Effect of Ultraviolet—A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators
Abstract
:1. Introduction
2. Experimental Works
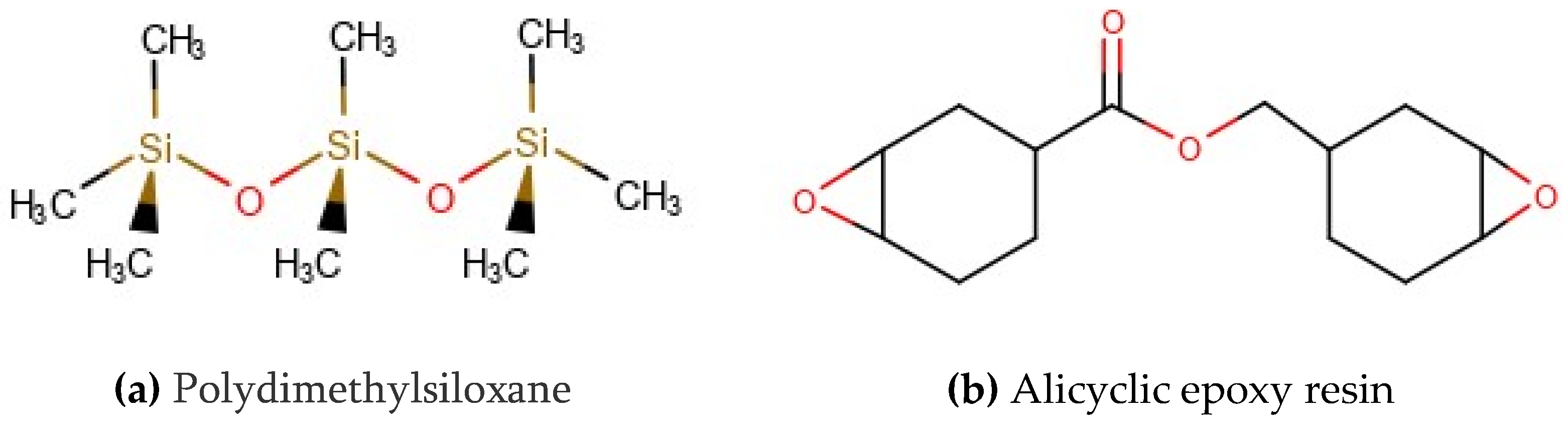
