New Class of Polymer Materials—Quasi-Nematic Colloidal Particle Self-Assemblies: The Case of Assemblies of Prolate Spheroidal Poly(Styrene/Polyglycidol) Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Suspensions of P(S/PGL) Spheroids for Deposition on Glass/Silicon Supports
2.2.2. Determination of PVA Traces Irreversibly Adsorbed on the Surface of Spherical and Spheroidal Particles
2.2.3. Recording of UV-Vis Angle-Resolved Reflectance Spectra
2.2.4. Measurements of Water Contact Angle Deposited on Colloidal Multilayers
2.2.5. Measurements of Mechanical Properties by Nanoindentation Method
3. Results and Discussion
3.1. Preparation and Basic Characteristics of Spherical and Spheroidal P(S/PGL) Particles
Chemical Composition of (P/S/PGL) Microspheres and Microspheroids. Determination of Adsorbed PVA on the Particle Surface
3.2. Properties of Multilayers of Microspheroids
3.2.1. AFM Studies of Particle Interfacial Layers and Particle Assemblies
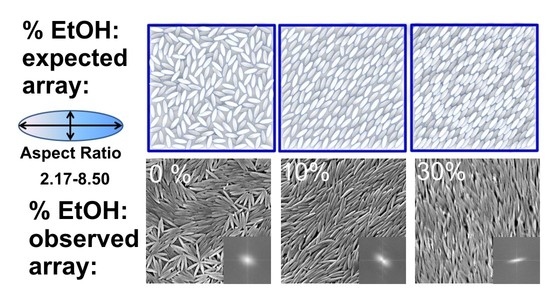
3.2.2. SEM Studies of Particle Assemblies
3.2.3. Angle-Resolved Reflectance Spectroscopy Studies of Particle Assemblies
3.2.4. Mechanical Properties of Particle Assemblies Determined by Nanoindentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.-H.; Park, H.S.; Choi, J.H.; Shim, J.W.; Yang, S.-M. Integration of colloidal photonic crystals toward miniaturized spectrometers. Adv. Mat. 2010, 22, 946–950. [Google Scholar] [CrossRef]
- Lee, H.S.; Shim, T.S.; Hwang, H.; Yang, S.-M.; Kim, S.-H. Colloidal photonic crystals toward structural color palettes for security materials. Chem. Mater. 2013, 25, 2684–2690. [Google Scholar] [CrossRef]
- Lee, K.; Asher, S.A. Photonic crystal chemical sensors: pH and ionic strength. J. Am. Chem. Soc. 2000, 122, 9534–9537. [Google Scholar] [CrossRef]
- Ben-Moshe, M.; Alexeev, V.L.; Asher, S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006, 78, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, M.; Boehm, A.K.; Grandjean, A.; Jung, G.; Gallei, M. Embedding Photoacids into Polymer Opal Structures: Synergistic effects on optical and stimuli-responsive features. Molecules 2021, 26, 7350. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wan, Y.; Wang, L.; Zhou, L.; Ma, N.; Qian, W. Construction of optical interference fibrin and thrombolysis analysis with silica colloidal crystal films. Langmuir 2021, 37, 7264–7272. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Wakayama, Y.; Onodera, T.; Takaya, Y.; Oikawa, H. Light propagation within colloidal crystal wire fabricated by a dewetting process. Nano Lett. 2008, 8, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Furst, E.M. Directed self-assembly. Soft Matter 2013, 9, 9039–9045. [Google Scholar] [CrossRef]
- Jiang, P.; Hwang, K.S.; Mittleman, D.M.; Bertone, J.F.; Colvin, V.L. Template-directed preparation of macroporous polymers with oriented and crystalline arrays of voids. J. Am. Chem. Soc. 1999, 121, 11630–11637. [Google Scholar] [CrossRef]
- Gu, Z.-Z.; Fujishima, A.; Sato, O. Fabrication of high-quality opal films with controllable thickness. Chem. Mater. 2002, 14, 760–765. [Google Scholar] [CrossRef]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Ibisate, M.; John, S.; Leonard, S.W.; Lopez, C.; Meseguer, F.; Miguez, H.; Mondia, J.P.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap near 1.5 micrometers. Nature 2000, 405, 437–439. [Google Scholar] [CrossRef]
