Automated Parallel Dialysis for Purification of Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reversible Addition–Fragmentation Chain-Transfer (RAFT) Polymerizations
2.3. Characterization
2.3.1. Size-Exclusion Chromatography (SEC)
2.3.2. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
2.4. VMR PP 4083 Peristaltic Pump
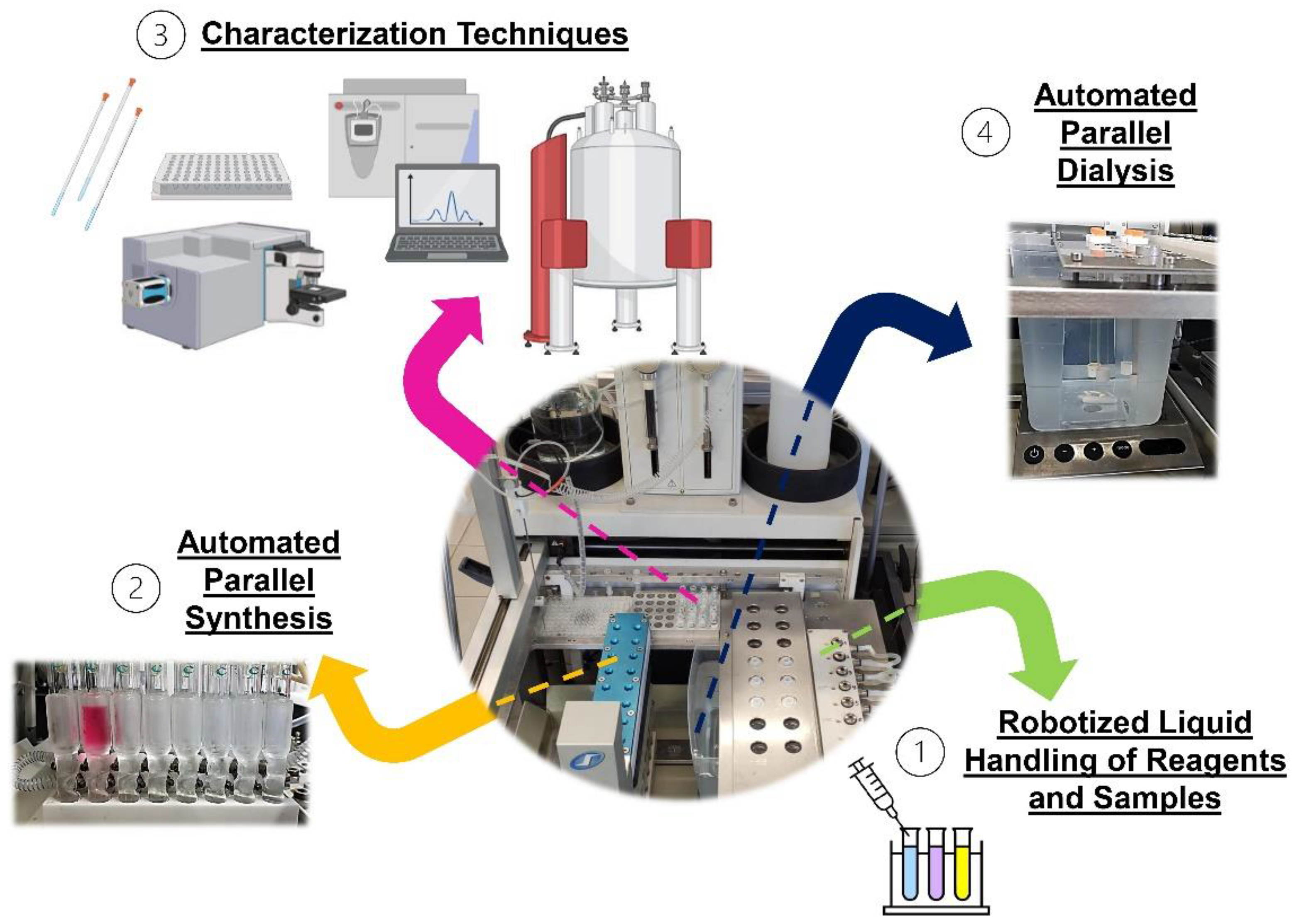
2.5. Chemspeed ASW2000 Automated Parallel Synthesizer (APS)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kimmig, J.; Zechel, S.; Schubert, U.S. Digital Transformation in Materials Science: A Paradigm Change in Material’s Development. Adv. Mater. 2021, 33, 2004940. [Google Scholar] [CrossRef]
- Lechuga-Islas, V.D.; Guerrero-Sanchez, C.; Guerrero-Santos, R.; Vitz, J.; Schubert, U.S. High-Throughput/High-Output Experimentation in Polymer Research. In Macromolecular Engineering; Wiley: New York, NY, USA, 2022; pp. 1–26. ISBN 9783527815562. [Google Scholar]
- Guerrero-Sanchez, C.; Yañez-Macias, R.; Rosales-Guzmán, M.; de Jesus-Tellez, M.A.; Piñon-Balderrama, C.; Haven, J.J.; Moad, G.; Junkers, T.; Schubert, U.S. High-Throughput/High-Output Experimentation in RAFT Polymer Synthesis. In RAFT Polymerization; Wiley: New York, NY, USA, 2021; pp. 1051–1076. ISBN 9783527821358. [Google Scholar]
- Guerrero-Sanchez, C.; Zhang, J.; Vitz, J.; Schubert, U.S. High-Throughput Synthesis of Polymers. In Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2018; pp. 1–21. ISBN 9780471440260. [Google Scholar]
- Armarego, W.L.F. Common Physical Techniques Used in Purification. In Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–70. [Google Scholar]
- Hagnauer, G.L.; Pearce, P.J. Effects of Impurities on Hydrolytic Stability and Curing Behavior. In Epoxy Resin Chemistry II; ACS Publications: Washington, DC, USA, 1983; pp. 193–209. [Google Scholar]
- Suzuki, Y.; Steinhart, M.; Kappl, M.; Butt, H.-J.; Floudas, G. Effects of Polydispersity, Additives, Impurities and Surfaces on the Crystallization of Poly(Ethylene Oxide)(PEO) Confined to Nanoporous Alumina. Polymer 2016, 99, 273–280. [Google Scholar] [CrossRef]
- Lechuga-Islas, V.D.; Trejo-Maldonado, M.; Stumpf, S.; Guerrero-Santos, R.; Elizalde-Herrera, L.; Schubert, U.S.; Guerrero-Sanchez, C. Separation of Volatile Compounds from Polymers by Physisorption. Eur. Polym. J 2021, 159, 110748. [Google Scholar] [CrossRef]
