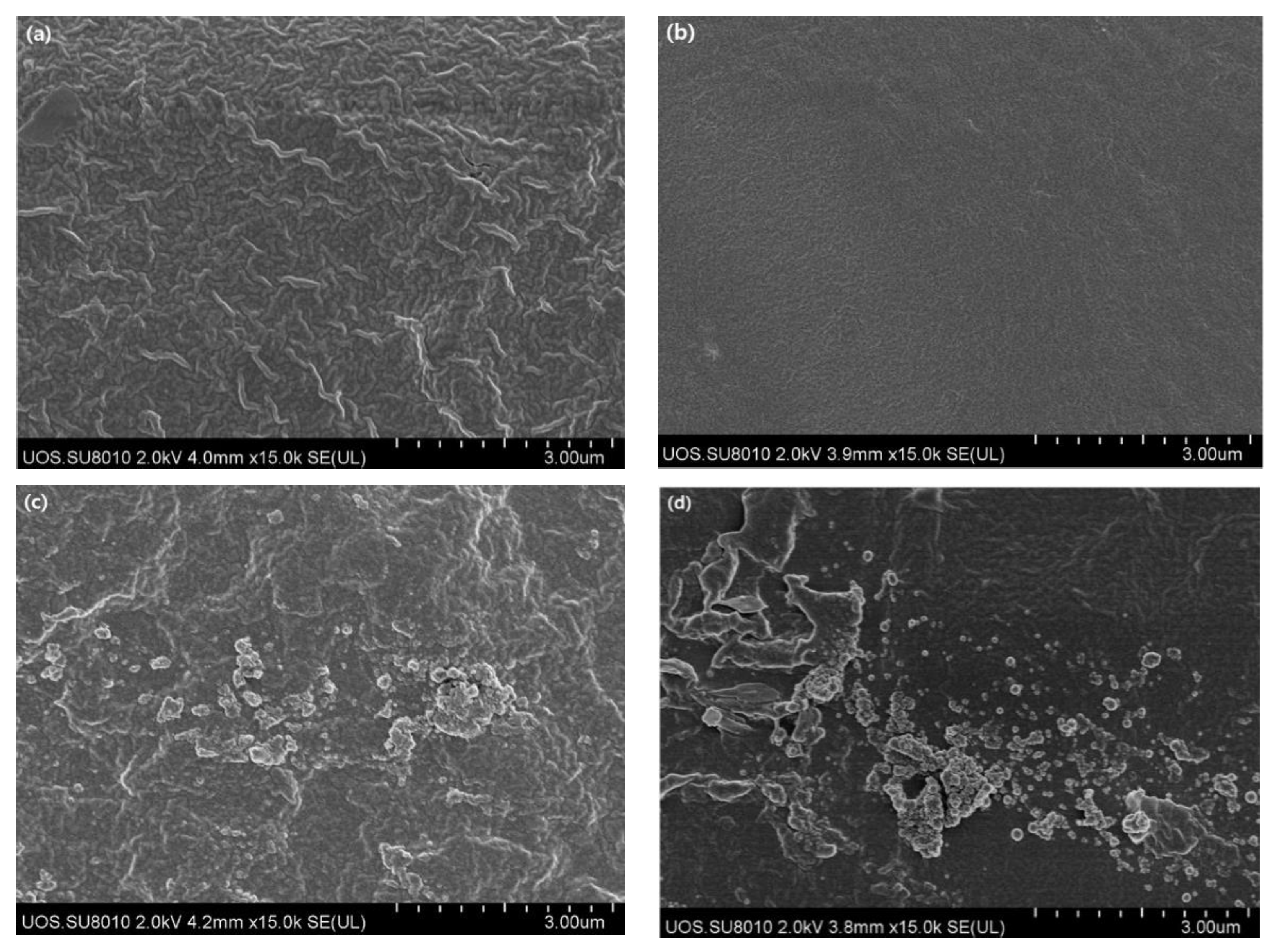
3.1. Morphology, FT-IR, and UV-Vis Spectrum
3.2. Color and Mechanical Properties
3.3. Optical Properties
3.4. Water Vapor Barrier Property (WVP) and Water Contact Angle (WCA)
3.5. Antimicrobial Activity
3.6. Cytotoxicity and Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priyadarshi, R.; Roy, S.; Purohit, S.D.; Ghosh, T. Biopolymers for food packaging and biomedical applications: Options or obligations? Coatings 2022, 12, 1261. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Purohit, S.D.; Roy, S.; Ghosh, T.; Rhim, J.; Han, S.S. Antiviral biodegradable food packaging and edible coating materials in the COVID-19 era: A mini-review. Coatings 2022, 12, 577. [Google Scholar] [CrossRef]
- Ghosh, T.; Priyadarshi, R.; de Souza, C.K.; Angioletti, B.L.; Rhim, J. Advances in pullulan utilization for sustainable applications in food packaging and preservation: A mini-review. Trends Food Sci. Technol. 2022, 125, 43–53. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Knuth, J. Material increase manufacturing by rapid prototyping technique. Ann. CIPP 1999, 40, 603–604. [Google Scholar]
- Heller, T.B.; Hill, R.M.; Saggal, A.F. Apparatus for Forming a Solid Three-Dimensional Article from a Liquid Medium. US5071337A, 10 December 1991. [Google Scholar]
- Malone, E.; Lipson, H. Fab@ Home: The personal desktop fabricator kit. Rapid Prototyp. J. 2007, 13, 245–255. [Google Scholar] [CrossRef]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Lee, M.P.; Cooper, G.J.; Hinkley, T.; Gibson, G.M.; Padgett, M.J.; Cronin, L. Development of a 3D printer using scanning projection stereolithography. Sci. Rep. 2015, 5, 9875. [Google Scholar] [CrossRef] [Green Version]
- Crump, S. Apparatus and Method for Creating Three-Dimensional Objects. U.S. Patent 5121329, 30 October 1989. [Google Scholar]
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Shah, F.A.; Omar, O.; Suska, F.; Snis, A.; Matic, A.; Emanuelsson, L.; Norlindh, B.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting. Acta Biomater. 2016, 36, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible scaffolds based on non-mammalian biopolymers for myoblast growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, C.A.; Orellana, N.; Avarias, K.; Ortiz, R.; Benavente, D.; Prieto, P. Micropatterning technology to design an edible film for in vitro meat production. Food Bioprocess Technol. 2018, 11, 1267–1273. [Google Scholar] [CrossRef]
