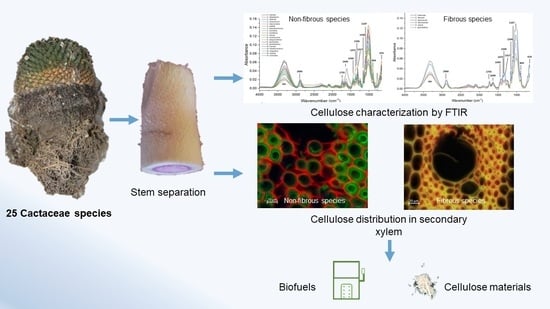
Cellulose in Secondary Xylem of Cactaceae: Crystalline Composition and Anatomical Distribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystalline Analysis of Cellulose
2.2. Epifluorescence Microscopy
3. Results
3.1. Cellulose Composition
3.2. Staining Methods for Fluorescence
3.3. Cellulose Distribution in Cells of the Secondary Xylem
4. Discussion
4.1. Crystalline Indexes
4.2. Staining Methods for Fluorescence
4.3. Cellulose Distribution and Crystalline Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, V.; McKee, L.S.; Bulone, V. Plant Cell Walls. In eLS.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–17. [Google Scholar]
- Bidhendi, A.J.; Chebli, Y.; Geitmann, A. Fluorescence visualization of cellulose and pectin in the primary plant cell wall. J. Microsc. 2020, 278, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Kuki, H.; Yokoyama, R.; Kuroha, T.; Nishitani, K. Xyloglucan is not essential for the formation and integrity of the cellulose network in the primary cell wall regenerated from Arabidopsis protoplasts. Plants 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J. Primary and secondary plant cell walls: A comparative overview. N. Zeal. J. For. Sci. 2006, 36, 36–53. [Google Scholar]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef] [Green Version]
- Kubicki, J.D.; Yang, H.; Sawada, D.; O’Neill, H.; Oehme, D.; Cosgrove, D. The shape of native plant cellulose microfibrils. Sci. Rep. 2018, 8, 13983. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef] [Green Version]
- Festucci-Buselli, R.A.; Otoni, W.C.; Joshi, C.P. Structure, organization, and functions of cellulose synthase complexes in higher plants. Brazilian J. Plant Physiol. 2007, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [Green Version]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020. [Google Scholar] [CrossRef]
- Carere, C.R.; Sparling, R.; Cicek, N.; Levin, D.B. Third generation biofuels via direct cellulose fermentation. Int. J. Mol. Sci. 2008, 9, 1342–1360. [Google Scholar] [CrossRef] [Green Version]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose-structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Alves, A.; Simoes, R.; Stackpole, D.J.; Vaillancourt, R.E.; Potts, B.M.; Schwanninger, M.; Rodrigues, J. Determination of the syringyl/guaiacyl ratio of Eucalyptus globulus wood lignin by near infrared-based partial least squares regression models using analytical pyrolysis as the reference method. J. Near Infrared Spectrosc. 2011, 19, 343–348. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Kesten, C.; Gámez-Arjona, F.M.; Menna, A.; Scholl, S.; Dora, S.; Huerta, A.I.; Huang, H.; Tintor, N.; Kinoshita, T.; Rep, M.; et al. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019, 38, e101822. [Google Scholar] [CrossRef]
- Hidalgo-Reyes, M.; Caballero-Caballero, M.; HernáNdez-Gómez, L.H.; Urriolagoitia-Calderón, G. Chemical and morphological characterization of Agave angustifolia bagasse fibers. Bot. Sci. 2015, 93, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Rivera, J.; Canché-Escamilla, G.; Soto-Hernández, M.; Terrazas, T. Wood chemical composition in species of Cactaceae the relationship between lignification and stem morphology. PLoS ONE 2015, 10, e0123919. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Terrazas, T. Chemical composition of cacti wood and comparison with the wood of other taxonomic groups. Chem. Biodivers. 2018, 15, e1700574. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Differences in the structural chemical composition of the primary xylem of Cactaceae: A topochemical perspective. Front. Plant Sci. 2019, 10, 1497. [Google Scholar] [CrossRef] [Green Version]