- Bittner, B.; Morlock, M.; Koll, H.; Winter, G.; Kissel, T. Recombinant Human Erythropoietin (RhEPO) Loaded Poly(Lactide-Co-Glycolide) Microspheres: Influence of the Encapsulation Technique and Polymer Purity on Microsphere Characteristics. Eur. J. Pharm. Biopharm. 1998, 45, 295–305. [Google Scholar] [CrossRef]
- Kedjarune, U.; Charoenworaluk, N.; Koontongkaew, S. Release of Methyl Methacrylate from Heat-Curved and Autopolymerized Resins: Cytotoxicity Testing Related to Residual Monomer. Aust. Dent. J. 1999, 44, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, A.; Bek, B.; Çevik, N.N.; Usanmaz, A. The Effect of Preparation Conditions of Acrylic Denture Base Materials on the Level of Residual Monomer, Mechanical Properties and Water Absorption. J. Dent. 1995, 23, 313–318. [Google Scholar] [CrossRef]
- Novais, P.M.R.; Giampaolo, E.T.; Vergani, C.E.; Machado, A.L.; Pavarina, A.C.; Jorge, J.H. The Occurrence of Porosity in Reline Acrylic Resins. Effect of Microwave Disinfection. Gerodontology 2009, 26, 65–71. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Jamshidi, K.; Ikada, Y. Effects of Residual Monomer on the Degradation OfDL-Lactide Polymer. Polym. Int. 1998, 46, 196–202. [Google Scholar] [CrossRef]
- Bittner, B.; Ronneberger, B.; Zange, R.; Volland, C.; Anderson, J.M.; Kissel, T. Bovine Serum Albumin Loaded Poly(Lactide-Co-Glycolide) Microsphe: The Influence of Polymer Purity on Particle Characteristics. J. Microencapsul. 1998, 15, 495–514. [Google Scholar] [CrossRef]
- Schwach, G.; Oudry, N.; Delhomme, S.; Lück, M.; Lindner, H.; Gurny, R. Biodegradable Microparticles for Sustained Release of a New GnRH Antagonist—Part I: Screening Commercial PLGA and Formulation Technologies. Eur. J. Pharm. Biopharm. 2003, 56, 327–336. [Google Scholar] [CrossRef]
- Mufula, A.I.; Neuse, E.W. Macromolecular Carriers for Methotrexate and Ferrocene in Cancer Chemotherapy. J. Inorg. Organomet. Polym. Mater. 2011, 21, 511–526. [Google Scholar] [CrossRef]
- Neufeld, C.H.H.; Marvel, C.S. The Use of Dialysis in Polymer Purification. J. Polym. Sci. A1 1966, 4, 2907–2908. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H.; Johns, S.R.; Willing, R.I. Fate of the Initiator in the Azobisisobutyronitrile-Initiated Polymerization of Styrene. Macromolecules 1984, 17, 1094–1099. [Google Scholar] [CrossRef]