2.1. Materials
2.2. Methods
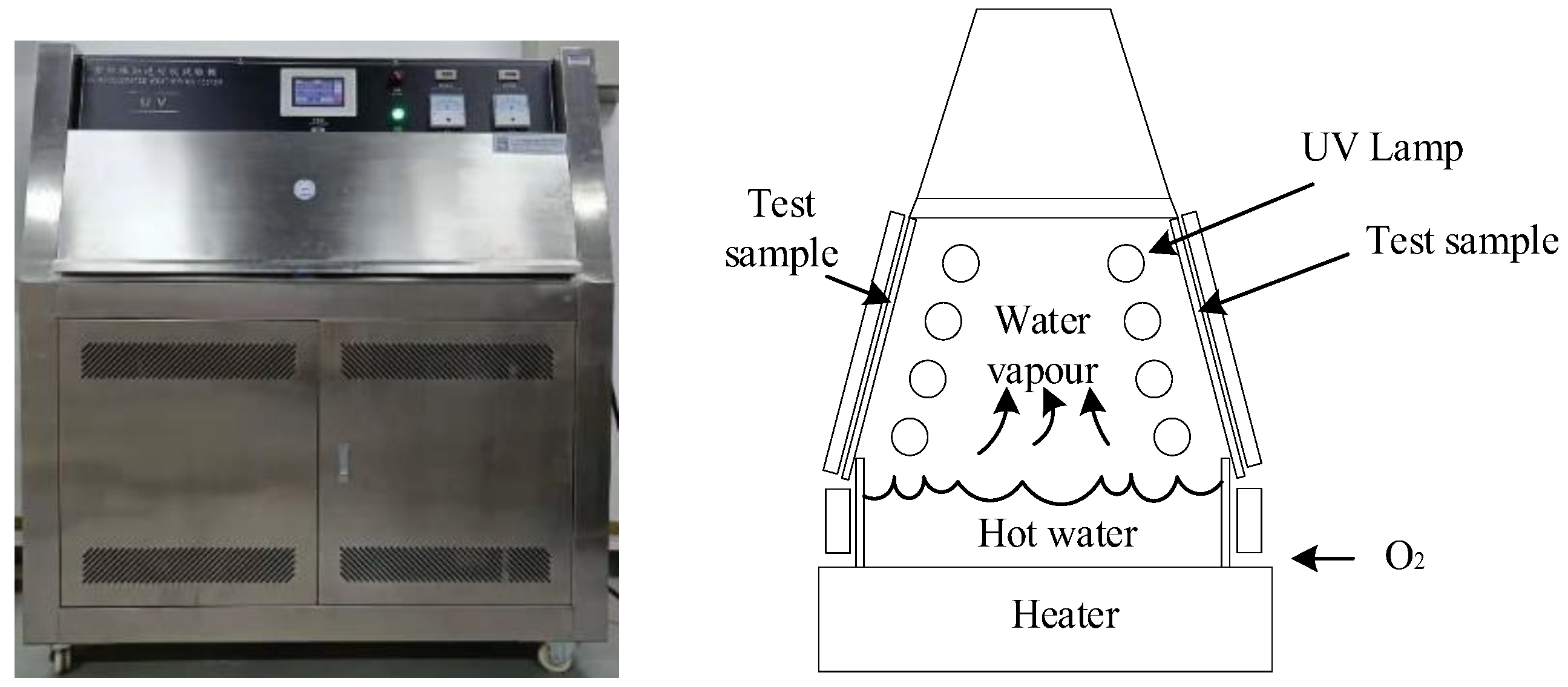
2.2.1. UV-A Aging Process
2.2.2. Scanning Electron Microscope