- Maceda, A.; Reyes-Rivera, J.; Soto-Hernández, M.; Terrazas, T. Distribution and chemical composition of lignin in secondary xylem of Cactaceae. Chem. Biodivers. 2021, 18, e2100431. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, M.; Carl, S.; Mayer, J.A.; Cushman, J.C.; Tian, E.; Lin, H. Biomass characterization of Agave and Opuntia as potential biofuel feedstocks. Biomass Bioenergy 2015, 76, 43–53. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin analysis by HPLC and FTIR. Methods Mol. Biol. 2017, 1544, 193–211. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Cichosz, S.; Masek, A. IR study on cellulose with the varied moisture contents: Insight into the supramolecular structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Kitin, P.; Nakaba, S.; Hunt, C.G.; Lim, S.; Funada, R. Direct fluorescence imaging of lignocellulosic and suberized cell walls in roots and stems. AoB Plants 2020, 12, plaa032. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 2007, 82, 209–216. [Google Scholar] [CrossRef]
- Nakaba, S.; Kitin, P.; Yamagishi, Y.; Begum, S.; Kudo, K.; Nugroho, W.D.; Funada, R. Three-dimensional imaging of cambium and secondary xylem cells by confocal laser scanning microscopy. In Plant Microtechniques and Protocols; Springer: Cham, Switzerland, 2015; pp. 431–465. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999; ISBN 0195089561. [Google Scholar]
- Loza-Cornejo, S.; Terrazas, T. Anatomía del tallo y de la raíz de dos especies de Wilcoxia Britton & Rose (Cactaceae) del noreste de México. Bot. Sci. 1996, 59, 13–23. [Google Scholar] [CrossRef]
- Baldacci-Cresp, F.; Spriet, C.; Twyffels, L.; Blervacq, A.; Neutelings, G.; Baucher, M.; Hawkins, S. A rapid and quantitative safranin-based fluorescent microscopy method to evaluate cell wall lignification. Plant J. 2020, 102, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring wood degradation during weathering by cellulose crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Pistor, V.; Santana, R.M.C.; Zattera, A.J. Materials produced from plant biomass: Part II: Evaluation of crystallinity and degradation kinetics of cellulose. Mater. Res. 2012, 15, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Hofmann, D.; Fink, H.P.; Philipp, B. Lateral crystallite size and lattice distortions in cellulose II samples of different origin. Polymer 1989, 30, 237–241. [Google Scholar] [CrossRef]
- Kljun, A.; Benians, T.A.S.; Goubet, F.; Meulewaeter, F.; Knox, J.P.; Blackburn, R.S. Comparative analysis of crystallinity changes in cellulose I polymers using ATR-FTIR, X-ray diffraction, and carbohydrate-binding module probes. Biomacromolecules 2011, 12, 4121–4126. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.R.; Ralph, S.A. Estimation of cellulose crystallinity of lignocelluloses using near-IR FT-Raman spectroscopy and comparison of the Raman and Segal-WAXS methods. J. Agric. Food Chem. 2013, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Orrabalis, C.; Rodríguez, D.; Pampillo, L.G.; Londoño-Calderón, C.; Trinidad, M.; Martínez-García, R. Characterization of nanocellulose obtained from Cereus forbesii (a South American cactus). Mater. Res. 2019, 22, 20190243. [Google Scholar] [CrossRef] [Green Version]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Biorefinery 2020, 12, 5165–5174. [Google Scholar] [CrossRef]