- Zhang, C.; Bates, M.W.; Geng, Z.; Levi, A.E.; Vigil, D.; Barbon, S.M.; Loman, T.; Delaney, K.T.; Fredrickson, G.H.; Bates, C.M.; et al. Rapid Generation of Block Copolymer Libraries Using Automated Chromatographic Separation. J. Am. Chem. Soc. 2020, 142, 9843–9849. [Google Scholar] [CrossRef]
- Bahramian, B.; Ma, Y.; Rohanizadeh, R.; Chrzanowski, W.; Dehghani, F. A New Solution for Removing Metal-Based Catalyst Residues from a Biodegradable Polymer. Green Chem. 2016, 18, 3740–3748. [Google Scholar] [CrossRef]
- Quadri, G.P. Purification of Polymers from Solvents by Steam or Gas Stripping. Ind. Eng. Chem. Res. 1998, 37, 2850–2863. [Google Scholar] [CrossRef]
- Templeman, M.B.; Marshall, L.M. Dialysis of Certain Sugars through Cellophane. Science 1960, 132, 153–154. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Han, J.; Ni, L.; Tang, X.; Hu, Y.; Chen, T. Integrated Method of Thermosensitive Triblock Copolymer–Salt Aqueous Two Phase Extraction and Dialysis Membrane Separation for Purification of Lycium Barbarum Polysaccharide. Food Chem. 2016, 194, 257–264. [Google Scholar] [CrossRef]
- Kim, D.-M.; Choi, C.-Y. A Semicontinuous Prokaryotic Coupled Transcription/Translation System Using a Dialysis Membrane. Biotechnol. Prog. 1996, 12, 645–649. [Google Scholar] [CrossRef]
- Spirin, A.S.; Swartz, J.R. Cell-Free Protein Synthesis; Spirin, A.S., Swartz, J.R., Eds.; Wiley: New York, NY, USA, 2007; ISBN 9783527316496. [Google Scholar]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef]
- Leo, E.; Cameroni, R.; Forni, F. Dynamic Dialysis for the Drug Release Evaluation from Doxorubicin–Gelatin Nanoparticle Conjugates. Int. J. Pharm. 1999, 180, 23–30. [Google Scholar] [CrossRef]
- Kumar, R.; Le, N.; Tan, Z.; Brown, M.E.; Jiang, S.; Reineke, T.M. Efficient Polymer-Mediated Delivery of Gene-Editing Ribonucleoprotein Payloads through Combinatorial Design, Parallelized Experimentation, and Machine Learning. ACS Nano 2020, 14, 17626–17639. [Google Scholar] [CrossRef]
- Judzewitsch, P.R.; Zhao, L.; Wong, E.H.H.; Boyer, C. High-Throughput Synthesis of Antimicrobial Copolymers and Rapid Evaluation of Their Bioactivity. Macromolecules 2019, 52, 3975–3986. [Google Scholar] [CrossRef]
- Repligen Corporation Dialysis Technologies. Available online: https://www.repligen.com/technologies/dialysis (accessed on 4 October 2022).