2.2.3. Mechanical Characterization
2.2.4. Hydrophobicity and its Transfer Characteristics
2.2.5. Fourier Transform Infrared Spectroscopy
2.2.6. X-ray Photoelectron Spectroscopy
3. Results and Discussion
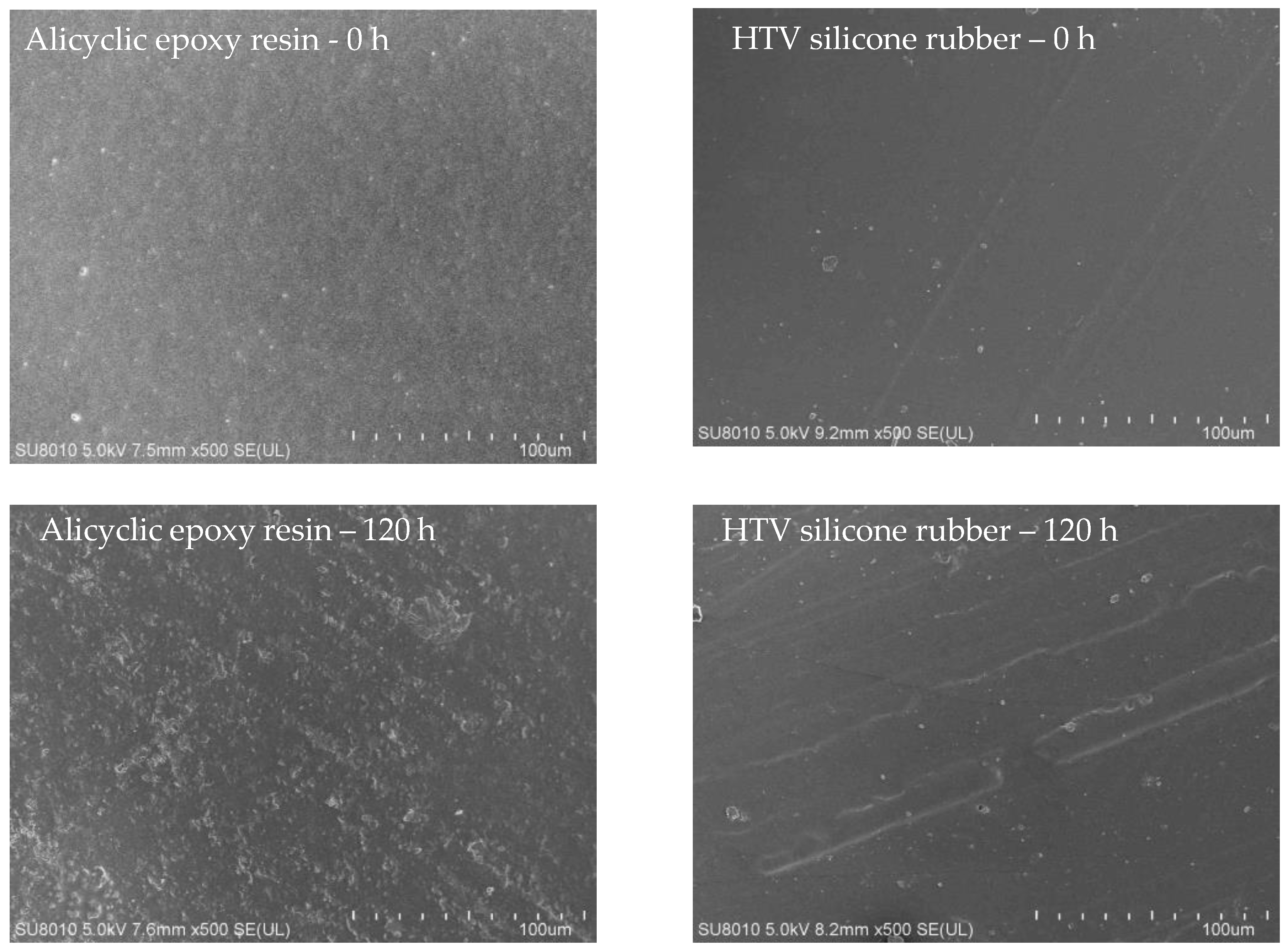
3.1. Appearance Analysis and SEM Studies
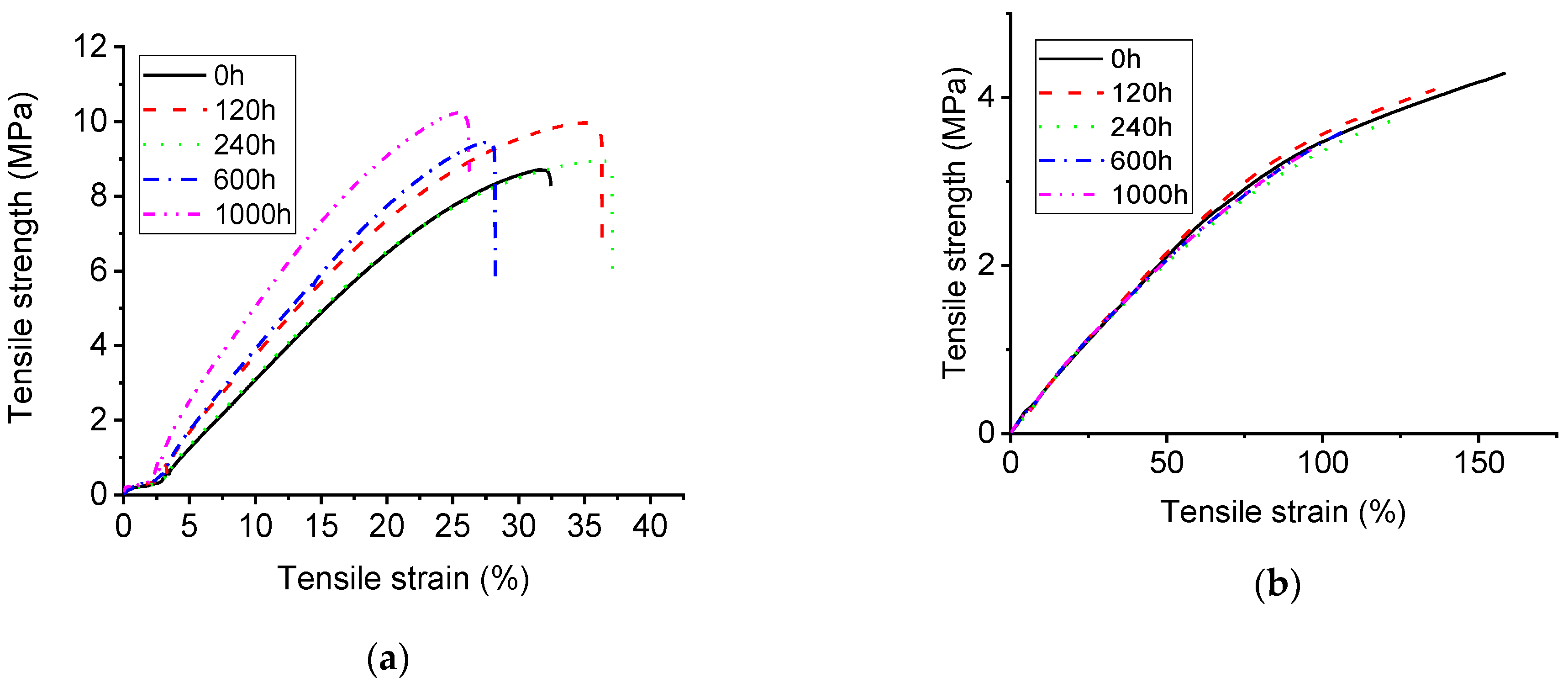
