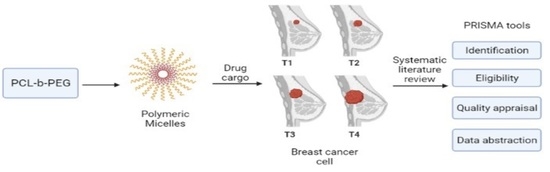
Poly(caprolactone)-b-poly(ethylene glycol)-Based Polymeric Micelles as Drug Carriers for Efficient Breast Cancer Therapy: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. The Review Protocol—PRISMA
2.2. Formulation of the Research Question
2.3. Resources
2.4. Systematic Searching Strategies
2.4.1. Identification
2.4.2. Screening
2.4.3. Eligibility
2.5. Quality Appraisal
2.6. Data Abstraction and Analysis
3. Results
3.1. Selected Articles’ Background
3.2. Themes and Sub-Themes
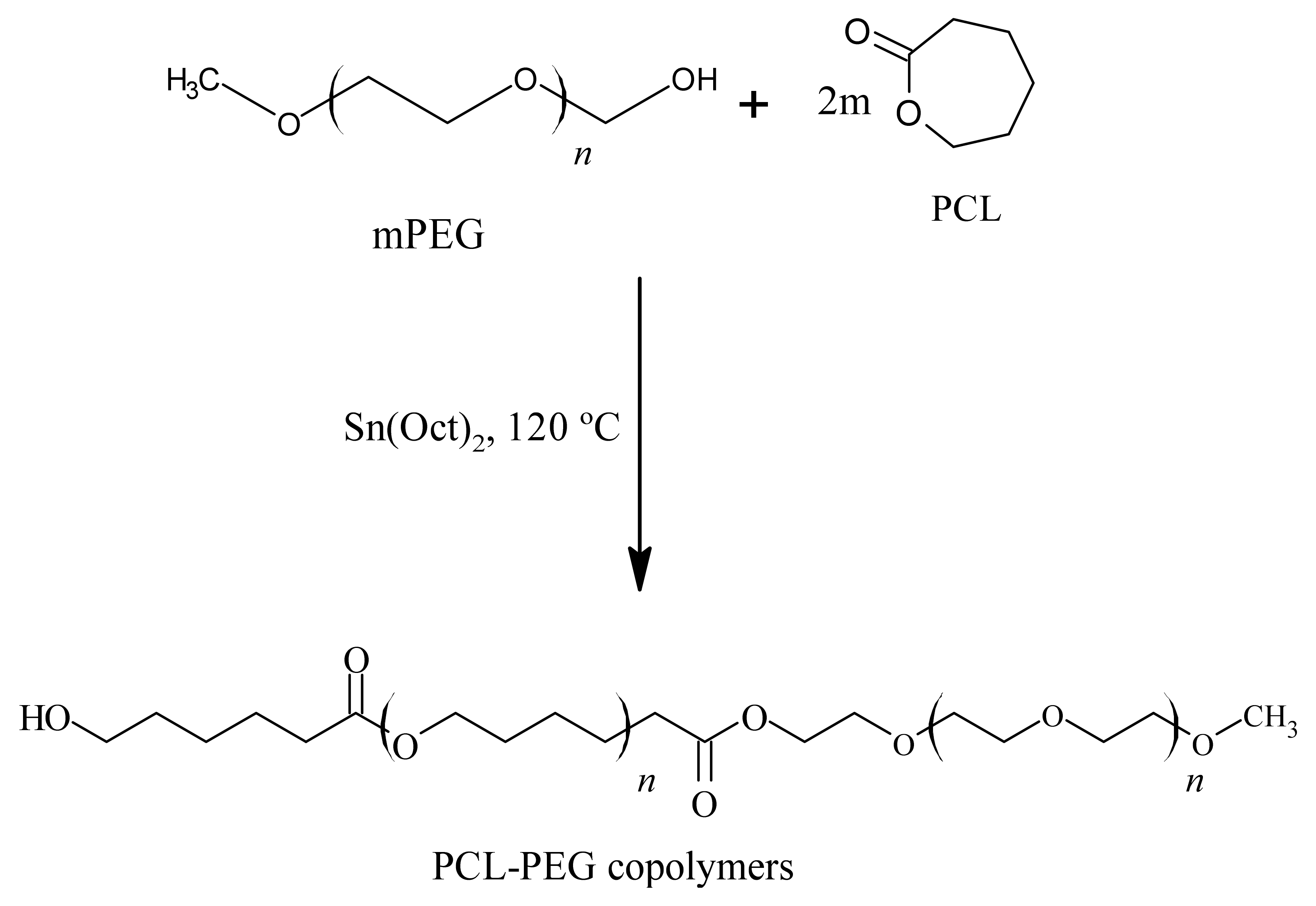
3.2.1. Synthesis and Characterisation of PCL-PEG Copolymers
3.2.2. Preparation of Micelles
3.2.3. Characterisation of Micelles
Morphology of Micelles
Particle Size and Zeta Potential
Drug Loading (DL) and Encapsulation Efficiency (EE)
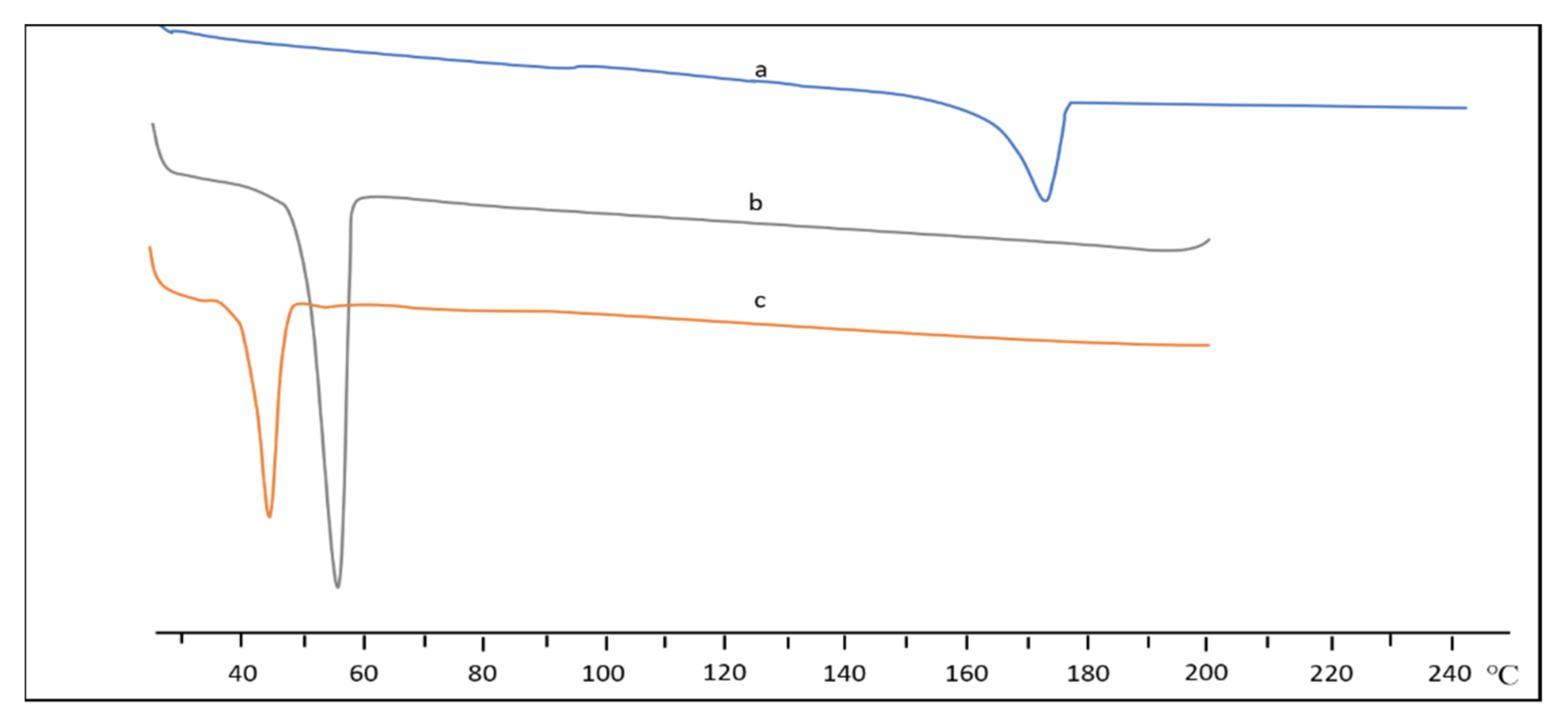
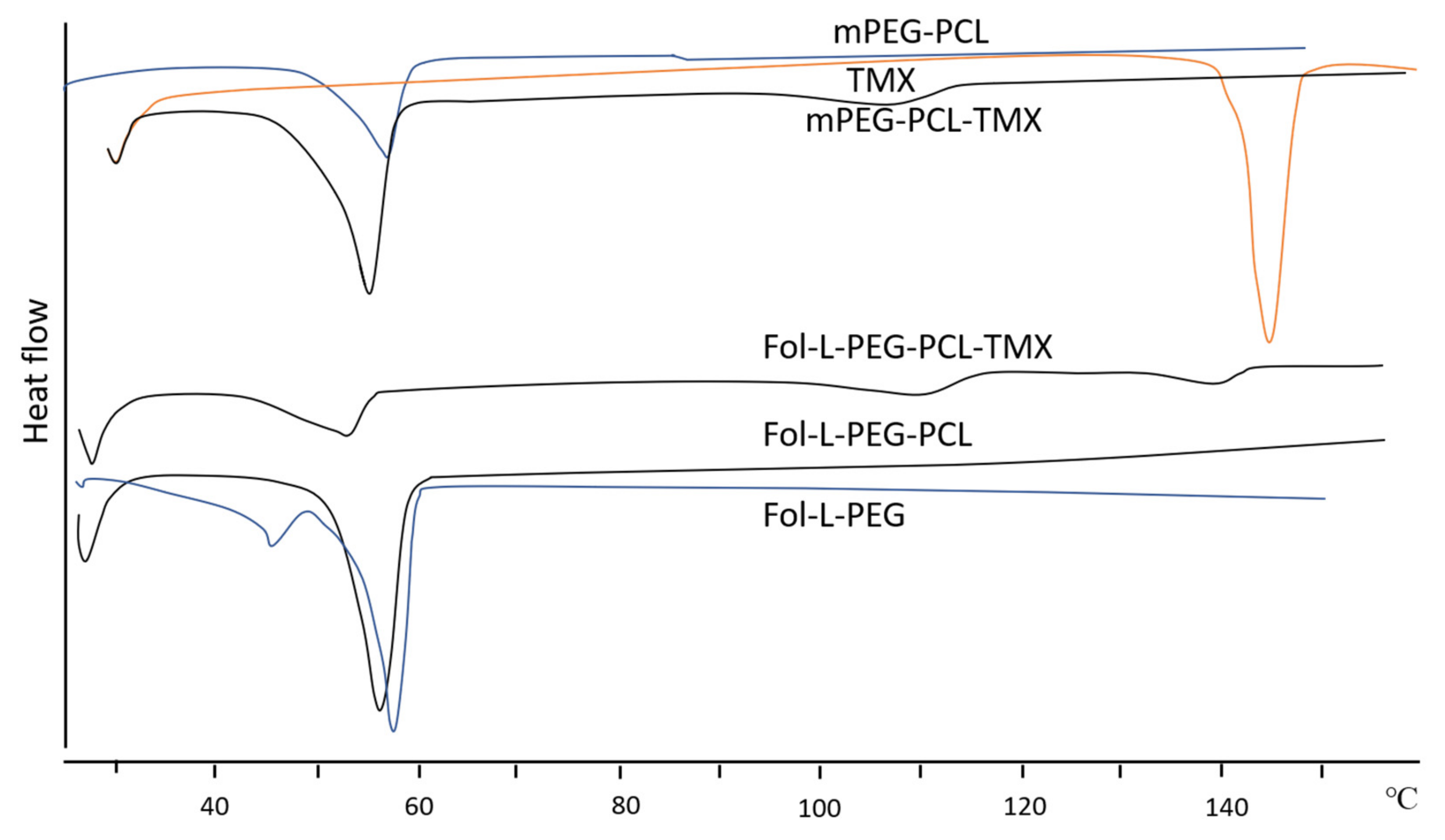
Thermal Analysis
3.2.4. Drug Release Study
Bioavailability of Drugs
3.2.5. Anti-Tumour Activity
Cell Viability and Cytotoxicity Study
Anti-Tumour Efficacy
4. Discussion
5. Recommendation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region 2000–2019; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- National Cancer Institute. Types of Cancer Treatment; National Institutes of Health (NIH): Rockville, MD, USA. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 20 June 2021).
- Bernstein, S. Chemotherapy: How the Drugs That Treat Cancer Work; WebMD LLC: New York, NY, USA, 2020; Available online: https://www.webmd.com/cancer/how-chemo-works (accessed on 10 August 2021).