- Aoki, M.; Matsuda, T.; Tomo, Y.; Miyata, Y.; Inoue, M.; Kigawa, T.; Yokoyama, S. Automated System for High-Throughput Protein Production Using the Dialysis Cell-Free Method. Protein Expr. Purif. 2009, 68, 128–136. [Google Scholar] [CrossRef]
- Schuett, T.; Geitner, R.; Zechel, S.; Schubert, U.S. Dialysis Diffusion Kinetics in Polymer Purification. Macromolecules 2021, 54, 9410–9417. [Google Scholar] [CrossRef]
- Schuett, T.; Kimmig, J.; Zechel, S.; Schubert, U.S. Automated Polymer Purification Using Dialysis. Polymers 2020, 12, 2095. [Google Scholar] [CrossRef]
- Schuett, T.; Kimmig, J.; Zechel, S.; Schubert, U.S. Fully Automated Multi-Step Synthesis of Block Copolymers. Polymers 2022, 14, 292. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Keddie, D.J. A Guide to the Synthesis of Block Copolymers Using Reversible-Addition Fragmentation Chain Transfer (RAFT) Polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- RAFT: Choosing the Right Agent to Achieve Controlled Polymerization. Available online: https://www.sigmaaldrich.com/DE/en/technical-documents/technical-article/materials-science-and-engineering/polymer-synthesis/raft-polymerization (accessed on 3 November 2022).
- Balakshin, M.; Capanema, E. On the Quantification of Lignin Hydroxyl Groups with 31P and 13C NMR Spectroscopy. J. Wood Chem. Technol. 2015, 35, 220–237. [Google Scholar] [CrossRef]
- Bak, J.M.; Lee, H. Novel Thermoresponsive Fluorinated Double-Hydrophilic Poly{[N-(2,2-Difluoroethyl)Acrylamide]-b-[N-(2-Fluoroethyl)Acrylamide]} Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1976–1982. [Google Scholar] [CrossRef]
