Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes
Abstract
:1. Introduction
2. Methodology
2.1. Materials
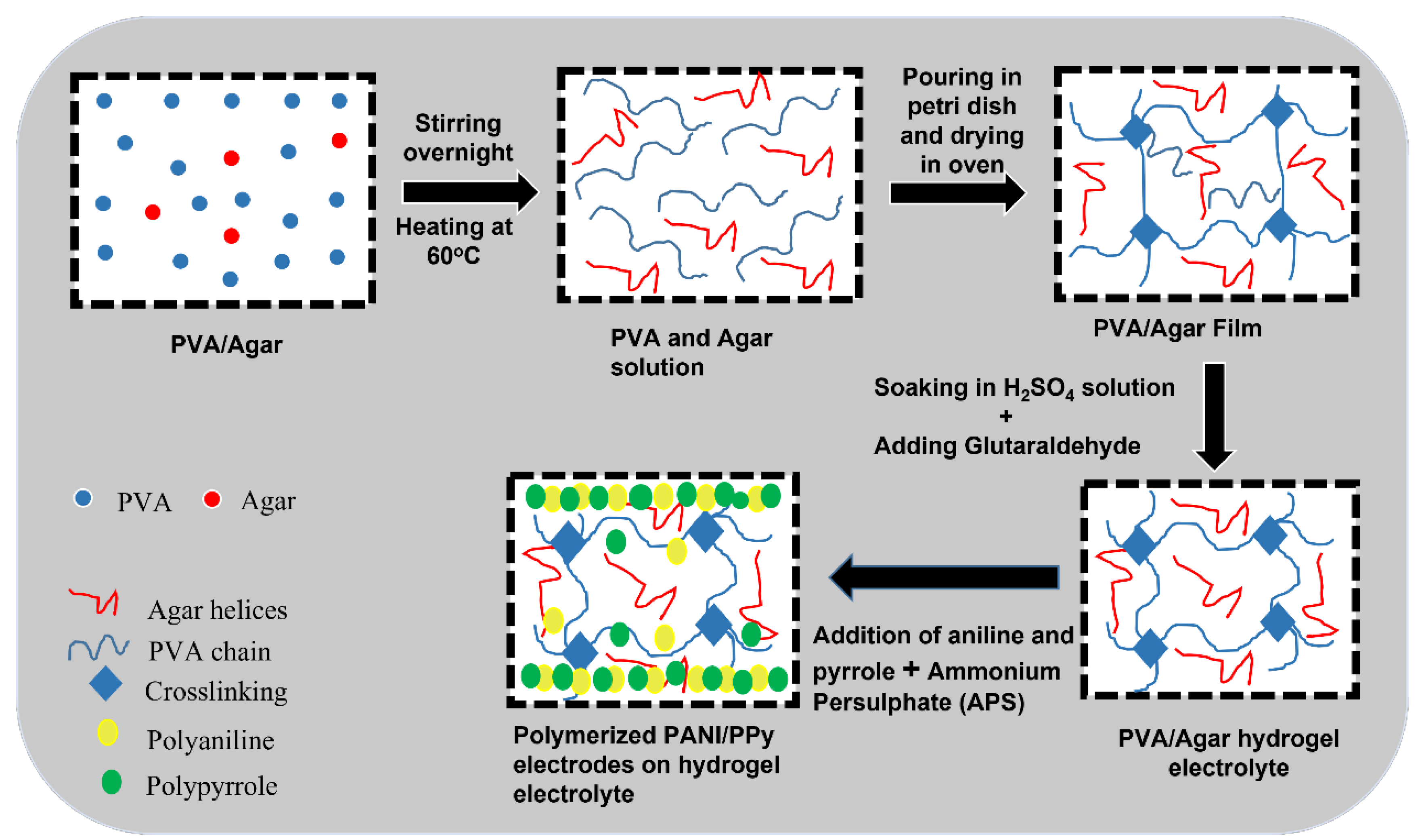
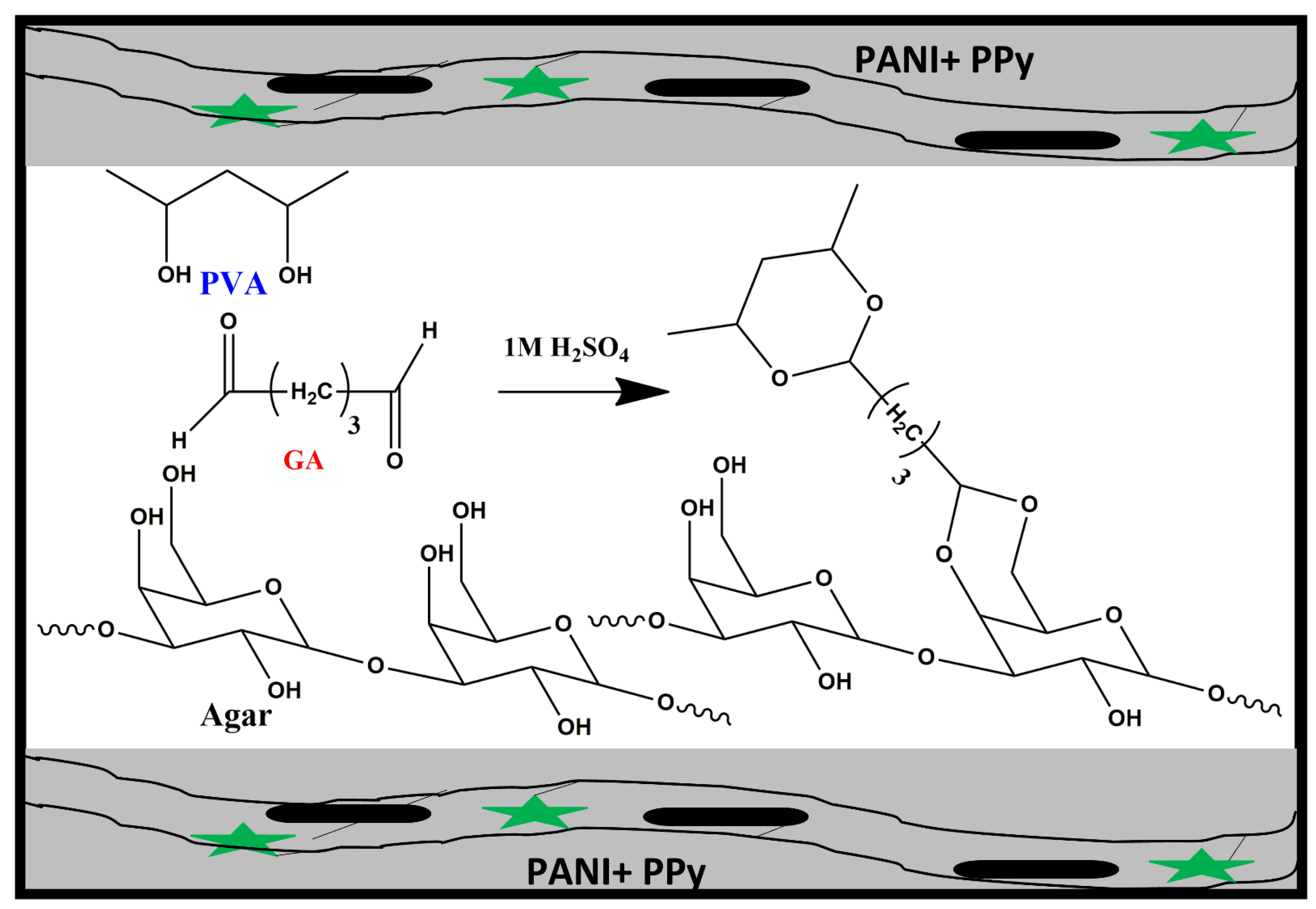
2.2. Methods
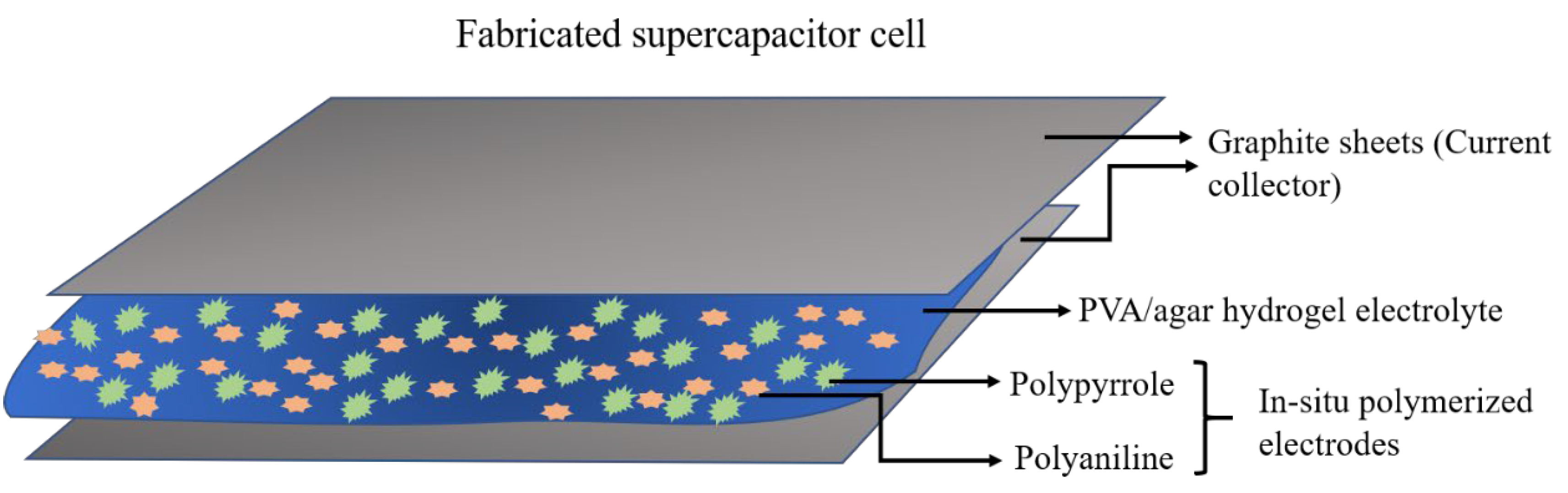
In Situ Preparation of Binder Free PANI-PPy Electrodes
2.3. Characterizations
2.4. Electrochemical Studies
3. Results and Discussion
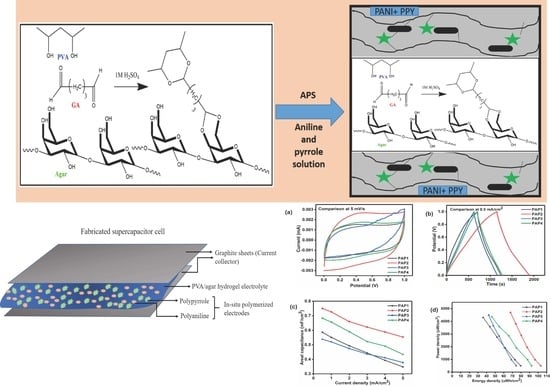
3.1. Synthesis of Hydrogel Electrolytes and Conducting Polymer Electrodes
3.2. Characterizations
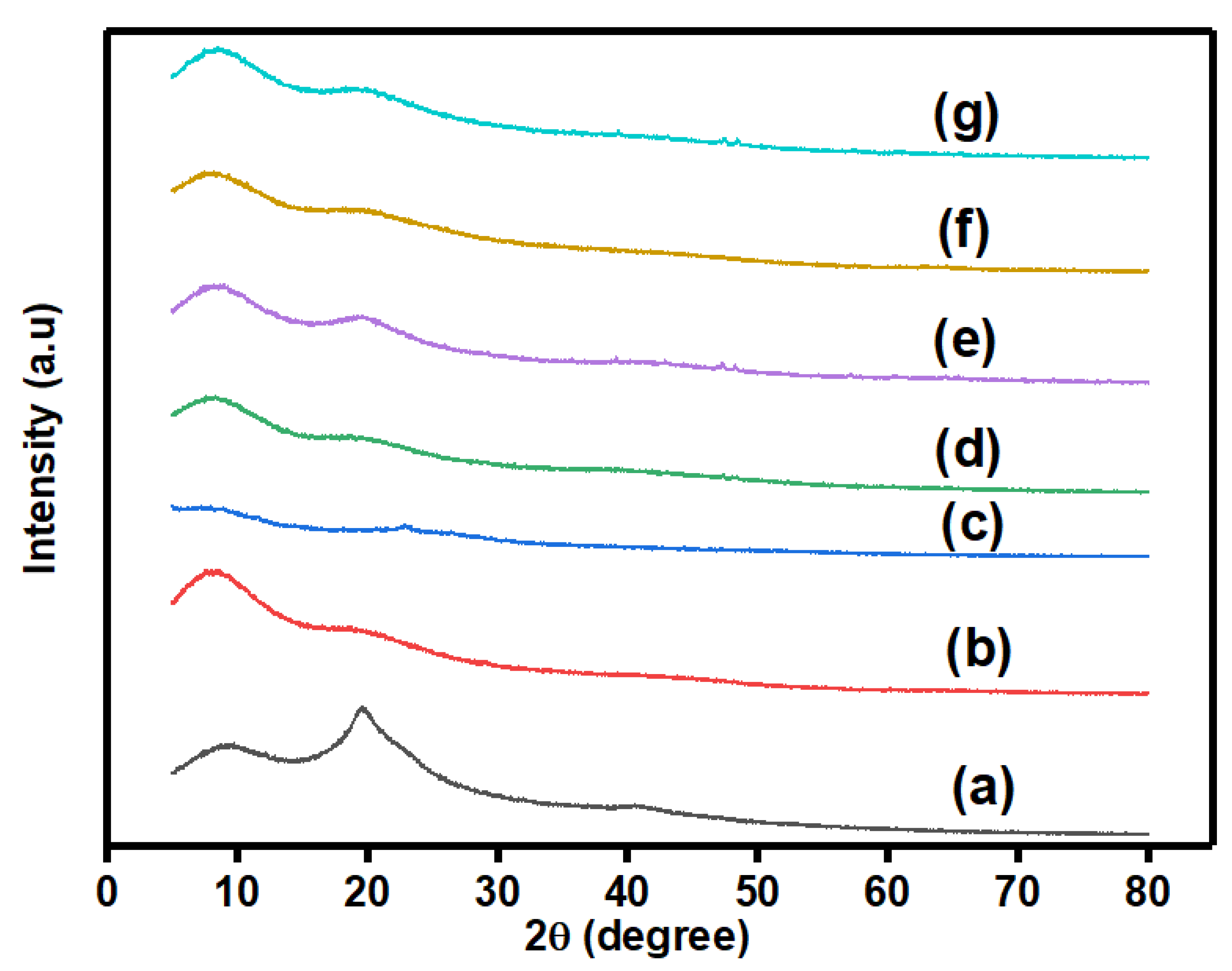
3.2.1. X-ray Diffraction
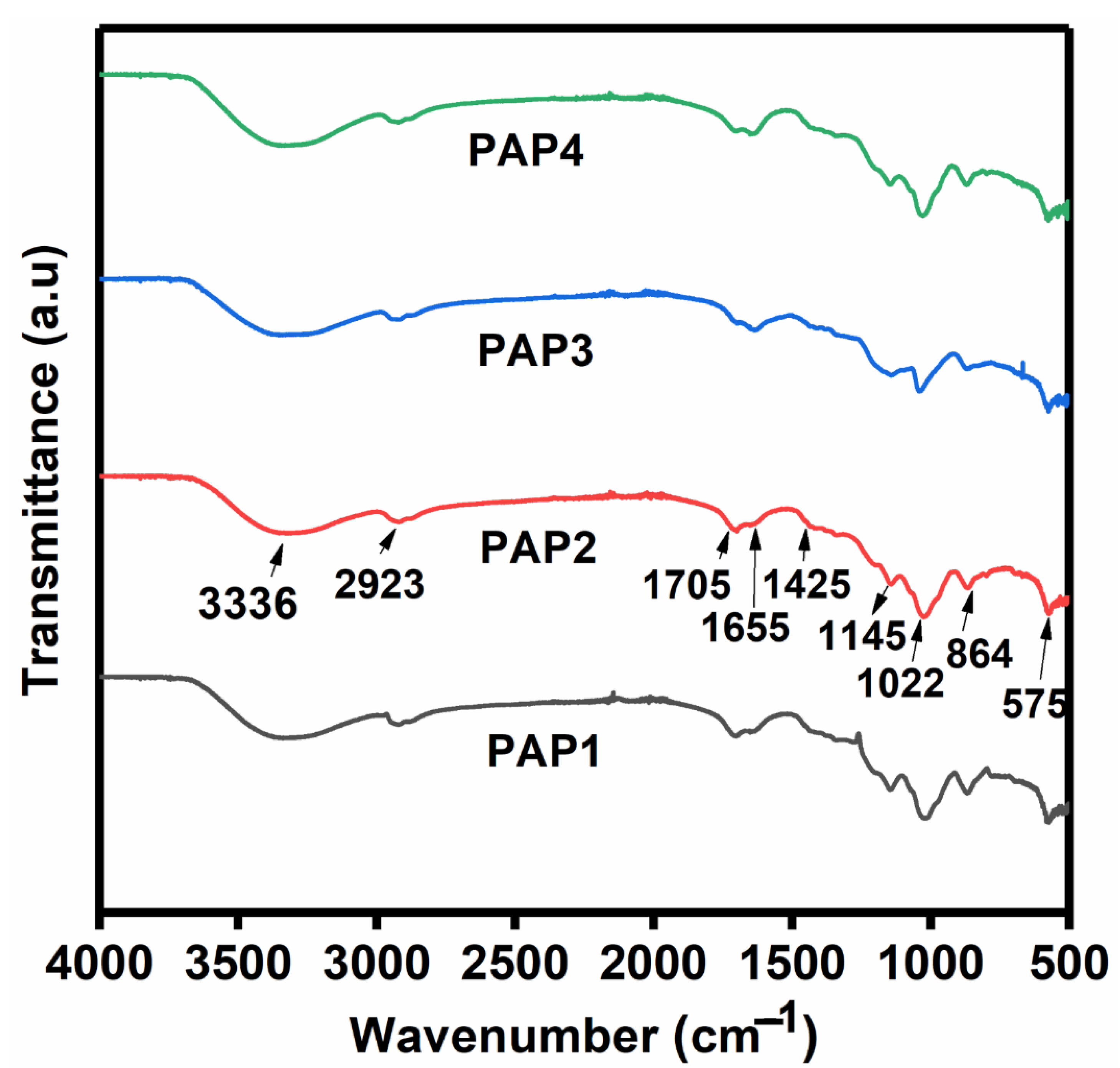
3.2.2. Fourier Transform Infrared Analysis
3.2.3. Surface Morphology
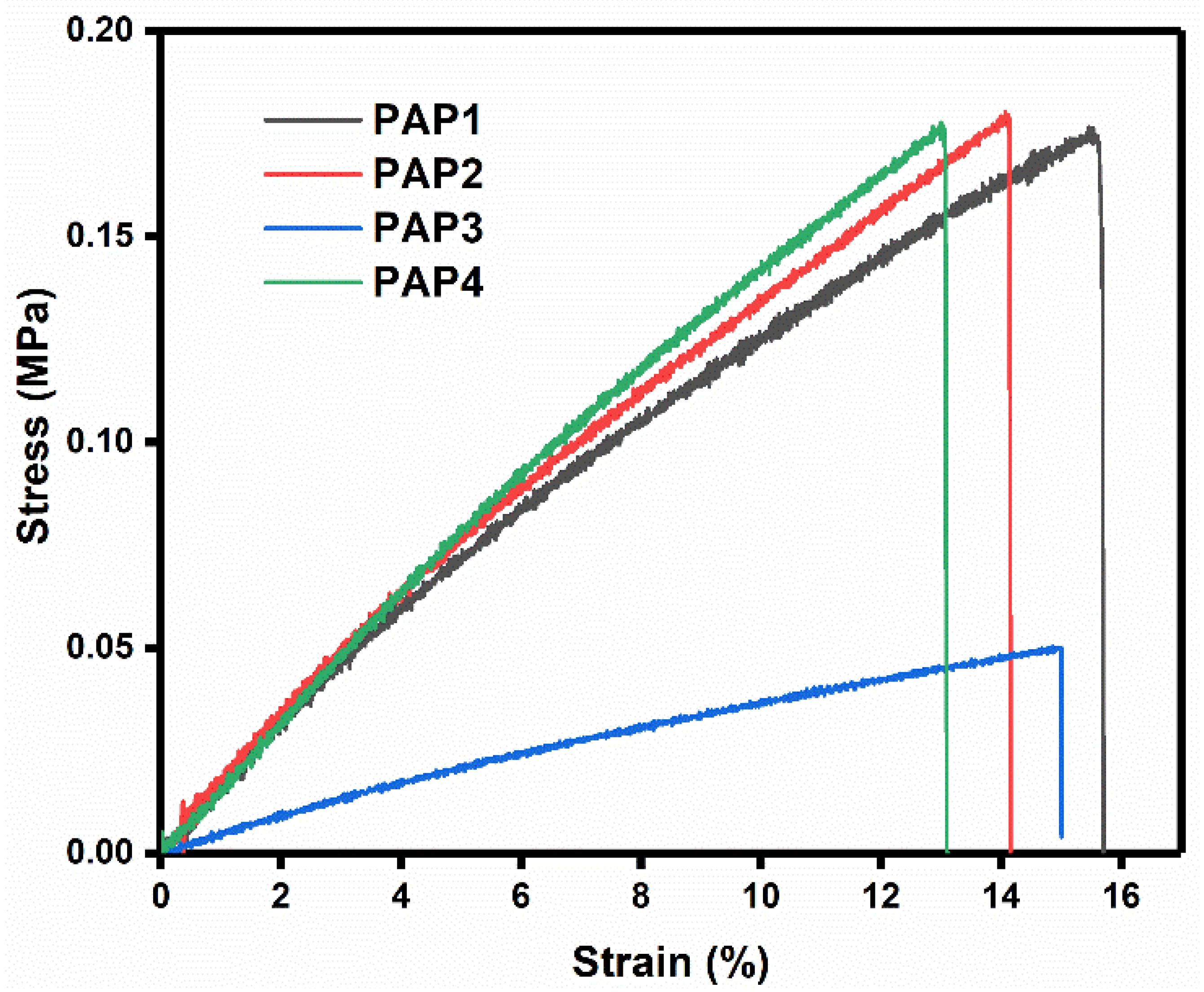
3.2.4. Tensile Testing
3.3. Electrochemical Analysis
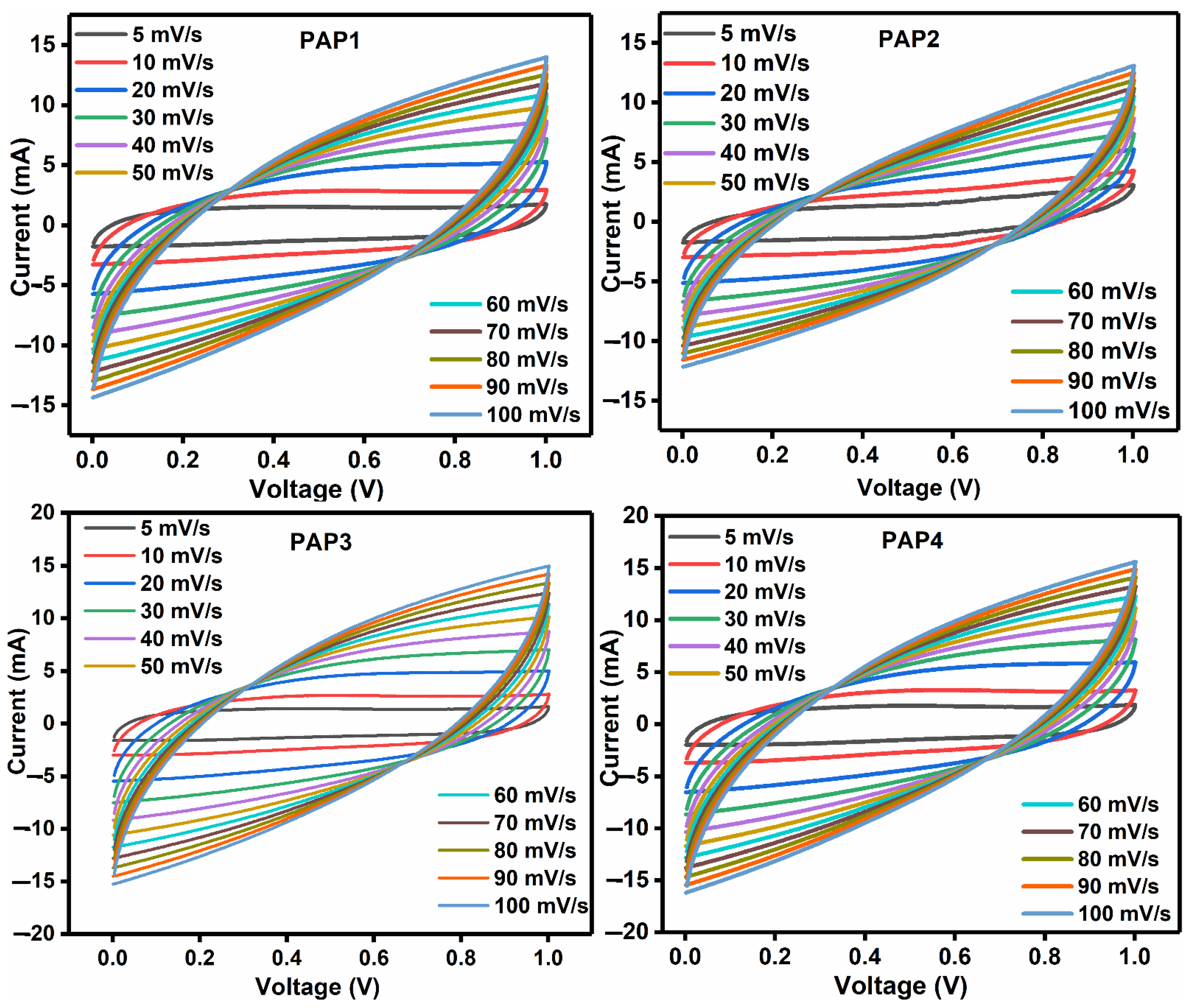
3.3.1. Cyclic Voltammetry
3.3.2. Galvanic Charge-Discharge
3.3.3. Electrochemical Impedance Spectroscopy and Life Cycle Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, J.; Luo, X.; Huang, H.; Li, L.; Lai, W.; Yu, X. Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor. J. Power Sources 2018, 407, 105–111. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Hu, J.; Yu, J.; Feng, G.; Yang, B.; Li, C.; Zhao, N.; Zhu, C.; Xu, J. Microgel-Enhanced Double Network Hydrogel Electrode with High Conductivity and Stability for Intrinsically Stretchable and Flexible All-Gel-State Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 19323–19330. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, L.; Wang, Y.; Zhang, C.; Liu, T. Polyaniline/graphene nanocomposites towards high-performance supercapacitors: A review. In Composites Communications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 8, pp. 83–91. [Google Scholar] [CrossRef]
- Moon, W.G.; Kim, G.-P.; Lee, M.; Song, H.D.; Yi, J. A biodegradable gel electrolyte for use in high-performance flexible supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3503–3511. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef]
- Liu, X.; Osaka, T. All-solid-state electric double-layer capacitor with isotropic high-density graphite electrode and polyethylene oxide/LiClO4 polymer electrolyte. J. Electrochem. Soc. 1996, 143, 3982–3986. [Google Scholar] [CrossRef]
- Menzel, J.; Frąckowiak, E.; Fic, K. Agar-based aqueous electrolytes for electrochemical capacitors with reduced self-discharge. Electrochim. Acta 2020, 332, 135435. [Google Scholar] [CrossRef]
- Bashir, S.; Hasan, K.; Hina, M.; Ali Soomro, R.; Mujtaba, M.A.; Ramesh, S.; Ramesh, K.; Duraisamy, N.; Manikam, R. Conducting polymer/graphene hydrogel electrodes based aqueous smart Supercapacitors: A review and future prospects. J. Electroanal. Chem. 2021, 898, 115626. [Google Scholar] [CrossRef]
- Wang, H.; Dai, L.; Chai, D.; Ding, Y.; Zhang, H.; Tang, J. Recyclable and tear-resistant all-in-one supercapacitor with dynamic electrode/electrolyte interface. J. Colloid Interface Sci. 2020, 561, 629–637. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Silvaraj, D.S.; Bashir, S.; Hina, M.; Iqbal, J.; Gunalan, S.; Ramesh, S.; Ramesh, K. Tailorable solid-state supercapacitors based on poly (N-hydroxymethylacrylamide) hydrogel electrolytes with high ionic conductivity. J. Energy Storage 2021, 35, 102320. [Google Scholar] [CrossRef]
- Gong, Q.; Li, Y.; Liu, X.; Xia, Z.; Yang, Y. A facile preparation of polyaniline/cellulose hydrogels for all-in-one flexible supercapacitor with remarkable enhanced performance. Carbohydr. Polym. 2020, 245, 116611. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Liu, W.; Su, J. Recent advances in all-in-one flexible supercapacitors. Sci. China Mater. 2020, 64, 27–45. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Q.; Sun, K.; Peng, H.; Xie, X.; Hamouda, H.A.; Ma, G. A novel all-in-one integrated flexible supercapacitor based on self-healing hydrogel electrolyte. J. Alloys Compd. 2021, 888, 161554. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, K.; Wan, P. A Flexible Stretchable Hydrogel Electrolyte for Healable All-in-One Configured Supercapacitors. Small 2018, 14, e1704497. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Rouelle, N.; Qiu, A.; Oh, J.-A.; Kempaiah, D.M.; Whittle, J.; Aakyiir, M.; Xing, W.; Ma, J. Hydrogen Bonding-Reinforced Hydrogel Electrolyte for Flexible, Robust, and All-in-One Supercapacitor with Excellent Low-Temperature Tolerance. ACS Appl. Mater. Interfaces 2020, 12, 37977–37985. [Google Scholar] [CrossRef]
- Guo, L.; Ma, W.-B.; Wang, Y.; Song, X.-Z.; Ma, J.; Han, X.-D.; Tao, X.-Y.; Guo, L.-T.; Fan, H.-L.; Liu, Z.-S.; et al. A chemically crosslinked hydrogel electrolyte based all-in-one flexible supercapacitor with superior performance. J. Alloys Compd. 2020, 843, 155895. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Wang, L.; Yang, X.; Yang, W.; Zheng, J.; Hou, H. Advanced Supercapacitors Based on Porous Hollow Carbon Nanofiber Electrodes with High Specific Capacitance and Large Energy Density. ACS Appl. Mater. Interfaces 2020, 12, 4777–4786. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Sun, K.; Feng, E.; Zhao, G.; Peng, H.; Wei, G.; Lv, Y.; Ma, G. A Single Robust Hydrogel Film Based Integrated Flexible Supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 165–173. [Google Scholar] [CrossRef]
- Zang, L.; Liu, Q.; Qiu, J.; Yang, C.; Wei, C.; Liu, C.; Lao, L. Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Q.; Song, Q.; Lu, H.; Ma, M. Strong Stretchable Polypyrrole Hydrogels with Biphase Microstructure as Electrodes for Substrate-Free Stretchable Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1900133. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Alzaid, M.A. A facile approach to investigate the charge storage mechanism of MOF/PANI based supercapattery devices. Solid State Ion. 2020, 354, 115411. [Google Scholar] [CrossRef]
- Li, X.; Song, H.; Zhang, Y.; Wang, H.; Du, K.; Li, H.; Yuan, Y.; Huang, J. Enhanced Electrochemical Capacitance of Graphene Nanosheets Coating With Polyaniline for Supercapacitors. Int. J. Electrochem. Sci. 2012, 7, 5163–5171. Available online: www.electrochemsci.org (accessed on 7 June 2022).