- Wang, X.; Wu, Z.; Li, J.; Pan, G.; Shi, D.; Ren, J. Preparation, characterization, biotoxicity, and biodistribution of thermo-responsive magnetic complex micelles formed by Mn0.6Zn0.4Fe2O4 and a PCL/PEG analogue copolymer for controlled drug delivery. J. Mater. Chem. B 2016, 5, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cao, J.; Shen, A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Feng, C.; Huang, X. Polymer Brushes: Efficient Synthesis and Applications. Accounts Chem. Res. 2018, 51, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Shen, Y.; Leng, M.; Yu, H.; Zhang, Q.; Luo, X.; Gregersen, H.; Wang, G.; Liu, X. Effect of Amphiphilic PCL-PEG Nano-Micelles on HepG2 Cell Migration. Macromol. Biosci. 2014, 15, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Khoee, S.; Kavand, A. Preparation, co-assembling and interfacial crosslinking of photocurable and folate-conjugated amphiphilic block copolymers for controlled and targeted drug delivery: Smart armored nanocarriers. Eur. J. Med. Chem. 2014, 73, 18–29. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.R. Delivering Combination Chemotherapies and Targeting Oncogenic Pathways via Polymeric Drug Delivery Systems. Polymers 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Gou, M.; Wei, X.; Men, K.; Wang, B.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. PCL/PEG Copolymeric Nanoparticles: Potential Nanoplatforms for Anticancer Agent Delivery. Curr. Drug Targets 2011, 12, 1131–1150. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lee, H.J.; Park, S.; Kim, Y.; Jeong, B. Fast Degradable Polycaprolactone for Drug Delivery. Biomacromolecules 2018, 19, 2302–2307. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Cao, J.; Wu, Z.; Cheng, F.; Chen, Y.; Luo, X. In vitro and in vivo anti-tumor efficiency comparison of phosphorylcholine micelles with PEG micelles. Colloids Surf. B Biointerfaces 2017, 157, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, S.A.; Washington, K.E.; Biewer, M.C.; Stefan, M.C. PEG based anti-cancer drug conjugated prodrug micelles for the delivery of anti-cancer agents. J. Mater. Chem. B 2015, 4, 360–370. [Google Scholar] [CrossRef]
- Douglas, P.; Albadarin, A.; Sajjia, M.; Mangwandi, C.; Kuhs, M.; Collins, M.N.; Walker, G. Effect of poly ethylene glycol on the mechanical and thermal properties of bioactive poly(ε-caprolactone) melt extrudates for pharmaceutical applications. Int. J. Pharm. 2016, 500, 179–186. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, X.; Gong, T.; Zhang, Z.-R.; Fu, Y. d -Fructose Modification Enhanced Internalization of Mixed Micelles in Breast Cancer Cells via GLUT5 Transporters. Macromol. Biosci. 2017, 17, 1600529. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Dastanpour, L.; Tehrani, Z.M. Magnetic micellar nanocarrier based on pH-sensitive PEG-PCL-PEG triblock copolymer: A potential carrier for hydrophobic anticancer drugs. J. Nanopart. Res. 2018, 20, 282. [Google Scholar] [CrossRef]
- Yu, T.; Huang, X.; Liu, J.; Fu, Q.; Wang, B.; Qian, Z. Polymeric nanoparticles encapsulating α-mangostin inhibit the growth and metastasis in colorectal cancer. Appl. Mater. Today 2019, 16, 351–366. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Hou, J.; Jin, X.; Ke, Z.; Liu, D.; Du, M.; Jia, X.; Lv, H. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int. J. Nanomed. 2017, 12, 7653–7667. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gou, M.; Huang, M.; Huang, N.; Qian, Z.; Wei, X.; Wang, B.; Yang, B.; Men, K.; Rao, W.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef]