- Xia, Z.; Akim, L.G.; Argyropoulos, D.S. Quantitative 13C NMR Analysis of Lignins with Internal Standards. J. Agric. Food Chem. 2001, 49, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, S.T.; Hatzis, A.; Rothchild, R. Quantitative NMR Assay for Aspirin, Phenacetin, and Caffeine Mixtures with 1,3,5-Trioxane as Internal Standard. J. Pharm. Biomed. Anal. 1986, 4, 147–154. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Lohmeijer, B.G.G.; Meier, M.A.R.; Schubert, U.S. Synthesis of Terpyridine-Terminated Polymers by Anionic Polymerization. Macromolecules 2005, 38, 10388–10396. [Google Scholar] [CrossRef]
- Ventura-Hunter, C.; Lechuga-Islas, V.D.; Ulbrich, J.; Kellner, C.; Schubert, U.S.; Saldívar-Guerra, E.; Rosales-Guzmán, M.; Guerrero-Sánchez, C. Glycerol Methacrylate-Based Copolymers: Reactivity Ratios, Physicochemical Characterization and Cytotoxicity. Eur. Polym. J. 2022, 178, 111478. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Abeln, C.; Schubert, U.S. Automated Parallel Anionic Polymerizations: Enhancing the Possibilities of a Widely Used Technique in Polymer Synthesis. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4151–4160. [Google Scholar] [CrossRef]
- Dias, M.L.; Mano, E.B.; Azuma, C. Size Exclusion Behavior of Polymers in Amide Solvents—III. Elution Characteristics of Acrylic Polymers in N,N-Dimethylformamide. Eur. Polym. J. 1997, 33, 559–564. [Google Scholar] [CrossRef]
- Gričar, M.; Žigon, M.; Žagar, E. Elution Behavior of Poly(Lactide-Co-Succinimide) Copolymers Studied by SEC-MALS. Anal. Bioanal. Chem. 2009, 393, 1815–1823. [Google Scholar] [CrossRef]
- Saikin, S.K.; Kreisbeck, C.; Sheberla, D.; Becker, J.S.; Aspuru-Guzik, A. Closed-Loop Discovery Platform Integration Is Needed for Artificial Intelligence to Make an Impact in Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 1–4. [Google Scholar] [CrossRef]
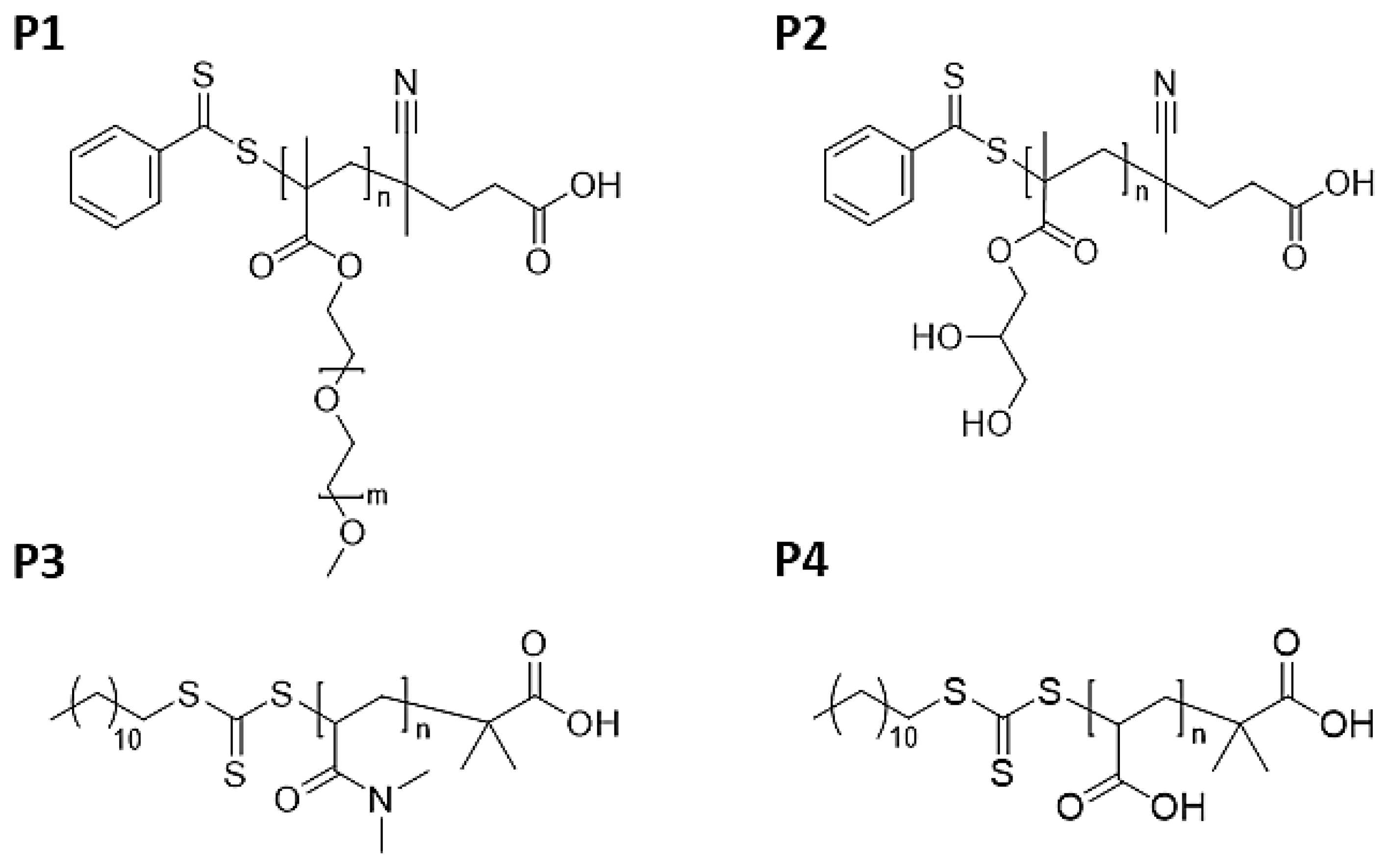



| Entry | Monomer (M) /mmol | Chain Transfer Agent (CTA) /mmol | Initiator (I) /mmol | Solvent/mL | M:CTA:I Ratio a | Temp. (°C) | Reaction Time (h) |
|---|---|---|---|---|---|---|---|