- Bracci, R.; Maccaroni, E.; Cascinu, S. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Handral, H.K.; Tay, H.S.; Chan, W.W.; Choudhury, D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2022, 62, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bang, Y.; Yoon, K.S.; Priyadarshi, R.; Rhim, J.-W. Pine needle (Pinus densiflora) extract-mediated synthesis of silver nanoparticles and the preparation of carrageenan-based antimicrobial packaging films. J. Nanomater. 2022, 2022, 8395302. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bang, Y.; Yoon, K.S.; Rhim, J.-W. UV barrier and antimicrobial activity of agar-based composite films incorporated with ZnO nanoparticles and grapefruit seeds extract. Korean J. Packag. Sci. Technol. 2019, 25, 69–77. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.; Rhim, J. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Kumar, B.; Rhim, J.-W. Green and facile synthesis of carboxymethylcellulose/ZnO nanocomposite hydrogels crosslinked with Zn2 ions. Int. J. Biol. Macromol. 2020, 162, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Soubhagya, A.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Hashem, M.; Abd El-Hady, M.; Sharaf, S. Development of CMC hydrogels loaded with silver nanoparticles for medical applications. Carbohydr. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.; Rhim, J. Carboxymethyl cellulose-based multifunctional film combined with zinc oxide nanoparticles and grape seed extract for the preservation of high-fat meat products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of layer thickness and raster angle on the mechanical properties of 3D-printed PEEK and a comparative mechanical study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Negi, Y.S. Effect of varying filler concentration on zinc oxide nanoparticle embedded chitosan films as potential food packaging material. J. Polym. Environ. 2017, 25, 1087–1098. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Effect of types of zinc oxide nanoparticles on structural, mechanical, and antibacterial properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composite films. Food Packag. Shelf Life 2019, 21, 100327. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.-W.; Kim, J. Cellulose nanofiber-based nanocomposite films reinforced with zinc oxide nanorods and grapefruit seed extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Zhou, J.; Song, B.; Qin, H.; Lu, W.; Zhao, G.; Huang, Z.; Han, G. Color-tunable and white emission of Tm3 doped transparent zinc silicate glass-ceramics embedding ZnO nanocrystals. J. Am. Ceram. Soc. 2020, 103, 1010–1017. [Google Scholar] [CrossRef]
- Hasheminya, S.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S. Physicochemical, mechanical, optical, microstructural and antimicrobial properties of novel kefiran-carboxymethyl cellulose biocomposite films as influenced by copper oxide nanoparticles (CuONPs). Food Packag. Shelf Life 2018, 17, 196–204. [Google Scholar] [CrossRef]