- Tazehkand, A.P.; Salehi, R.; Velaei, K.; Samadi, N. The potential impact of trigonelline loaded micelles on Nrf2 suppression to overcome oxaliplatin resistance in colon cancer cells. Mol. Biol. Rep. 2020, 47, 5817–5829. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sui, B.; Gou, J.; Liu, J.; Tang, X.; Xu, H.; Zhang, Y.; Jin, X. PSMA Ligand Conjugated PCL-PEG Polymeric Micelles Targeted to Prostate Cancer Cells. PLoS ONE 2014, 9, e112200. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Shaffril, H.A.M.; Krauss, S.E.; Samsuddin, S.F. A systematic review on Asian’s farmers’ adaptation practices towards climate change. Sci. Total Environ. 2018, 644, 683–695. [Google Scholar] [CrossRef]
- Mckenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2021. [Google Scholar]
- Busalim, A.H.; Hussin, A.R.C. Understanding social commerce: A systematic literature review and directions for further research. Int. J. Inf. Manag. 2016, 36, 1075–1088. [Google Scholar] [CrossRef]
- Siddaway, A.P.; Wood, A.M.; Hedges, L.V. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu. Rev. Psychol. 2018, 70, 703–718. [Google Scholar] [CrossRef]
- Kheiri Manjili, H.; Ghasemi, P.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur. J. Pharm. Biopharm. 2017, 116, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Manjili, H.K.; Sharafi, A.; Attari, E.; Danafar, H. Pharmacokinetics and in vitro and in vivo delivery of sulforaphane by PCL–PEG–PCL copolymeric-based micelles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif. Cells Nanomed. Biotechnol. 2017, 46, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Mahdaviani, P.; Bahadorikhalili, S.; Navaei-Nigjeh, M.; Vafaei, S.Y.; Esfandyari-Manesh, M.; Abdolghaffari, A.H.; Daman, Z.; Atyabi, F.; Ghahremani, M.H.; Amini, M.; et al. Peptide functionalized poly ethylene glycol-poly caprolactone nanomicelles for specific cabazitaxel delivery to metastatic breast cancer cells. Mater. Sci. Eng. C 2017, 80, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chin. Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef]
- Zamani, M.; Rostamizadeh, K.; Manjili, H.K.; Danafar, H. In vitro and in vivo biocompatibility study of folate-lysine-PEG-PCL as nanocarrier for targeted breast cancer drug delivery. Eur. Polym. J. 2018, 103, 260–270. [Google Scholar] [CrossRef]
- Kheiri, M.H.; Alimohammadi, N.; Danafar, H. Preparation of biocompatible copolymeric micelles as a carrier of atorvastatin and rosuvastatin for potential anticancer activity study. Pharm. Dev. Technol. 2018, 24, 303–313. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef]
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Synthesis and Multi-Responsive Self-Assembly of Cationic Poly(caprolactone)-Poly(ethylene glycol) Multiblock Copolymers. Chem.-A Eur. J. 2017, 23, 8166–8170. [Google Scholar] [CrossRef] [PubMed]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid Prepolymer-Based in Situ Formation of Degradable Poly(ethylene glycol)-Linked-Poly(caprolactone)-Linked-Poly(2-dimethylaminoethyl)methacrylate Amphiphilic Conetwork Gels Showing Polarity Driven Gelation and Bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhou, W.; Guo, X.; Zhong, R.; Wang, A.; Li, J.; Cen, Y.; You, C.; Tan, H.; Tian, M. Poly(ϵ-Caprolactone)-Methoxypolyethylene Glycol (PCL-MPEG)-Based Micelles for Drug-Delivery: The Effect of PCL Chain Length on Blood Components, Phagocytosis, and Biodistribution. Int. J. Nanomed. 2022, 17, 1613–1632. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Golder, S.; Rodriguez-Lopez, R. Citation searching: A systematic review case study of multiple risk behaviour interventions. BMC Med. Res. Methodol. 2014, 14, 73. [Google Scholar] [CrossRef]