- Park, S.H.; Xia, Y.N. Macroporous membranes with highly ordered and three-dimensionally interconnected spherical pores. Adv. Mater. 1998, 10, 1045–1048. [Google Scholar] [CrossRef]
- Velev, O.D.; Lenhoff, A.M.; Kaler, E.W. A class of microstructured particles through colloidal crystallization. Science 2000, 287, 2240–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massé, P.; Vallée, A.L.; Dechézelles, J.F.; Rosselgong, J.; Cloutet, E.; Cramail, H.; Zhao, X.S.; Ravaine, S. Effects of the position of a chemically or size-induced planar defect on the optical properties of colloidal crystals. J. Phys. Chem. C 2009, 113, 14487–14492. [Google Scholar] [CrossRef]
- Szamocki, R.; Massé, P.; Ravaine, S.; Ravaine, V.; Hempelmann, R.; Kuhn, A. Multicomponent macroporous materials with a controlled architecture. J. Mater. Chem. 2009, 19, 409–414. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.-W.; Lyu, Q.; Peng, B.; Zhong, R.; Zhao, M.; Xiong, B.; Yi, G.-R.; Zhang, L.; Zhu, J. Structure-tunable construction of colloidal photonic composites via kinetically controlled supramolecular crosslinking. Macromolecules 2022, 55, 8345–8354. [Google Scholar] [CrossRef]
- Ho, K.M.; Chan, C.T.; Soukoulis, C.M. Existence of a photonic gap in periodic dielectric structures. Phys. Rev. Lett. 1990, 65, 3152–3155. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, X.-H.; Gu, B.Y.; Yang, G.Z. Effect of shapes and orientations of scatterers and lattice symmetries on the photonic band gap in two-dimensional photonic crystals. J. Appl. Phys. 2001, 90, 4307–4313. [Google Scholar] [CrossRef]
- Gadzinowski, M.; Mickiewicz, D.; Basinska, T. Spherical versus prolate spheroidal particles in biosciences: Does the shape make a difference? Polym. Adv. Technol. 2021, 32, 3867–3876. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Li, Z.-Y.; Xia, Y. Colloidal crystals made of polystyrene spheroids: Fabrication and structural/optical characterization. Langmuir 2002, 18, 7722–7727. [Google Scholar] [CrossRef]
- Crassous, J.J.; Mihut, A.M.; Wernersson, E.; Pfleiderer, P.; Vermant, J.; Linse, P.; Schurtenberger, P. Field-induced assembly of colloidal ellipsoids into well-defined microtubules. Nat. Commun. 2014, 5, 5516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.A.; Kang, H.; Kohlstedt, K.L.; Ahn, K.H.; Glotzer, S.C.; Monroe, C.W.; Solomon, M.J. Liquid crystal order in colloidal suspensions of spheroidal particles by direct current electric field assembly. Small 2012, 8, 1551–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Besseling, T.H.; Hermes, M.; Demirörs, F.; Imhof, A.; van Blaaderen, A. Switching plastic crystals of colloidal rods with electric fields. Nat. Commun. 2014, 5, 3092. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Song, K.; Clays, K.; Tung, C.-H. Fabrication of 3D photonic crystals of ellipsoids: Convective self-assembly in magnetic field. Adv. Mater. 2009, 21, 1936–1940. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chow, E.; Hietala, V.; Villeneuve, P.R.; Joannopoulos, J.D. Experimental demonstration of guiding and bending of electromagnetic waves in a photonic crystal. Science 1998, 282, 274–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lele, P.P.; Furst, E.M. Assemble-and-stretch method for creating two- and three-dimensional structures of anisotropic particles. Langmuir 2009, 25, 8875–8878. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, Y.I.; Drozdov, A.S.; Solovyeva, A.S.; Fakhardo, A.F.; Vinogradov, V.V. Polyelectrolyte-based magnetic photonic crystals with anticoagulant activity. Mater. Today Chem. 2020, 100, 292. [Google Scholar] [CrossRef]
- Mukhija, D.; Solomon, M.J. Nematic order in suspensions of colloidal rods by application of a centrifugal field. Soft Matter 2011, 7, 540–545. [Google Scholar] [CrossRef]
- Savenko, S.V.; Dijkstra, M. Sedimentation and multiphase equilibria in suspensions of colloidal hard rods. Phys. Rev. E 2004, 70, 051401. [Google Scholar] [CrossRef] [Green Version]
- Donev, A.; Cisse, I.; Sachs, D.; Variano, E.A.; Stillinger, F.H.; Connelly, R.; Torquato, S.; Chaikin, P.M. Improving the density of jammed disordered packings using ellipsoids. Science 2004, 303, 990–993. [Google Scholar] [CrossRef]
- Man, W.; Donev, A.; Stillinger, F.H.; Sullivan, M.T.; Russel, W.B.; Heeger, D.; Inati, S.; Torquato, S.; Chaikin, P.M. Experiments on random packings of ellipsoids. Phys. Rev. Lett. 2005, 94, 198001. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, D.; Basinska, T.; Gosecka, M.; Gadzinowski, M.; Slomkowski, S. Colloidal liquid crystal type assemblies of spheroidal polystyrene core/polyglycidol-rich shell particles (P[S/PGL]) formed at the liquid-silicon-air interface by a directed dewetting process. Polym. Adv. Technol. 2019, 30, 1724–1731. [Google Scholar] [CrossRef]
- Wang, J.; Schwenger, J.; Ströbel, A.; Feldner, P.; Herre, P.; Romeis, S.; Peukert, W.; Merle, B.; Vogel, N. Mechanics of colloidal supraparticles under compression. Sci. Adv. 2021, 7, eabj0954. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, H.; Thompson, M.P.; Xiao, M.; Allen, M.C.; Zhou, X.; Ni, Q.Z.; Wang, Z.; Li, W.; Burkart, M.D.; et al. Structurally colored inks from synthetic melanin-based crosslinked supraparticles. ACS Mater. Lett. 2021, 3, 50–55. [Google Scholar] [CrossRef]
- Ho, C.C.; Keller, A.; Odell, J.A.; Ottewill, R.H. Preparation of monodisperse ellipsoidal polystyrene particles. Coll. Polym. Sci. 1993, 271, 469–479. [Google Scholar] [CrossRef]
- Komar, P.; Gosecka, M.; Gadzinowski, M.; Gosecki, M.; Makowski, T.; Slomkowski, S.; Basinska, T. Core-shell spheroidal microparticles with polystyrene cores and rich in polyglycidol shells. Polymer 2018, 146, 6–11. [Google Scholar] [CrossRef]
- Fariyal, A.; Alexandridis, P.; Neelamegham, S. Synthesis and application of fluorescein-labeled Pluronic block copolymers to the study of polymer−surface interactions. Langmuir 2001, 17, 537–546. [Google Scholar] [CrossRef]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Nanoindentation study of polydimethylsiloxane elastic modulus using Berkovich and flat punch tips. J. Appl. Polym. Sci. 2015, 132, 41384. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.A. Anisotropy of Young’s modulus and Poisson’s ratio in diamond. Mater. Res. Bull. 1992, 27, 1407–1414. [Google Scholar] [CrossRef]