| P1 | PEGMA /3.50 | CPAD /0.1 | AIBN /0.01 | Ethanol /5.38 | 32:1:0.1 | 65 | 10 |
| P2 | GMMA /6.55 | CPAD /0.06 | ACVA /0.006 | Ethanol /6.03 | 109:1:0.1 | 70 | 7 |
| P3 | DMA /13.94 | DTMPA /0.08 | AIBN /0.008 | Dioxane /5.53 | 174:1:0.1 | 70 | 0.5 |
| P4 | AA /13.99 | DTMPA /0.06 | AIBN /0.006 | Ethanol /6.04 | 233:1:0.1 | 65 | 3.5 |
| Entry | Conversion a (%) | Dialysis Time (h) | Residual Monomer a,b (%) | Mn theo. c (kg mol−1) | Mn d (kg mol−1) | Ð d | Mn d (kg mol−1) | Ð d |
|---|---|---|---|---|---|---|---|---|
| Before Dialysis | After Dialysis | |||||||
| P1 | 43 | 72 | 2.44 | 7.38 | 7.6 | 1.11 | 7.8 | 1.10 |
| P2 | 55 | 93 | 1.33 | 9.34 | 19.1 | 1.11 | 25.4 | 1.26 |
| P3 | 46 | 48 | 0.83 | 8.09 | 8.2 | 1.11 | 7.9 | 1.12 |
| P4 | 51 | 12 | 0.32 | 8.79 | 11.5 e | 1.47 e | 12.3 e | 1.47 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzioğlu, İ.; Ventura-Hunter, C.; Ulbrich, J.; Saldívar-Guerra, E.; Schubert, U.S.; Guerrero-Sánchez, C. Automated Parallel Dialysis for Purification of Polymers. Polymers 2022, 14, 4835. https://doi.org/10.3390/polym14224835
Terzioğlu İ, Ventura-Hunter C, Ulbrich J, Saldívar-Guerra E, Schubert US, Guerrero-Sánchez C. Automated Parallel Dialysis for Purification of Polymers. Polymers. 2022; 14(22):4835. https://doi.org/10.3390/polym14224835
Chicago/Turabian StyleTerzioğlu, İpek, Carolina Ventura-Hunter, Jens Ulbrich, Enrique Saldívar-Guerra, Ulrich S. Schubert, and Carlos Guerrero-Sánchez. 2022. "Automated Parallel Dialysis for Purification of Polymers" Polymers 14, no. 22: 4835. https://doi.org/10.3390/polym14224835
APA StyleTerzioğlu, İ., Ventura-Hunter, C., Ulbrich, J., Saldívar-Guerra, E., Schubert, U. S., & Guerrero-Sánchez, C. (2022). Automated Parallel Dialysis for Purification of Polymers. Polymers, 14(22), 4835. https://doi.org/10.3390/polym14224835









