Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
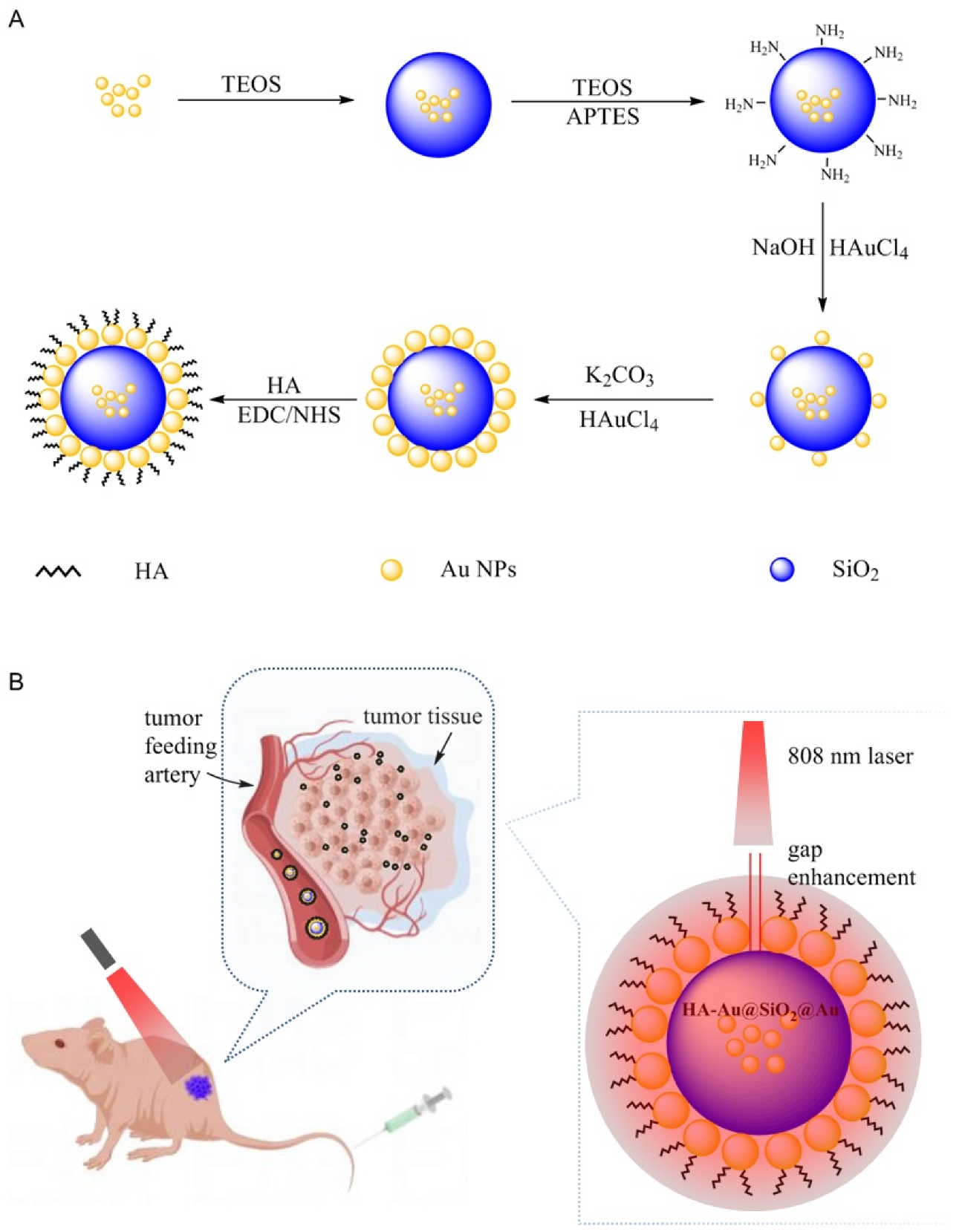
2.2. Synthesis of Au@SiO2@Au NPs
2.3. Synthesis of HA-Au@SiO2@Au NPs
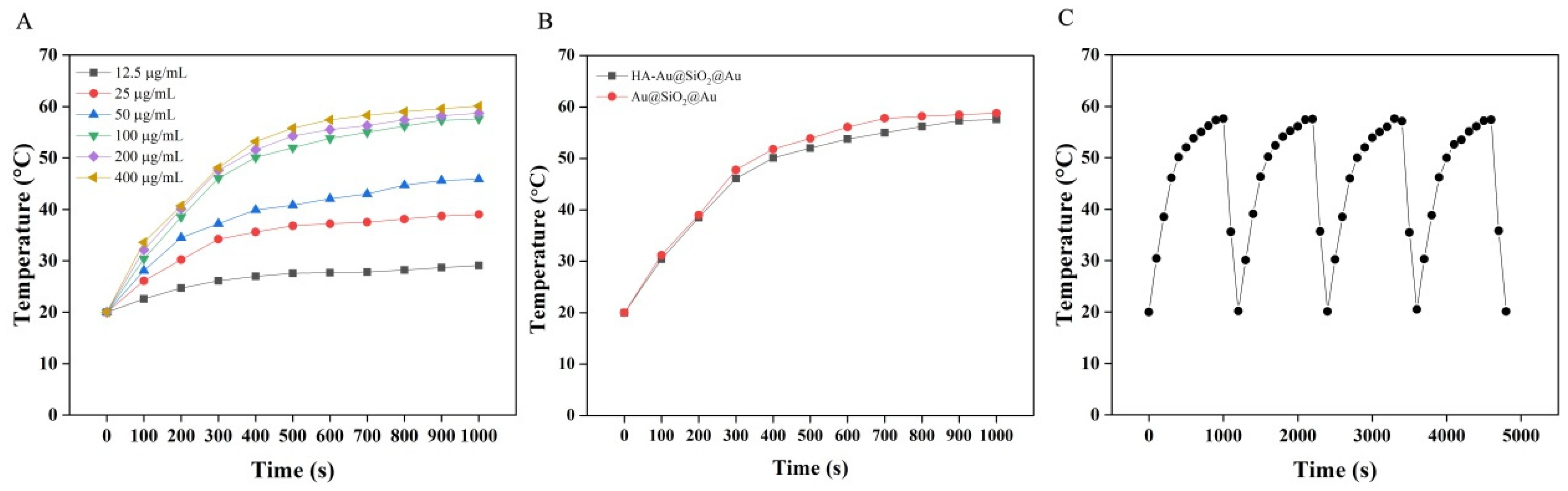
2.4. Photothermal Properties of HA-Au@SiO2@Au NPs
2.5. Cell Culture
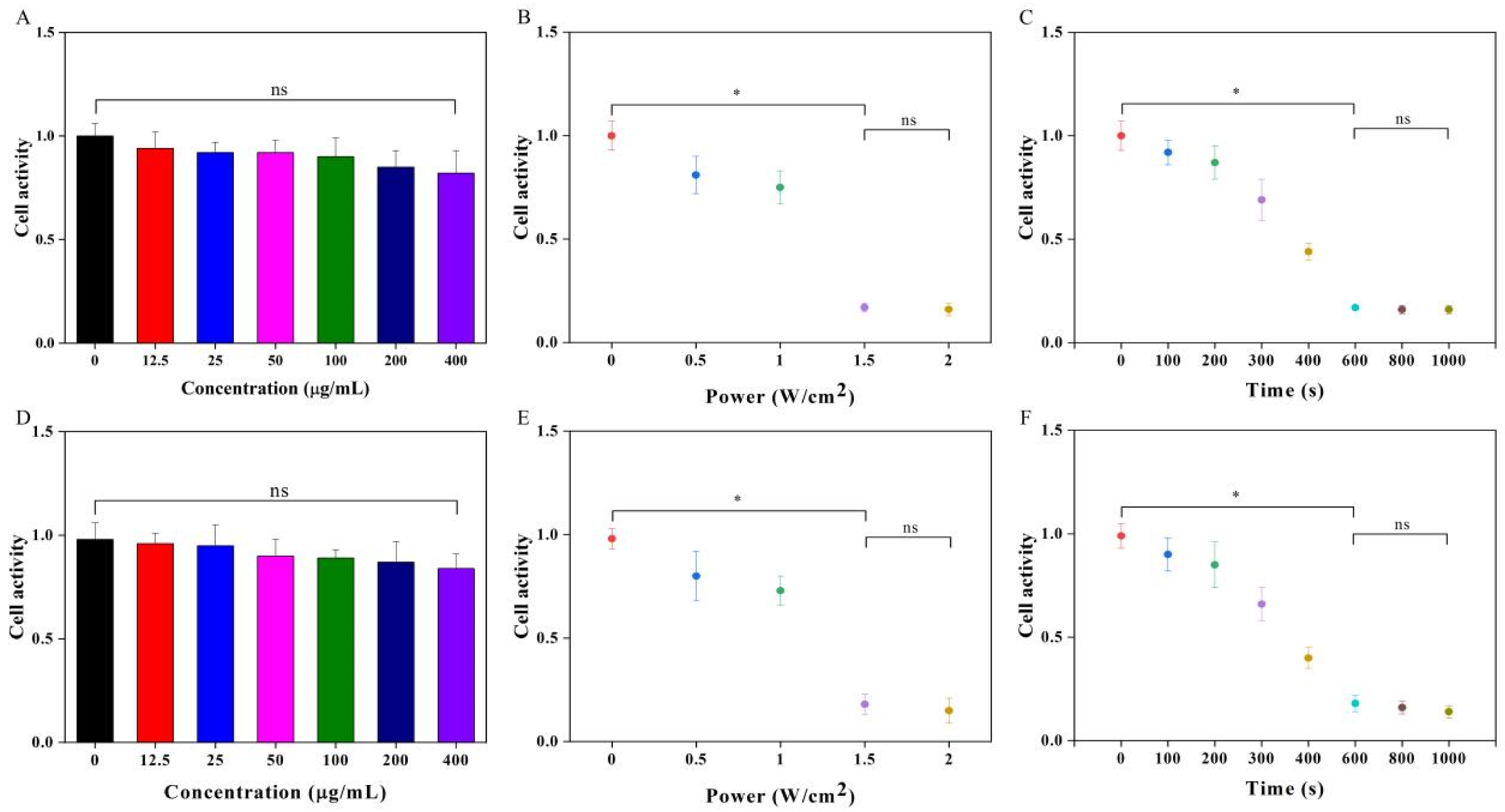
2.6. PTT of Cancer Cells In Vitro
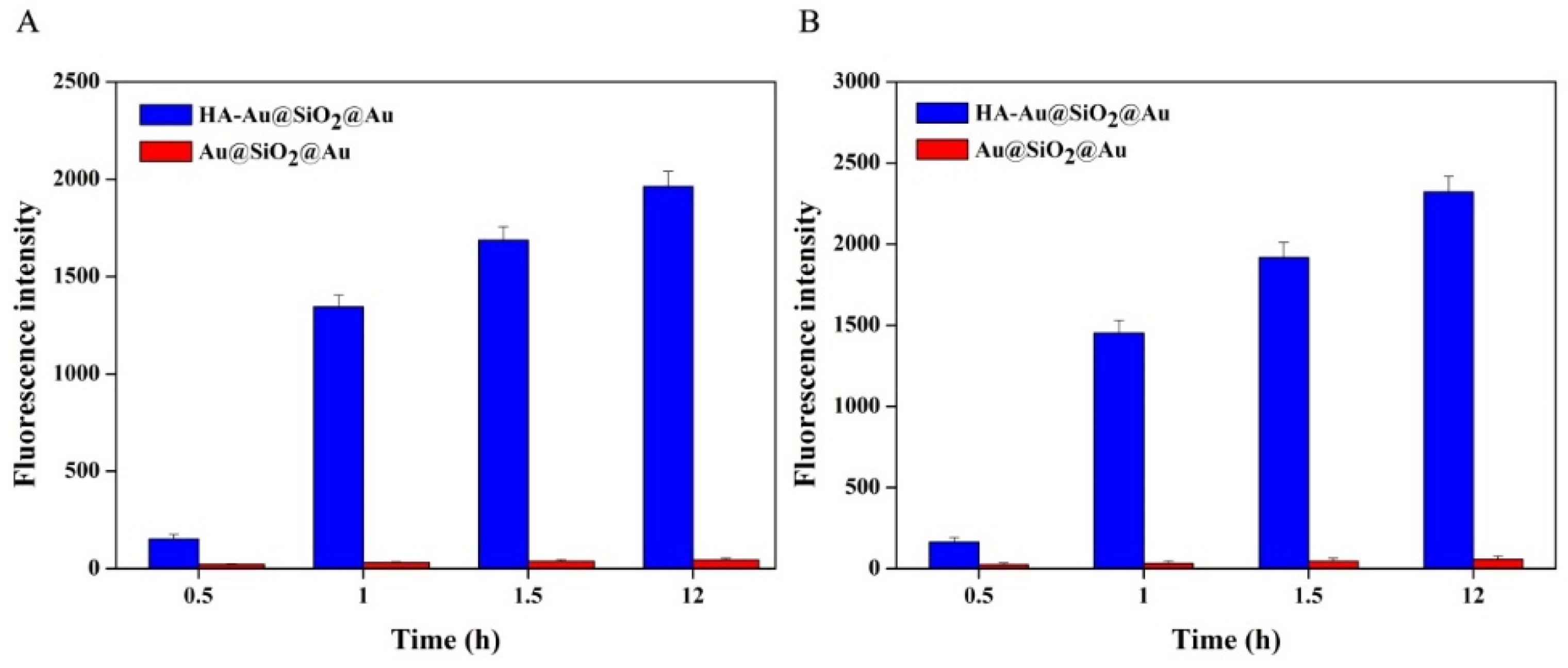
2.7. Quantitative Analysis of Cancer Cells Phagocytosis
2.8. PTT of Tumors In Vivo
3. Results and Discussion
3.1. Characterization
3.2. Photothermal Properties
3.3. PTT of Cancer Cells In Vitro
3.4. Fluorescence Imaging of Cancer Cells In Vitro
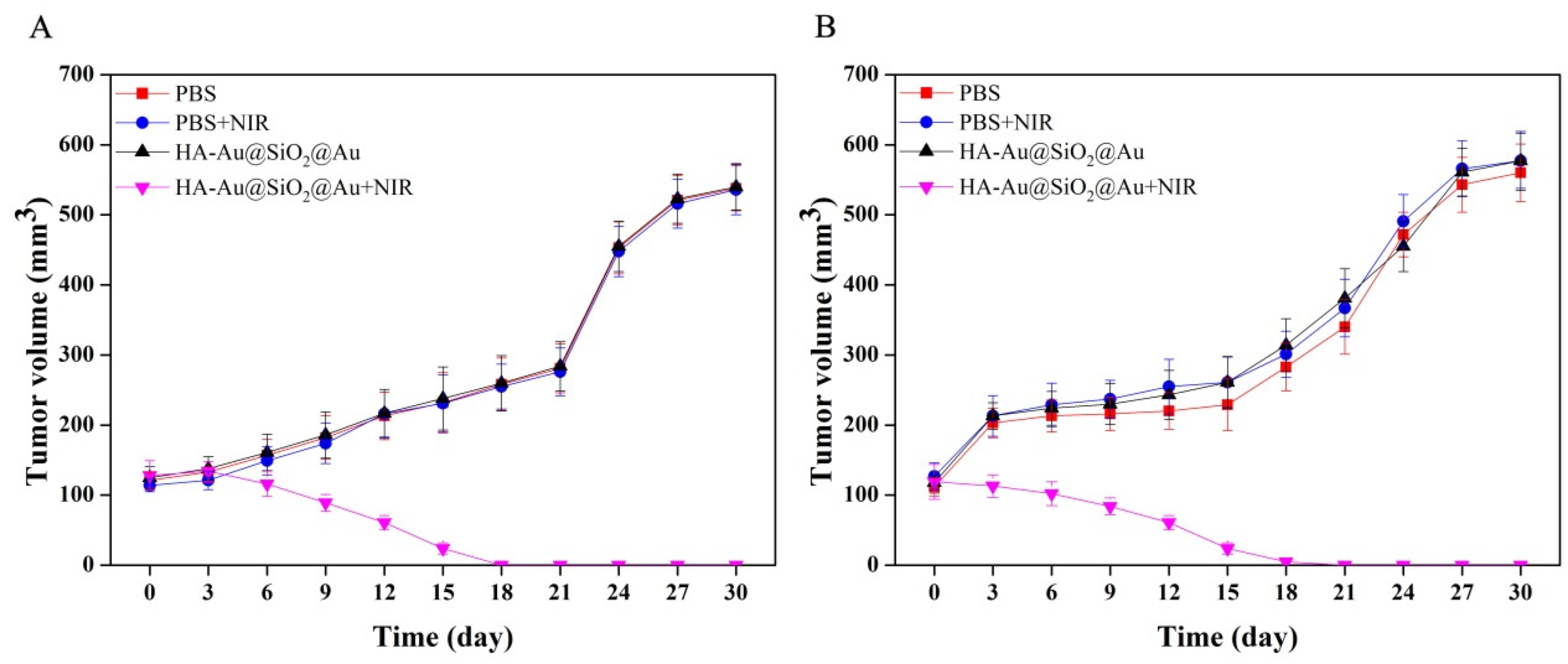
3.5. PTT of Tumors In Vivo
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, S. The impact of genetics on the classification of renal carcinoma. Histopathology 1993, 22, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.A.; Evans, S.; Wright, M.P. Prostate cancer and urinary incontinence. Maturitas 2009, 63, 323–328. [Google Scholar] [CrossRef]
- Ooi, E.H.; Popov, V.; Alfano, M.; Cheong, J.K.K. Influence of natural convection on gold nanorods-assisted photothermal treatment of bladder cancer in mice. Int. J. Hyperth. 2020, 37, 634–650. [Google Scholar] [CrossRef]