- Gosecka, M.; Basinska, T.; Slomkowski, S.; Tracz, A.; Chehimi, M.M. Mechanism of particle formation in radical emulsion copolymerization of styrene with α-tert-butoxy-ω-vinylbenzyl-polyglycidol macromonomer. Polymer 2014, 55, 788–797. [Google Scholar] [CrossRef]
- Basinska, T.; Slomkowski, S.; Dworak, A.; Panchev, I.; Chehimi, M.M. Synthesis and characterization of poly(syrene/α-t-butoxy-ω-vinylbenzyl-polyglycidol) microspheres. Colloid Polym. Sci. 2001, 279, 916–924. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Madivala, B.; Fransaer, J.; Vermant, J. Self-assembly and rheology of ellipsoidal particles at interfaces. Langmuir 2009, 25, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Lehle, H.; Noruzifar, E.; Oettel, M. Ellipsoidal particles at fluid interfaces. Eur. Phys. J. E 2008, 26, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Loudet, J.C.; Alsayed, A.M.; Zhang, J.; Yodh, A.G. Capillary interactions between anisotropic colloidal particles. Phys. Rev. Lett. 2005, 94, 018301. [Google Scholar] [CrossRef] [PubMed]
- Loudet, J.C.; Pouligny, B. How do mosquito eggs self-assemble on the water surface? Eur. Phys. J. E 2011, 34, 76. [Google Scholar] [CrossRef] [PubMed]
- Griffete, N.; Dybkowska, M.; Glebocki, B.; Basinska, T.; Connan, C.; Maître, A.; Chehimi, M.M.; Slomkowski, S.; Mangeney, C. Thermoresponsive colloidal crystals built from core-shell poly(styrene/α-tert-butoxy-ω-vinylbenzylpolyglycidol) microspheres. Langmuir 2010, 26, 11550–11557. [Google Scholar] [CrossRef]
- Ishii, M.; Harada, M.; Tsukigase, A.; Nakamura, H. Photonic band structures of colloidal crystals measured with angle-resolved reflection spectroscopy. Colloids Surf. B Biointerfaces 2007, 56, 224–230. [Google Scholar] [CrossRef]
- Nakamura, H.; Ishii, M. Effects of medium composition on optical properties and microstructures of non-close-packed colloidal crystalline arrays. Colloid Polym. Sci. 2007, 285, 833–837. [Google Scholar] [CrossRef]
- Nakamura, H.; Ishii, M. Optical properties of colloidal crystalline arrays composed of hollow polystyrene spheres. J. Appl. Polym. Sci. 2007, 103, 2364–2368. [Google Scholar] [CrossRef]
- Schutzmann, S.; Venditti, I.; Prosposito, P.; Casalboni, M.; Russo, M.V. High-energy angle resolved reflection spectroscopy on three-dimensional photonic crystals of self-organized polymeric nanospheres. Opt. Express 2008, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Nemtsev, I.V.; Tambasov, I.A.; Ivanenko, A.A.; Zyryanov, V.Y. Angle-resolved reflection spectroscopy of high-quality PMMA opal crystal. Photonics Nanostruct.-Fundam. Appl. 2018, 28, 37–44. [Google Scholar] [CrossRef]
- Pfleiderer, P.; Schilling, T. Simple monoclinic crystal phase in suspensions of hard ellipsoids. Phys. Rev. E 2007, 75, 020402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitton, A.O.; Hill, J.; Jane, D.E.; Millar, R. Synthesis of simple oxetanes carrying reactive 2-substituents. Synthesis-Stuttgart 1987, 12, 1140–1142. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Varma, R.S. Solvent-free tetrahydropyranylation (THP) of alcohols and phenols and their regeneration by catalytic aluminium chloride hexahydrate. Tetrahedron Lett. 2002, 43, 1143–1146. [Google Scholar] [CrossRef]