- Vignon, M.R.; Heux, L.; Malainine, M.-E.; Mahrouz, M. Arabinan-cellulose composite in Opuntia ficus-indica prickly pear spines. Carbohydr. Res. 2004, 339, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bustamante, M.Q.; Chanona-Pérez, J.J.; Güemes-Vera, N.; Cásarez-Santiago, R.; Pereaflores, M.J.; Arzate-Vázquez, I.; Calderón-Domínguez, G. Production and characterization of cellulose nanoparticles from nopal waste by means of high impact milling. Procedia Eng. 2017, 200, 428–433. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A. Rotational molding of biodegradable composites obtained with PLA reinforced by the wooden backbone of Opuntia ficus-indica cladodes. J. Appl. Polym. Sci. 2015, 132, 42447. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Habibi, Y.; Heux, L.; Mahrouz, M.; Vignon, M.R. Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2008, 72, 102–112. [Google Scholar] [CrossRef]
- Tribulová, T.; Kačík, F.; Evtuguin, D.V.; Čabalová, I.; Ďurkovič, J. The effects of transition metal sulfates on cellulose crystallinity during accelerated ageing of silver fir wood. Cellulose 2019, 26, 2625–2638. [Google Scholar] [CrossRef]
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77. [Google Scholar] [CrossRef] [Green Version]
- Durkovič, J.; Kačík, F.; Olčák, D.; Kučerová, V.; Krajňáková, J. Host responses and metabolic profiles of wood components in dutch elm hybrids with a contrasting tolerance to dutch elm disease. Ann. Bot. 2014, 114, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Hamann, T. The role of mechanoperception in plant cell wall integrity maintenance. Plants 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Polo, C.C.; Pereira, L.; Mazzafera, P.; Flores-Borges, D.N.A.; Mayer, J.L.S.; Guizar-Sicairos, M.; Holler, M.; Barsi-Andreeta, M.; Westfahl, H.; Meneau, F. Correlations between lignin content and structural robustness in plants revealed by X-ray ptychography. Sci. Rep. 2020, 10, 6023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.H.; Trevor Forsyth, V.; Šturcová, A.; Kennedy, C.J.; May, R.P.; Altaner, C.M.; Apperley, D.C.; Wess, T.J.; Jarvis, M.C. Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol. 2013, 161, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rytioja, J.; Hildén, K.; Yuzon, J.; Hatakka, A.; de Vries, R.P.; Mäkelä, M.R. Plant-polysaccharide-degrading enzymes from Basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef] [Green Version]
- Suchy, M.; Linder, M.B.; Tammelin, T.; Campbell, J.M.; Vuorinen, T.; Kontturi, E. Quantitative assessment of the enzymatic degradation of amorphous cellulose by using a quartz crystal microbalance with dissipation monitoring. Langmuir 2011, 27, 8819–8828. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Ruel, K.; Nishiyama, Y.; Joseleau, J.P. Crystalline and amorphous cellulose in the secondary walls of Arabidopsis. Plant Sci. 2012, 193, 48–61. [Google Scholar] [CrossRef]