- Horsley, T.; Dingwall, O.; Sampson, M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst. Rev. 2011, 2011, MR000026. [Google Scholar] [CrossRef]
- Tsafnat, G.; Glasziou, P.; Choong, M.K.; Dunn, A.; Galgani, F.; Coiera, E. Systematic review automation technologies. Syst. Rev. 2014, 3, 74. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Ioannidis, J.P.A. Content area experts as authors: Helpful or harmful for systematic reviews and meta-analyses? BMJ 2012, 345, e7031. [Google Scholar] [CrossRef]




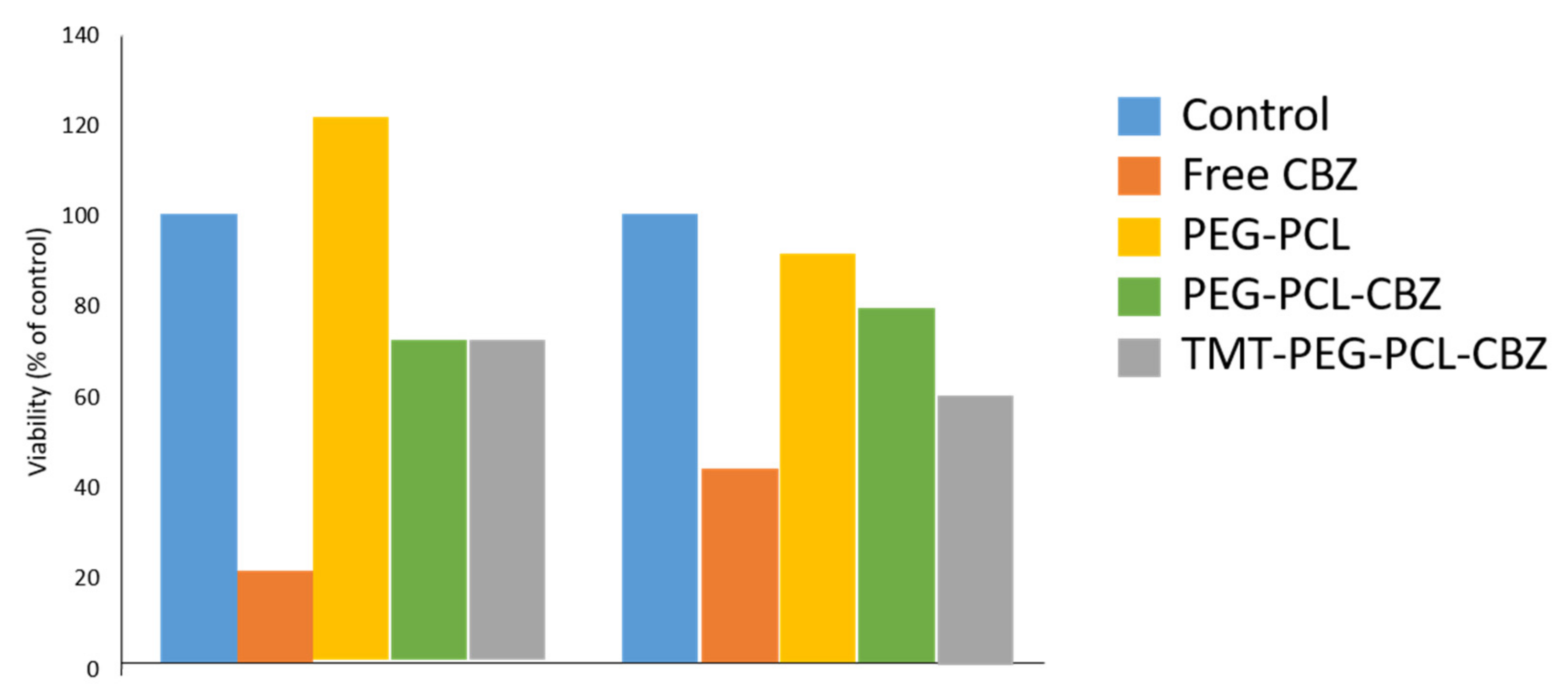
| Database | Search String |
|---|---|
| Scopus | TITLE-ABS-KEY (“PCL-PEG” OR “polycaprolactone polyethylene glycol” OR “PCLPEG”) AND (“micelle*s” OR “micellar”) AND (“drug delivery” OR “drug cargo” OR “drug carrier”) AND (“breast cancer”) |
| ScienceDirect | (“PCL-PEG” OR “polycaprolactone polyethylene glycol” OR “PCL PEG”) AND (“micelle” OR “micellar”) AND (“drug delivery” OR “drug cargo” OR “drug carrier”) AND (“breast cancer”) |
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Literature type | Journal article (empirical data) | Systematic reviews, review papers, meta-analyses, meta-syntheses, proceedings, books, book chapters, book series |
| Language | English | Non-English |
| Timeline | 2016–2021 | <2016 |
| Authors | Synthesis and Characterisation of PCL-PEG | Preparation of Micelles | Characterisation of Micelles | Drug Release Study | Anti-Tumour Activity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Morphology | Particle Size Distribution and Zeta Potential | Drug Loading and Encapsulation Efficiency | DSC Analysis | Cell Viability and Cytotoxicity Study | Anti-Tumour Efficacy | ||||
| Kheiri Manjili et al., (2016) [35] | / | / | / | / | / | / | / | / | / |
| Kheiri Manjili et al., (2017a) [36] | / | / | / | / | / | x | / | / | / |
| Kheiri Manjil et al., (2017b) [37] | / | / | / | / | / | / | / | / | / |
| Mahdaviani et al., (2017) [38] | / | / | / | / | / | x | x | / | / |
| Hu et al., (2017) [39] | / | / | / | / | / | / | x | x | / |
| Zamani et al., (2018) [40] | / | / | / | / | / | / | / | / | / |
| Kheiri Manjili et al., (2018) [41] | / | / | / | / | / | / | / | / | / |
| Peng et al., (2019) [42] | x | / | / | / | / | x | / | / | / |
| Authors | Amount of ε-CL | Amount of PEG | Catalyst | Reaction Condition | Drying Condition | Products Synthesised | GPC Analysis | DSC Analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn (Da) | Mw (Da) | PDI | Melting Temperature (°C) | |||||||
| Kheiri Manjili et al., (2016) [35] | 0.5, 1, 2, 4, 5, and 6 g | 1 g | Sn(Oct)2, 0.01 mol | 120 °C, 12 h, oil bath | 23 °C, 24 h | mPEG-PCL di-block copolymer | 9342–20,543 | 10,231–21,932 | 1.04–1.09 | 55.00 |
| Kheiri Manjili et al., (2017a) [36] | 1, 2, 4, 8, and 10 g | 2 g | Sn(Oct)2, 0.01 mol | 120 °C, 12 h, oil bath | 23 °C, 24 h | PCL-PEG-PCL tri-block copolymer | 8940–18,976 | 9731–21,321 | 1.05–1.12 | 54.72 |
| Kheiri Manjil et al., (2017b) [37] | 0.5, 1, and 2 g | 1 g | Sn(Oct)2, 0.01 mol | 120 °C, 12 h, oil bath | 23 °C, 24 h | PCL-PEG-PCL tri-block copolymer | 8722–11,257 | 10,200–15,961 | 1.16–1.41 | 52.34 |