- Halacheva, S.; Rangelov, S.; Tsvetanov, C. Poly(glycidol)-based analogues to Pluronic based copolymers. Synthesis and aqueous solution properties. Macromolecules 2006, 39, 6845–6852. [Google Scholar] [CrossRef]
- Basinska, T.; Slomkowski, S. Design of polyglycidol-containing microspheres for biomedical applications. Chem. Pap. 2012, 66, 352–368. [Google Scholar] [CrossRef]











| Symbol of Particles | Final Length of PVA Film Stripe, mm | PVA Film Elongation, % | AR of Spheroids | Length of Long Axis, nm | Std. Dev. | Length of Short Axis, Nm | Std. Dev. |
|---|---|---|---|---|---|---|---|
| P(S/PGL)s1 | 90 | 150 | 2.17 | 606 | 0.066 | 279 | 0.025 |
| P(S/PGL)s2 | 120 | 200 | 3.89 | 935 | 0.116 | 240 | 0.026 |
| P(S/PGL)s3 | 170 | 283 | 6.41 | 1419 | 0.148 | 221 | 0.025 |
| P(S/PGL)s4 | 220 | 366 | 8.50 | 1620 | 0.153 | 190 | 0.023 |
| Symbol of Particles | AR | C%, from XPS | O%, from XPS | fPGL, M, Calculated from XPS Data | PVA Irreversibly Adsorbed on Microspheres and Microspheroids *, % (wt/wt) |
|---|---|---|---|---|---|
| P(S/PGL)m | 1.00 | 92.28 | 7.43 | 0.216 | - |
| P(S/PGL)m-PVA | 1.00 | 86.89 | 12.89 | - | 5.0 |
| P(S/PGL)s3 | 6.41 | 91.71 | 8.14 | - | 5.8 |
| Symbol of Particles | Short Axis of Microspheroid, nm | EtOH Content in Suspending Water/Etanol Medium, % v/v | Interplanar Distance (d), nm |
|---|---|---|---|
| P(S/PGL)s1 | 279 | 0 | 169 |
| 10 | 175 | ||
| 20 | 175 | ||
| P(S/PGL)s2 | 240 | 0 | 164 |
| 10 | 173 | ||
| 20 | 166 | ||
| P(S/PGL)s3 | 221 | 0 | 164 |
| 10 | 174 | ||
| 20 | 167 | ||
| P(S/PGL)s4 | 190 | 0 | 216 |
| 10 | 223 | ||
| 20 | 211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickiewicz, D.; Gadzinowski, M.; Makowski, T.; Szymański, W.; Slomkowski, S.; Basinska, T. New Class of Polymer Materials—Quasi-Nematic Colloidal Particle Self-Assemblies: The Case of Assemblies of Prolate Spheroidal Poly(Styrene/Polyglycidol) Particles. Polymers 2022, 14, 4859. https://doi.org/10.3390/polym14224859
Mickiewicz D, Gadzinowski M, Makowski T, Szymański W, Slomkowski S, Basinska T. New Class of Polymer Materials—Quasi-Nematic Colloidal Particle Self-Assemblies: The Case of Assemblies of Prolate Spheroidal Poly(Styrene/Polyglycidol) Particles. Polymers. 2022; 14(22):4859. https://doi.org/10.3390/polym14224859
Chicago/Turabian StyleMickiewicz, Damian, Mariusz Gadzinowski, Tomasz Makowski, Witold Szymański, Stanislaw Slomkowski, and Teresa Basinska. 2022. "New Class of Polymer Materials—Quasi-Nematic Colloidal Particle Self-Assemblies: The Case of Assemblies of Prolate Spheroidal Poly(Styrene/Polyglycidol) Particles" Polymers 14, no. 22: 4859. https://doi.org/10.3390/polym14224859
APA StyleMickiewicz, D., Gadzinowski, M., Makowski, T., Szymański, W., Slomkowski, S., & Basinska, T. (2022). New Class of Polymer Materials—Quasi-Nematic Colloidal Particle Self-Assemblies: The Case of Assemblies of Prolate Spheroidal Poly(Styrene/Polyglycidol) Particles. Polymers, 14(22), 4859. https://doi.org/10.3390/polym14224859