- Iqbal, J.; Numan, A.; Ansari, M.O.; Jafer, R.; Jagadish, P.R.; Bashir, S.; Hasan, P.M.Z.; Bilgrami, A.L.; Mohamad, S.; Ramesh, K.; et al. Cobalt Oxide Nanograins and Silver Nanoparticles Decorated Fibrous Polyaniline Nanocomposite as Battery-Type Electrode for High Performance Supercapattery. Polymers 2020, 12, 2816. [Google Scholar] [CrossRef] [PubMed]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-based conducting hydrogels. J. Mater. Sci. 2019, 54, 974–996. [Google Scholar] [CrossRef]



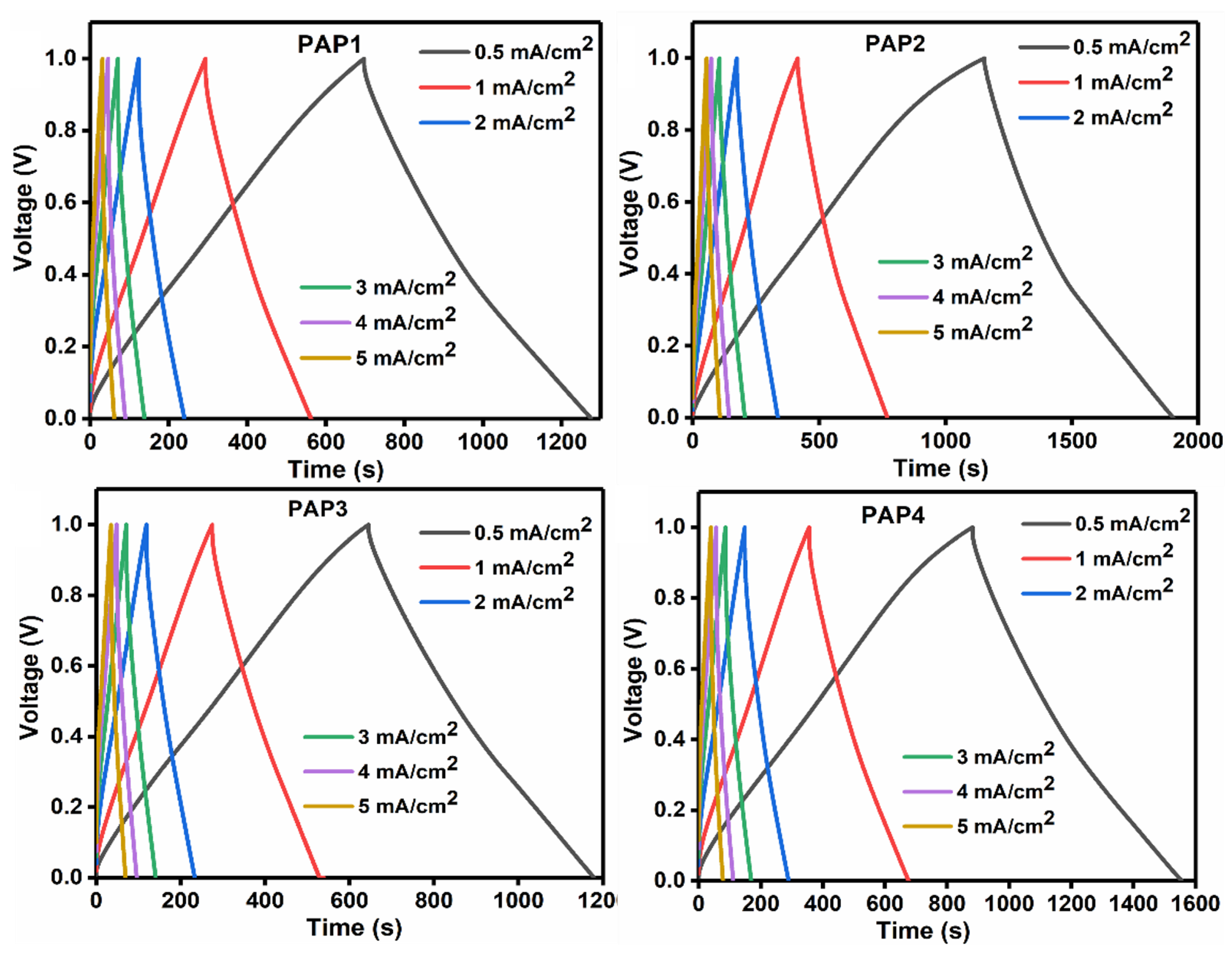
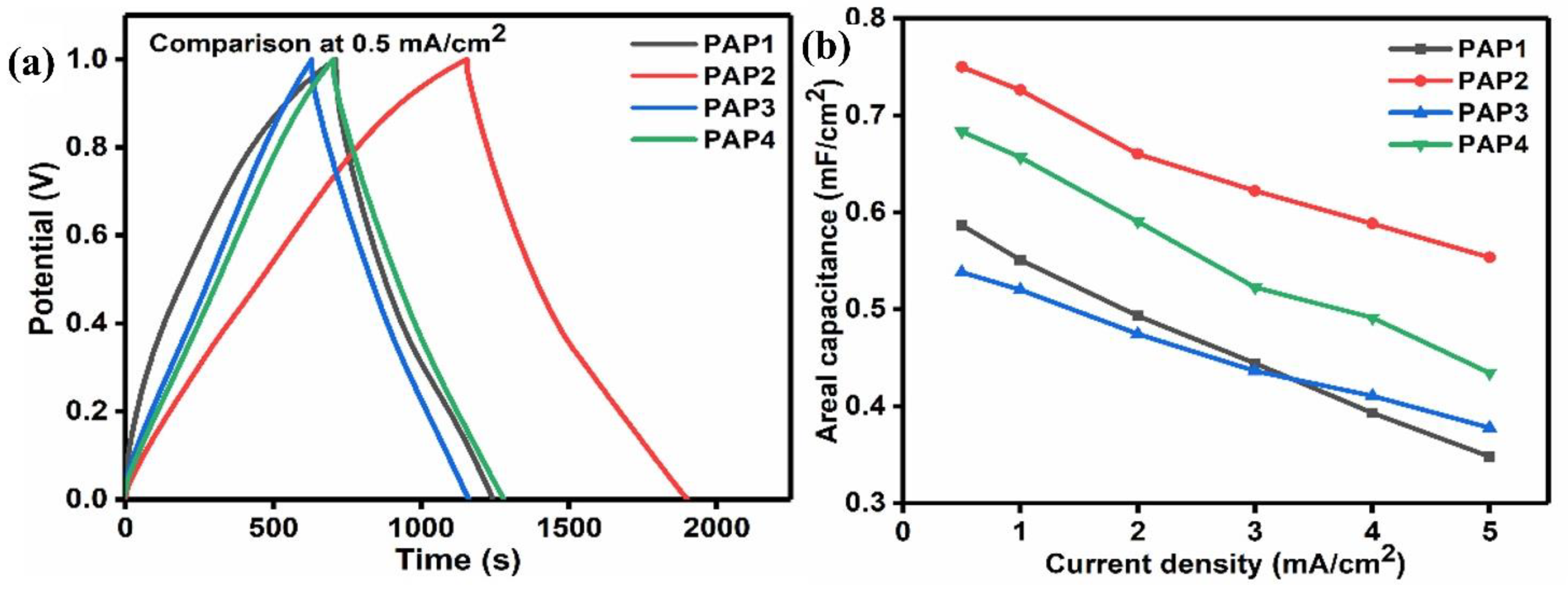
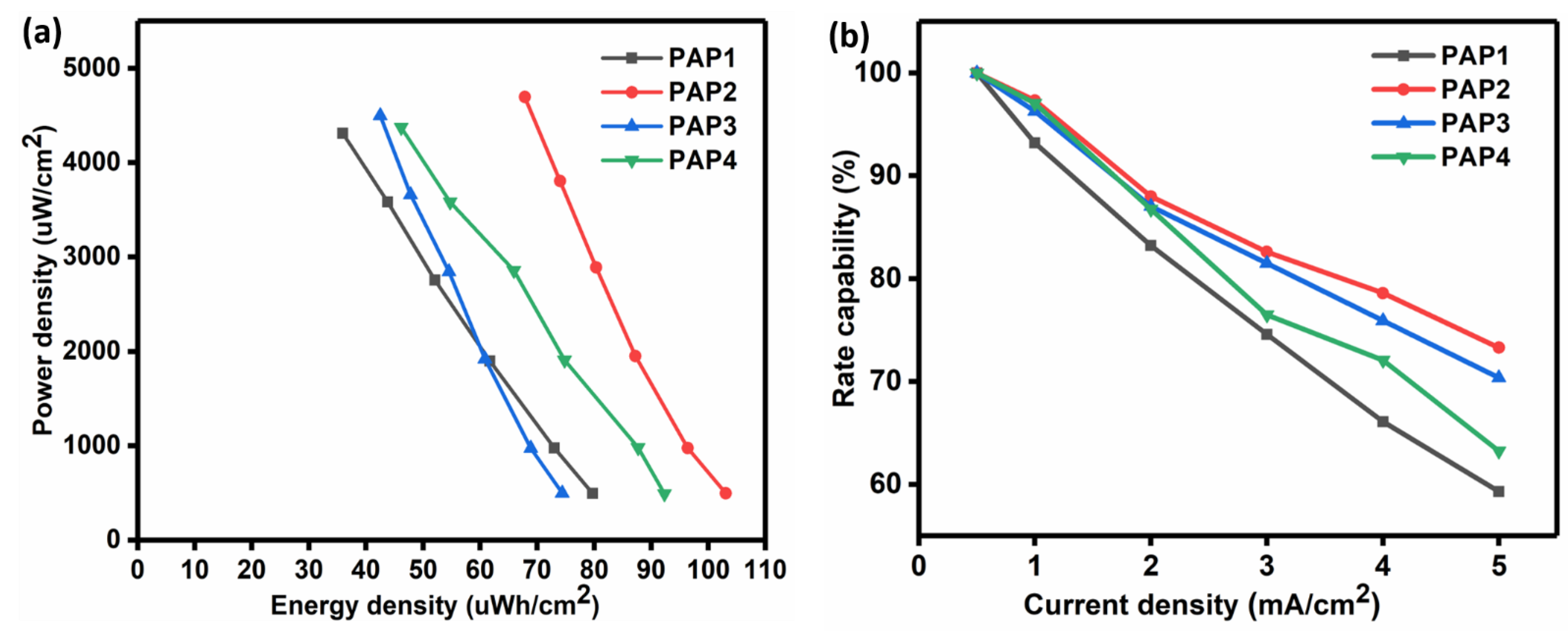
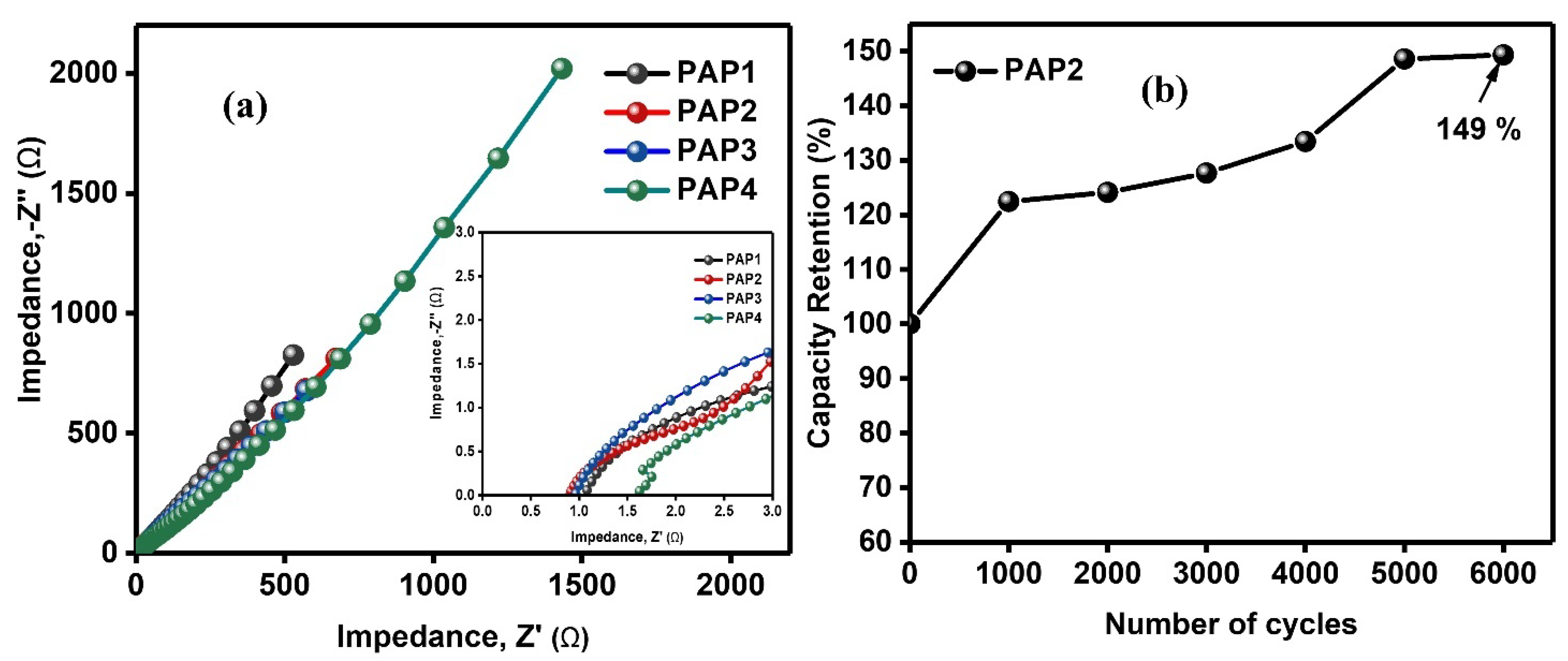
| Formulation | PVA 5 wt.% | Agar 5 wt.% | 1 M H2SO4 | GA 4 v/v% | Aniline 1 M | Pyrrole 1 M | APS 1 wt.% |
|---|---|---|---|---|---|---|---|
| PAP1 | 5 mL | 5 mL | 2 mL | 1 mL | 5 mL | 5 mL | 2 mL |
| PAP2 | 5 mL | 3 mL | 2 mL | 1 mL | 5 mL | 5 mL | 2 mL |