- Lin, T.; Yuan, A.; Zhao, X.; Lian, H.; Zhuang, J.; Chen, W.; Zhang, Q.; Liu, G.; Zhang, S.; Chen, W.; et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017, 53, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, Q.; Zhao, X.; Zhang, Q.; Chen, W.; Xu, L.; Zhao, S.; Wang, K.; Liu, T.; Guo, H. NIR molecule induced self-assembled nanoparticles for synergistic in vivo chemo-photothermal therapy of bladder cancer. Mater. Res. Express 2021, 8, 045017. [Google Scholar] [CrossRef]
- Luczynska, E.; Aniol, J. Neoangiogenesis in prostate cancer. Contemp. Oncol. 2013, 17, 229–233. [Google Scholar]
- Elalouf, V.; Xylinas, E.; Klap, J.; Pignot, G.; Delongchamps, N.B.; Saighi, D.; Peyromaure, M.; Flam, T.; Zerbib, M. Bladder recurrence after radical nephroureterectomy: Predictors and impact on oncological outcomes. Int. J. Urol. 2013, 20, 1078–1083. [Google Scholar] [CrossRef]
- Bauer, S.R.; Richman, E.L.; Sosa, E.; Weinberg, V.; Song, X.; Witte, J.S.; Carroll, P.R.; Chan, J.M. Antioxidant and vitamin E transport genes and risk of high-grade prostate cancer and prostate cancer recurrence. Prostate 2013, 73, 1786–1795. [Google Scholar] [CrossRef]
- Sasaki, N.; Ishi, K.; Kudo, N.; Nakayama, S.M.M.; Nakamura, K.; Morishita, K.; Ohta, H.; Ishizuka, M.; Takiguchi, M. Spatial and temporal profile of cisplatin delivery by ultrasound-assisted intravesical chemotherapy in a bladder cancer model. PLoS ONE 2017, 12, e0188093. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bing, Y.; Cong, H. Emerging Advanced Nanomaterials for Cancer Photothermal Therapy. Rev. Adv. Mater. Sci. 2018, 53, 131–146. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Niu, G.; Lan, M.; Jia, Q.; Liang, Q. Near-Infrared Organic Dye-Based Nanoagent for the Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2016, 8, 29899–29905. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, J.; Yang, D.; Wang, X.; Liu, B.; He, N.; Wang, Z. ICG@ZIF-8:One-step encapsulation of indocyanine green in ZIF-8 and use as a therapeutic nanoplatform. Chin. Chem. Lett. 2018, 29, 1421–1424. [Google Scholar] [CrossRef]
- Chen, H.W.; Chen, J.C.; Chen, N.S.; Huang, J.L.; Huang, M. Applications of Peptide Conjugated Photosensitizers in Photodynamic Therapy*: Applications of Peptide Conjugated Photosensitizers in Photodynamic Therapy. Prog. Biochem. Biophys. 2009, 36, 1106–1113. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.; Pentok, M.; Sauli, E.; He, N.; Zen, X.; Li, X.; Liu, T. Molecular Mechanism and Changes of Antioxidant Enzyme in ZnO Nanoparticles Against Fungus. J. Biomed. Nanotechnol. 2019, 15, 647–661. [Google Scholar] [CrossRef]
- Lesley, J.; Howes, N.; Perschl, A.; Hyman, R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J. Exp. Med. 1994, 180, 383–387. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Dong, S.; Tian, Y.; Li, G.; Vianney, J.M.; Buza, J.; Liu, J.; He, Q. Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules 2020, 10, 110. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; He, Q.; Yang, S.; Xie, M.; Deng, P.; Xia, Y.; Li, G. Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan. Biomolecules 2019, 9, 245. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, H.; Wang, Y.; Su, X.; Jin, L.; Wu, Y.; Deng, Y.; Li, S.; Chen, Z.; Chen, H.; et al. Fast and Accurate Control Strategy for Portable Nucleic Acid Detection (PNAD) System Based on Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 407–415. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chen, W.C.; Chen, M.F. Predictive Value of CD44 in Muscle-Invasive Bladder Cancer and Its Relationship with IL-6 Signaling. Ann. Surg. Oncol. 2018, 25, 3518–3526. [Google Scholar] [CrossRef]
- Lou, W.; Krill, D.; Dhir, R.; Becich, M.J.; Dong, J.T.; Frierson, H.F., Jr.; Isaacs, W.B.; Isaacs, J.T.; Gao, A.C. Methylation of the CD44 metastasis suppressor gene in human prostate cancer. Cancer Res. 1999, 59, 2329–2331. [Google Scholar] [PubMed]
- Zhu, J.; Li, J.J.; Zhao, J.W. The Study of Surface Plasmon Resonance in Au-Ag-Au Three-Layered Bimetallic Nanoshell: The Effect of Separate Ag Layer. Plasmonics 2014, 9, 435–441. [Google Scholar] [CrossRef]