- Edwards, E.J.; Donoghue, M.J. Pereskia and the origin of the cactus life-form. Am. Nat. 2006, 167, 777–793. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [Green Version]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.; Trejo, C.; Terrazas, T. Lignina: Composición, síntesis y evolución. Madera Bosques 2021, 27, e2722137. [Google Scholar] [CrossRef]
- Annis, S.L.; Goodwin, P.H. Production and regulation of polygalacturonase isozymes in Canadian isolates of Leptosphaeria maculans differing in virulence. Can. J. Plant Pathol. 1997, 19, 358–365. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.Y.; Huynh, C.-V.; North, G.B. Root contraction helps protect the “living rock” cactus Ariocarpus fissuratus from lethal high temperatures when growing in rocky soil. Am. J. Bot. 2010, 97, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Dlouhá, J.; Suryanegara, L.; Yano, H. The role of cellulose nanofibres in supercritical foaming of polylactic acid and their effect on the foam morphology. Soft Matter 2012, 8, 8704–8713. [Google Scholar] [CrossRef]
- Wegner, T.H.; Jones, P.E. Advancing cellulose-based nanotechnology. Cellulose 2006, 13, 115–118. [Google Scholar] [CrossRef]
- Robles, N.F.; Saucedo, A.R.; Delgado, E.; Sanjuán, R.; Turrado, J. Effect of cellulose microfibers on paper with high contents of recycled fiber. Rev. Mex. Ciencias For. 2014, 5, 70–78. [Google Scholar]
- Zheng, Y.; Pan, Z.; Zhang, R. Overview of biomass pretreatment for cellulosic ethanol production. Int. J. Agric. Biol. Eng. 2009, 2, 51–68. [Google Scholar] [CrossRef]
- Khamis, G.; Papenbrock, J. Newly established drought-tolerant plants as renewable primary products as source of bioenergy. Emirates J. Food Agric. 2014, 26, 1067–1080. [Google Scholar] [CrossRef]






| Subfamily | Species | Collection Number | Wood Type | Stem |
|---|---|---|---|---|
| Cactoideae | Coryphantha clavata (Scheidw.) Backeb. | BV2535 | Non-fibrous | Cylindrical |
| C. cornifera (DC.) Lem. | BV2534 | Non-fibrous | Cylindrical | |
| C. delaetiana (Quehl) A. Berger | BV2542 | Non-fibrous | Globose | |
| C. delicata L. Bremer | SA1927 | Non-fibrous | Cylindrical | |
| C. hintoriorum Dicht & A. Lüthy | BV2539 | Non-fibrous | Cylindrical | |
| C. macromeris (Engelmann) Lemaire | BV2600 | Non-fibrous | Globose | |
| C. pallida Britton & Rose | SA860 | Non-fibrous | Globose | |
| C. pseudoechinus Boed. | BV2543 | Non-fibrous | Cylindrical | |
| C. ramillosa Cutak | HSM3775 | Non-fibrous | Globose | |
| C. retusa Britton & Rose | SG55 | Non-fibrous | Globose | |
| Echinocereus cinerascens (DC.) Lem. subsp. tulensis | SA1744 | Non-fibrous | Cylindrical | |
| E. dasyacanthus Engelm. | SA2077 | Non-fibrous | Cylindrical | |
| E. pectinatus (Scheidw.) Engelm. | SA1918 | Non-fibrous | Cylindrical | |
| E. pentalophus (DC.) Lem. | SA1740 | Non-fibrous | Cylindrical | |
| Mammillaria carnea Zucc. Ex Pfeiff. | DA241 | Non-fibrous | Cylindrical | |
| M. dixathocentron Backeb. Ex Mottram | CPNL133 | Non-fibrous | Cylindrical | |
| M. magnifica Buchenau | UG1411 | Non-fibrous | Columnar | |
| M. mystax Mart. | DA238 | Non-fibrous | Cylindrical | |
| Neolloydia conoidea (DC.) Britton & Rose | BV2595 | Non-fibrous | Cylindrical | |
| Opuntioideae | Cylindropuntia imbricata (Haw.) F. M. Knuth | TT990 | Fibrous | Tree |
| C. kleiniae (DC.) F. M. Knuth | TT1000 | Fibrous | Shrub | |
| C. leptocaulis (DC.) F. M. Knuth | TT994 | Fibrous | Shrub | |
| Opuntia stenopetala Lem. | TT997 | Fibrous | Shrub | |
| O. stricta (Haw.) Haw. | TT998 | Fibrous | Shrub | |