| PAP3 | 5 mL | 5 mL | 2 mL | 1 mL | 2.5 mL | 2.5 mL | 2 mL |
| PAP4 | 5 mL | 3 mL | 2 mL | 1 mL | 2.5 mL | 2.5 mL | 2 mL |
| Results | PAP1 | PAP2 | PAP3 | PAP4 |
|---|---|---|---|---|
| Areal capacitance ( | 586.75 | 750.13 | 538.39 | 683.43 |
| Energy density ) | 79.62 | 103.02 | 74.38 | 92.31 |
| Power density ( ) | 494.00 | 497.22 | 495.92 | 493.10 |
| Rate capability | 59.32 | 73.30 | 70.37 | 63.23 |
| Material | Reported Specific Capacitance |
|---|---|
| PVA–PANI prepared by physical mixing | 11.3 mF/cm2 |
| PVA–PANI prepared by freeze–thawing method | 420 mF/cm2 |
| N-doped nanocarbon material derived from PANI | 25.7 mF/cm2 |
| PVA–PANI hydrogel | 488 mF/cm2 |
| Our work | 750.13 mF/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, K.; Bashir, S.; Subramaniam, R.; Kasi, R.; Kamran, K.; Iqbal, J.; Algarni, H.; Al-Sehemi, A.G.; Wageh, S.; Pershaanaa, M.; et al. Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes. Polymers 2022, 14, 4784. https://doi.org/10.3390/polym14214784
Hasan K, Bashir S, Subramaniam R, Kasi R, Kamran K, Iqbal J, Algarni H, Al-Sehemi AG, Wageh S, Pershaanaa M, et al. Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes. Polymers. 2022; 14(21):4784. https://doi.org/10.3390/polym14214784
Chicago/Turabian StyleHasan, Khadija, Shahid Bashir, Ramesh Subramaniam, Ramesh Kasi, Kashif Kamran, Javed Iqbal, Hamed Algarni, Abdullah G. Al-Sehemi, S. Wageh, M. Pershaanaa, and et al. 2022. "Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes" Polymers 14, no. 21: 4784. https://doi.org/10.3390/polym14214784
APA StyleHasan, K., Bashir, S., Subramaniam, R., Kasi, R., Kamran, K., Iqbal, J., Algarni, H., Al-Sehemi, A. G., Wageh, S., Pershaanaa, M., & Kamarulazam, F. (2022). Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes. Polymers, 14(21), 4784. https://doi.org/10.3390/polym14214784