- Yin, N.; Wu, P.; Wang, M.; Li, P.; Chen, W.; Liu, L. Anti-Body Guided Au–Ag Nanoframes with Highly Targeted Photothermal Properties for Cancer Cell Therapy. Sci. Adv. Mater. 2017, 9, 1334–1339. [Google Scholar] [CrossRef]
- Schlather, A.E.; Manjavacas, A.; Lauchner, A.; Marangoni, V.S.; DeSantis, C.J.; Nordlander, P.; Halas, N.J. Hot Hole Photoelectrochemistry on Au@SiO2@Au Nanoparticles. J. Phys. Chem. Lett. 2017, 8, 2060–2067. [Google Scholar] [CrossRef]
- Khanna, S.; Utsav; Marathey, P.; Chaliyawala, H.; Rajaram, N.; Roy, D.; Banerjee, R.; Mukhopadhyay, I. Fabrication of long-ranged close-packed monolayer of silica nanospheres by spin coating. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 520–527. [Google Scholar] [CrossRef]
- Utsav; Khanna, S.; Makani, N.H.; Paneliya, S.; Mukhopadhyay, I.; Banerjee, R. Thermal crowning mechanism in gold-silica nanocomposites: Plasmonic-photonic pairing in archetypal two-dimensional structures. Phys. Chem. Chem. Phys. 2021, 23, 17197–17207. [Google Scholar] [CrossRef]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, X.; Gao, J.; Chen, G.; Li, Z.; Wu, X.; Yu, Z.; Xing, J.; Sun, L.; Ruan, H.; et al. A Flexible Caterpillar-Like Gold Nanoparticle Assemblies with Ultrasmall Nanogaps for Enhanced Dual-Modal Imaging and Photothermal Therapy. Small 2018, 14, e1800094. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.G.; Liu, P.; Gu, N.; Chen, Z. Enhanced fluorescence of gold nanoclusters composed of HAuCl4 and histidine by glutathione: Glutathione detection and selective cancer cell imaging. Small 2014, 10, 5170–5177. [Google Scholar]