| Kheiri Manjili et al., (2018) [41] | 2 g | 1 g | Sn(Oct)2, 0.01 mol | 120 °C, 12 h, oil bath | 23 °C, 24 h | PCL-PEG-PCL tri-block copolymer | 16,987 | 18,765 | 1.10 | 62.84 |
| Mahdaviani et al., (2017) [38] | Not mentioned | Not mentioned | 1.2 mmol EDC and 2.5 mmol NHS | Room temperature, 24 h | At reduced pressure | PCL-PEG di-block copolymer | Not stated | 45,000 | Not stated | - |
| Hu et al., (2017) [39] | Not mentioned | Not mentioned | Sn(Oct)2 | 120 °C, 48 h, oil bath | Vacuum-dried | PCL-PEG-PCL tri-block copolymer | 12,875–27,844 | 13,615–33,084 | 1.057–1.218 | Not stated |
| Zamani et al., (2018) [40] | 3 g | 1 g | Sn(Oct)2, 0.01 mol | 120 °C, 12 h | Room temperature, vacuum-dried | mPEG-PCL di-block copolymer | - | - | - | 57.0 |
| Authour | Method Preparation | Type of Drug | Solvent Used | Selected Micelles in the Study | Size (nm) | DL % | EE % | Zeta Potential | Melting Temperature (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Micelles | |||||||||
| Kheiri Manjili et al., (2016) [35] | Nanoprecipitation | Curcumin (CUR) | Acetone | 0.25 (CUR/copolymer mass ratio) | 173.56 | 45.92 | ||||
| Kheiri Manjili et al., (2017a) [36] | Nanoprecipitation | Sulforaphane (SF) | Acetone | 0.25 (SF/copolymer mass ratio) | 81.70 | 20.65 | 89.32 | −11.5 | - | - |
| Kheiri Manjil et al., (2017b) [37] | Nanoprecipitation | Artemisinin (ART) | Acetone | 0.25 (ART/copolymer mass ratio) | 114.00 | 19.33 | 87.1 | −7.57 | 154.35 | 52.34 |
| Kheiri Manjili et al., (2018) [41] | Nanoprecipitation | Atorvastatin and rosuvastatin | Acetone | Atorvastatin-loaded | 83.22 | 18.62 | 89.23 | −15.45 | 167.73 | 55.48 |
| Mahdaviani et al., (2017) [38] | Cosolvent evaporation | Cabazitaxel (CBZ) | Acetone | Rosuvastatin-loaded | 55.66 | 20.0 | 88.19 | −7.72 | 75.51 | 51.12 |
| Hu et al., (2017) [39] | Thin-film hydration and ultrasonic dispersion | Paclitaxel (PTX) | Methylene chloride | CBZ-loaded | 53.72 | 13.21 | 59.01 | −2.99 | - | - |
| Zamani et al., (2018) [40] | Cosolvent evaporation | Tamoxifen (TMX) | Acetone | PTX-loaded | 110.00 | 8.5 | 82.5 | Not mentioned | - | - |
| Peng et al., (2019) [42] | Emulsion solvent evaporation | Paclitaxel | Chloroform | 1:6 (TMX/copolymer mass ratio) | 255.80 | 8.87 | 87.97 | −17.9 | 142.00 | 55.00 |
| Authors | Special Solvent | Mobile Phase | Column | Temperature | Flow Rate | Sample Injection Volume | Sample Detection |
|---|---|---|---|---|---|---|---|
| Kheiri Manjili et al., (2017a) [36] | - | Acetonitrile and water (45:55, v/v) | C18 analytical column (250 mm × 4.6 mm, particle size 5 µm) | Not mentioned | 1.0 mL/min | 20 µL | λ max = 241 nm, SF |
| Kheiri Manjil et al., (2017b) [37] | - | Methanol and 5% (w/v) acetic acid (70:30, v/v) | C18 analytical column (150 mm × 4.6 mm, particle size 5 µm) | Not mentioned | 1.0 mL/min | 20 µL | λ max = 420 nm, ART |
| Mahdaviani et al., (2017) [38] | Acetonitrile (to dissolve CBZ) | Methanol | Agilent ZORBAX Eclipse Plus C18 column (5 μm, 4.6 mm × 150 mm) | Not mentioned | 1.0 mL/min | Not mentioned | λ max = 248 nm, CBZ |
| Hu et al., (2017) [39] | Acetonitrile (to dissolve PTX) | Acetonitirle and water (50:50, v/v) | Reverse-phase column (Symmetry, 150 mm × 4.6 mm, five μm) | 40 °C | 1.0 mL/min | Not mentioned | λ max = 227 nm, PTX |