| Pereskioideae | Leuenbergeria lychnidiflora (DC.) Lodé | TT967 | Fibrous | Tree |
| Wavenumber (cm−1) | Assignments |
|---|---|
| 3000–3600 | OH stretching [27] |
| 2900 | CH stretching [13,27] |
| 1430 | CH2 symmetric bending (crystalline and amorphous cellulose) [13,26,27] |
| 1372 | C-H and C-O bending vibration bonds [27] |
| 1336 | C-O-H in-plane bending (amorphous cellulose) [27] |
| 1315 | CH2 wagging vibration (crystalline cellulose) [26] |
| 1163 | C-O-C asymmetrical stretching [36] |
| 893 | Out-of-plane asymmetrical stretching of cellulose ring [13] |
| 670 | C-O-H out-of-plane stretching [37] |
| Crystalline Indexes | χ-Square | Df | Significance |
|---|---|---|---|
| TCI | 65.81053 | 24 | 9.25 × 10−6 |
| LOI | 52.00702 | 24 | 7.81 × 10−4 |
| HBI | 61.89333 | 24 | 3.44 × 10−5 |
| Species | TCI (A1370/A2900) | LOI (A1430/A893) | HBI (A3400/A1315) |
|---|---|---|---|
| C. pallida | 1.118 ± 0.019 a | 0.503 ± 0.007 a | 1.365 ± 0.032 a |
| C. clavata | 1.126 ± 0.012 ab | 0.507 ± 0.014 ab | 1.301 ± 0.048 ab |
| C. delaetiana | 1.263 ± 0.020 abcd | 0.515 ± 0.020 ab | 1.148 ± 0.018 ab |
| C. delicata | 1.227 ± 0.021 abcd | 0.523 ± 0.010 ab | 1.231 ± 0.033 ab |
| C. hintoriorum | 1.273 ± 0.022 abcd | 0.561 ± 0.009 ab | 1.131 ± 0.024 ab |
| C. macromeris | 1.291 ± 0.023 abcd | 0.565 ± 0.009 ab | 1.131 ± 0.024 ab |
| C. pseudoechinus | 1.197 ± 0.030 abcd | 0.540 ± 0.009 ab | 1.287 ± 0.030 ab |
| E. cinerascens | 1.198 ± 0.026 abcd | 0.538 ± 0.011 ab | 1.339 ± 0.037 ab |
| E. dasyacanthus | 1.234 ± 0.039 abcd | 0.535 ± 0.014 ab | 1.202 ± 0.034 ab |
| E. pentalophus | 1.165 ± 0.018 abcd | 0.540 ± 0.012 ab | 1.270 ± 0.028 ab |
| M. dixathocentron | 1.243 ± 0.021 abcd | 0.513 ± 0.012 ab | 1.279 ± 0.026 ab |
| M. magnifica | 1.219 ± 0.025 abcd | 0.532 ± 0.012 ab | 1.289 ± 0.025 ab |
| M. mystax | 1.179 ± 0.028 abcd | 0.511 ± 0.013 ab | 1.252 ± 0.017 ab |
| N. conoidea | 1.212 ± 0.015 abcd | 0.513 ± 0.012 ab | 1.280 ± 0.030 ab |
| C. imbricata | 1.141 ± 0.021 abcd | 0.526 ± 0.013 ab | 1.277 ± 0.021 ab |
| C. kleiniae | 1.192 ± 0.015 abcd | 0.528 ± 0.006 ab | 1.293 ± 0.034 ab |
| C. leptocaulis | 1.242 ± 0.021 abcd | 0.567 ± 0.010 ab | 1.250 ± 0.019 ab |
| O. stenopetala | 1.299 ± 0.028 abcd | 0.535 ± 0.011 ab | 1.185 ± 0.042 ab |
| O. stricta | 1.287 ± 0.023 abcd | 0.541 ± 0.012 ab | 1.139 ± 0.035 ab |
| L. lychnidiflora | 1.202 ± 0.034 abcd | 0.510 ± 0.010 ab | 1.270 ± 0.037 ab |
| M. carnea | 1.323 ± 0.024 abcd | 0.540 ± 0.009 ab | 1.097 ± 0.028 ab |
| C. ramillosa | 1.362 ± 0.027 abcd | 0.472 ± 0.011 a | 1.085 ± 0.036 ab |
| C. retusa | 1.486 ± 0.039 bcd | 0.569 ± 0.017 ab | 1.030 ± 0.019 ab |
| E. pectinatus | 1.606 ± 0.042 cd | 0.647 ± 0.015 b | 0.516 ± 0.007 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maceda, A.; Soto-Hernández, M.; Terrazas, T. Cellulose in Secondary Xylem of Cactaceae: Crystalline Composition and Anatomical Distribution. Polymers 2022, 14, 4840. https://doi.org/10.3390/polym14224840
Maceda A, Soto-Hernández M, Terrazas T. Cellulose in Secondary Xylem of Cactaceae: Crystalline Composition and Anatomical Distribution. Polymers. 2022; 14(22):4840. https://doi.org/10.3390/polym14224840
Chicago/Turabian StyleMaceda, Agustín, Marcos Soto-Hernández, and Teresa Terrazas. 2022. "Cellulose in Secondary Xylem of Cactaceae: Crystalline Composition and Anatomical Distribution" Polymers 14, no. 22: 4840. https://doi.org/10.3390/polym14224840