- Rao, Y.-Y.; Li, Z.-L.; Huang, J.-H.; Jiang, Y.-H.; Zhao, X.-X. Preparation and SERS Properties of 3D Ordered Gold Nanoshells Arrays. Chin. J. Inorg. Chem. 2018, 34, 1231–1239. [Google Scholar]
- Wang, R.; Zhou, G.; Yang, Y.; Wang, S.; Gao, S.; Gao, D.; Wang, X. Prostate-Specific Membrane Antigen-1-Mediated Au@SiO2@Au Core-Shell Nanoparticles: Targeting Prostate Cancer to Enhance Photothermal Therapy and Fluorescence Imaging. J. Biomed. Nanotechnol. 2022, 18, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Prestwich, G.D. Hyaluronic acid-N-hydroxysuccinimide: A useful intermediate for bioconjugation. Bioconjug. Chem. 2001, 12, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Luo, Y.; Yang, S.; Lin, J.; Gao, D.; Zhao, Y.; Liu, J.; Shi, X.; Wang, X. Hyaluronic acid-modified manganese-chelated dendrimer-entrapped gold nanoparticles for the targeted CT/MR dual-mode imaging of hepatocellular carcinoma. Sci. Rep. 2016, 6, 33844. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Ren, B.; Yang, X.; Cai, Z.C.; Zhao, X.J.; Zhao, M.X. Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity. Pharmaceuticals 2021, 14, 101. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Wei, P.; Li, J.; Ding, L.; Zhang, G.; Shi, X.; Shen, M. Facile synthesis of hyaluronic acid-modified Fe3O4/Au composite nanoparticles for targeted dual mode MR/CT imaging of tumors. J. Mater. Chem. B 2015, 3, 9098–9108. [Google Scholar] [CrossRef]
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic nanomaterials for chemo/photothermal therapy: A promising horizon on effective cancer treatment. Biophys. Rev. 2019, 11, 335–352. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Du, N.; Jin, L.; Chen, W.; Ma, Z.; Zhang, T.; Xu, J.; Zhang, W.; Wang, X.; Li, M. Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors. Polymers 2022, 14, 4772. https://doi.org/10.3390/polym14214772
Wang R, Du N, Jin L, Chen W, Ma Z, Zhang T, Xu J, Zhang W, Wang X, Li M. Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors. Polymers. 2022; 14(21):4772. https://doi.org/10.3390/polym14214772
Chicago/Turabian StyleWang, Ruizhi, Nan Du, Liang Jin, Wufei Chen, Zhuangxuan Ma, Tianyu Zhang, Jie Xu, Wei Zhang, Xiaolin Wang, and Ming Li. 2022. "Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors" Polymers 14, no. 21: 4772. https://doi.org/10.3390/polym14214772
APA StyleWang, R., Du, N., Jin, L., Chen, W., Ma, Z., Zhang, T., Xu, J., Zhang, W., Wang, X., & Li, M. (2022). Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors. Polymers, 14(21), 4772. https://doi.org/10.3390/polym14214772