- Hari, K.D.; Garcia, C.V.; Shin, G.; Kim, J. Improvement of the UV barrier and antibacterial properties of crosslinked pectin/zinc oxide bionanocomposite films. Polymers 2021, 13, 2403. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Kim, S.; Rhim, J. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [Green Version]
- Tarasevich, Y.I. The surface energy of hydrophilic and hydrophobic adsorbents. Colloid J. 2007, 69, 212–220. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.D.F.F.; Coimbra, J.S.D.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]






| Components | Proportion (%) |
|---|---|
| Pectin | 3.33% |
| Sodium carboxymethyl cellulose | 1.67% |
| Glycerin | 1.50% |
| Calcium chloride | 0.11% |
| Water | 93.39% |
| Parameter | Setting Value | Parameter | Setting Value |
|---|---|---|---|
| Nozzle size of syringe (Gauge) | 25 | Printing speed (mm/s) | 10 |
| Nozzle size (mm) | 0.6 | Move speed (mm/s) | 2 |
| Hight of layer (mm) | 0.2 | Retraction speed (mm/s) | 10 |
| Density (%) | 100 | Z HOP (mm) | 0.4 |
| Infill pattern | Concentric | Pressure (KPa) | 150 |
| The angle of infill rotation | 90° | - | - |
| Bed temperature | Room temperature | - | - |
| Syringe temperature | Room temperature | - | - |
| Samples | L* | a* | b* | ∆E | TS (MPa) | EB (%) |
|---|---|---|---|---|---|---|
| Pec/CMC-SC | 90.07 ± 0.05 b | 4.31 ± 0.01 b | 2.70 ± 0.02 c | 5.51 ± 0.02 c | 12.8 ± 0.6 a | 27.9 ± 3.2 c |
| Pec/CMC/ZnO-SC | 88.38 ± 0.19 d | 4.23 ± 0.01 c | 9.67 ± 0.33 a | 7.98 ± 0.31 a | 24.1 ± 1.5 b | 26.1 ± 2.6 c |
| Pec/CMC-3D | 89.67 ± 0.27 c | 4.36 ± 0.01 a | 2.39 ± 0.18 c | 5.83 ± 0.08 b | 27.6 ± 2.7 b | 19.2 ± 1.6 a |
| Pec/CMC/ZnO-3D | 90.56 ± 0.08 a | 3.72 ± 0.02 d | 4.12 ± 0.05 b | 4.48 ± 0.04 d | 41.3 ± 5.8 c | 22.1 ± 2.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Priyadarshi, R.; Kim, J.-W.; Kim, J.; Alekseev, D.G.; Rhim, J.-W. 3D-Printed Pectin/Carboxymethyl Cellulose/ZnO Bio-Inks: Comparative Analysis with the Solution Casting Method. Polymers 2022, 14, 4711. https://doi.org/10.3390/polym14214711
Kim YH, Priyadarshi R, Kim J-W, Kim J, Alekseev DG, Rhim J-W. 3D-Printed Pectin/Carboxymethyl Cellulose/ZnO Bio-Inks: Comparative Analysis with the Solution Casting Method. Polymers. 2022; 14(21):4711. https://doi.org/10.3390/polym14214711
Chicago/Turabian StyleKim, Yeon Ho, Ruchir Priyadarshi, Jin-Wook Kim, Jangwhan Kim, Denis G. Alekseev, and Jong-Whan Rhim. 2022. "3D-Printed Pectin/Carboxymethyl Cellulose/ZnO Bio-Inks: Comparative Analysis with the Solution Casting Method" Polymers 14, no. 21: 4711. https://doi.org/10.3390/polym14214711
APA StyleKim, Y. H., Priyadarshi, R., Kim, J.-W., Kim, J., Alekseev, D. G., & Rhim, J.-W. (2022). 3D-Printed Pectin/Carboxymethyl Cellulose/ZnO Bio-Inks: Comparative Analysis with the Solution Casting Method. Polymers, 14(21), 4711. https://doi.org/10.3390/polym14214711