| Peng et al., (2019) [42] | Acetonitrile (to dissolve PTX) | Acetonitrile and water (50:50, v/v) | C18 column (5 μm, 4.6 × 150 mm) | 30 °C | 1.0 mL/min | Not mentioned | λ max = 227 nm, PTX |
| Authors | Release Medium | pH | Molecular Weight Cut-Off | Incubation Temperature | Shaking Speed | Method of Drug Concentration Analysis | Cumulative Drug Release |
|---|---|---|---|---|---|---|---|
| Kheiri Manjili et al., (2016) [35] | PBS with 5% (v/v) Tween 80 | 7.4 | 120,000 Da | 37 °C | Not mentioned | UV-Vis | ~45.32% |
| 5.5 | ~76.8% | ||||||
| Human plasma | ~63.21% | ||||||
| Kheiri Manjili et al., (2017a) [36] | PBS | 7.4 | 120,000 Da | 37 °C | Not mentioned | HPLC | ~56.75% |
| 5.5 | ~65.75% | ||||||
| Human plasma | ~63.21% | ||||||
| Kheiri Manjil et al., (2017b) [37] | PBS with 2% (v/v) tween 80 | 7.4 | 120,000 Da | 37 °C | Not mentioned | HPLC | ~38.0% |
| 5.5 | ~50.0% | ||||||
| Human plasma | ~42.0% | ||||||
| Kheiri Manjili et al., (2018) [41] | PBS with 5% (v/v) Tween 80 | 7.4 | 12,000 Da | 37 °C | Not mentioned | UV-Vis | ~55.76 % |
| 5.5 | ~60.12 % | ||||||
| Zamani et al., (2018) [40] | PBS | 7.4 | 140,000 Da | 37 °C | 100 rpm | UV-Vis | ~25.0% |
| 5.5 | ~55.0% | ||||||
| Peng et al., (2019) [42] | PBS with 1% Tween 80 | 7.4 | 10,000 Da | 37 °C | 120 rpm | HPLC | ~60.0% |
| 6.5 | ~63.5% |
| Authors | Solution | Dose | Values of Area under the Plasma Concentration—Time Curve (AUC0-∞) | Apparent Volume of Distribution (Vd) |
|---|---|---|---|---|
| Kheiri Manjili et al., (2016) [35] | CUR aqueous solution | 50 mg/kg | 8.734 ± 1.09 h ng/mL | 8769.132 ± 1321.3 L/kg |
| CUR-loaded micelles | 488.17 ± 1.23 h ng/mL | 807.123 ± 342.9 L/kg | ||
| Kheiri Manjili et al., (2017a) [36] | SF aqueous solution | 30 mg/kg | 8.3425 ± 1.564 h ng/mL | 9420.132 ± 2221.3 L/kg |
| SF-loaded micelles | 465.87 ± 34.2 h ng/mL | 909.123 ± 252.1 L/kg | ||
| Kheiri Manjil et al., (2017b) [37] | ART aqueous solution | 25 mg/kg | 320 ± 4.02 h ng/mL | - |
| ART-loaded micelles | 5234 ± 1.13 h ng/mL | - | ||
| Peng et al., (2019) [42] | PTX-PCL-PEG-Her | 5 mg/kg | 1922.78 μg * h/L | 6.59 L/kg |
| PTX-PCL-PEG | 1874.83 μg * h/L | 11.93 L/kg | ||
| TAXOL® | 5591.67 μg * h/L | 3.32 L/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Shariff, S.H.; Wan Abdul Khodir, W.K.; Abd Hamid, S.; Haris, M.S.; Ismail, M.W. Poly(caprolactone)-b-poly(ethylene glycol)-Based Polymeric Micelles as Drug Carriers for Efficient Breast Cancer Therapy: A Systematic Review. Polymers 2022, 14, 4847. https://doi.org/10.3390/polym14224847
Ahmad Shariff SH, Wan Abdul Khodir WK, Abd Hamid S, Haris MS, Ismail MW. Poly(caprolactone)-b-poly(ethylene glycol)-Based Polymeric Micelles as Drug Carriers for Efficient Breast Cancer Therapy: A Systematic Review. Polymers. 2022; 14(22):4847. https://doi.org/10.3390/polym14224847
Chicago/Turabian StyleAhmad Shariff, Siti Hajar, Wan Khartini Wan Abdul Khodir, Shafida Abd Hamid, Muhammad Salahuddin Haris, and Mohamad Wafiuddin Ismail. 2022. "Poly(caprolactone)-b-poly(ethylene glycol)-Based Polymeric Micelles as Drug Carriers for Efficient Breast Cancer Therapy: A Systematic Review" Polymers 14, no. 22: 4847. https://doi.org/10.3390/polym14224847
APA StyleAhmad Shariff, S. H., Wan Abdul Khodir, W. K., Abd Hamid, S., Haris, M. S., & Ismail, M. W. (2022). Poly(caprolactone)-b-poly(ethylene glycol)-Based Polymeric Micelles as Drug Carriers for Efficient Breast Cancer Therapy: A Systematic Review. Polymers, 14(22), 4847. https://doi.org/10.3390/polym14224847