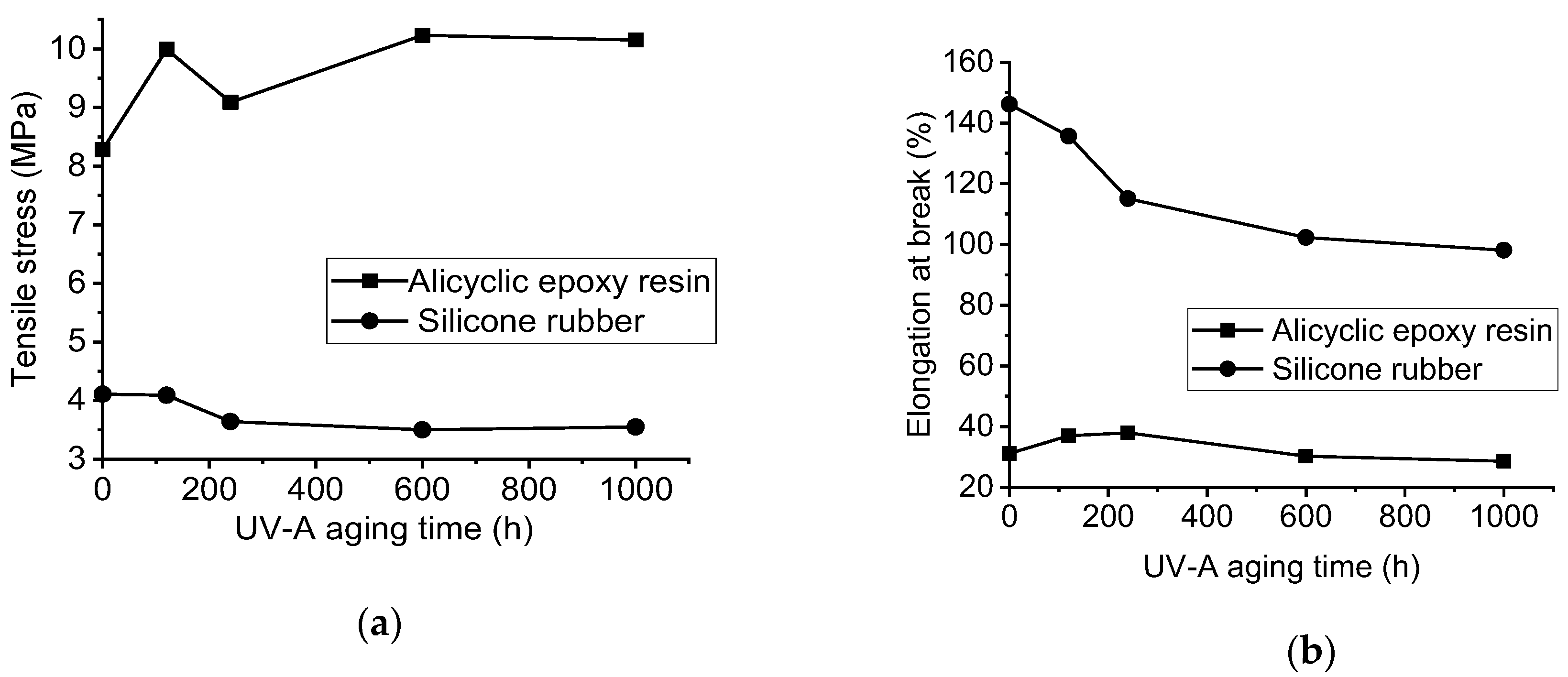
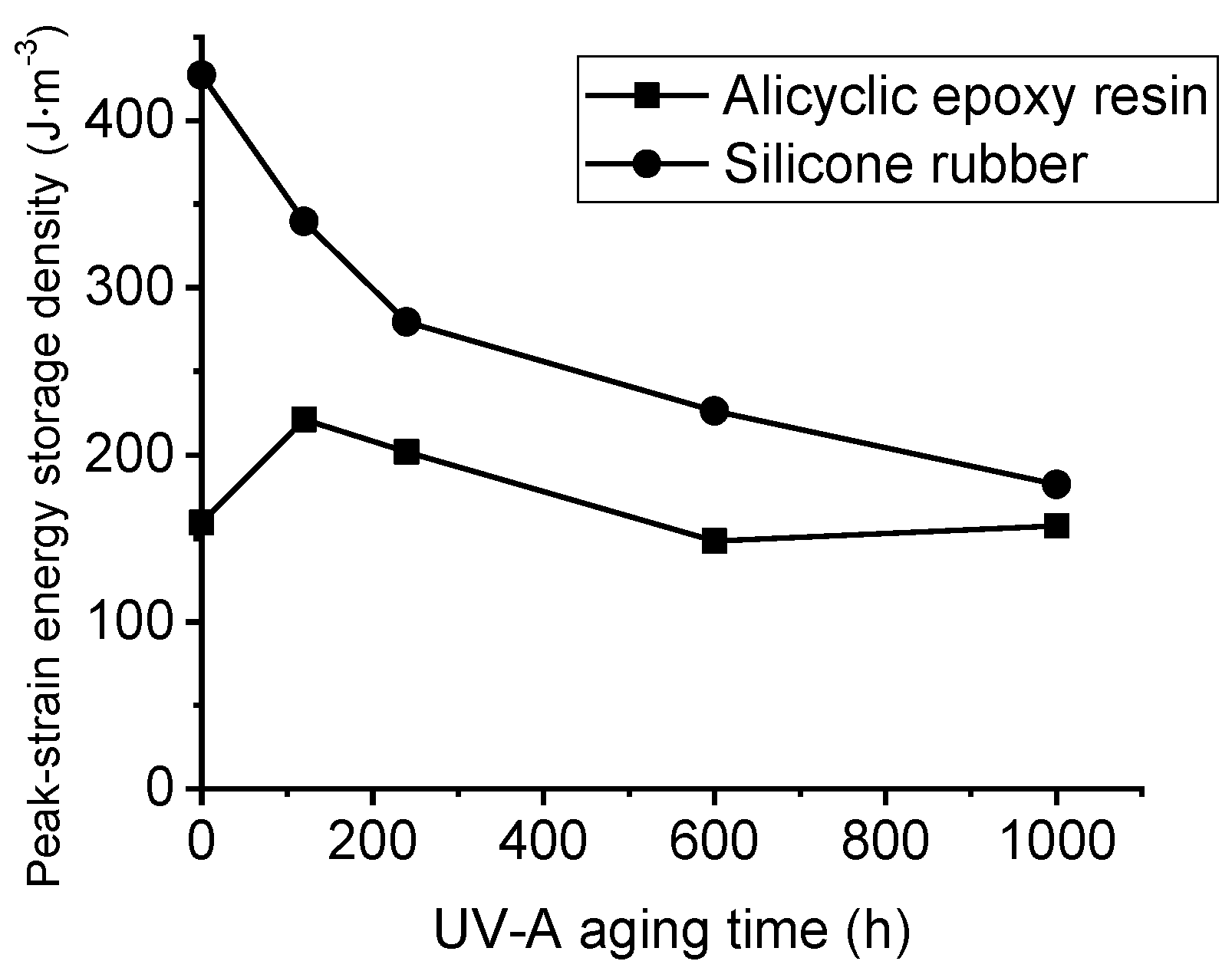
3.2. Mechanical Characterization with UV-A Aging Time
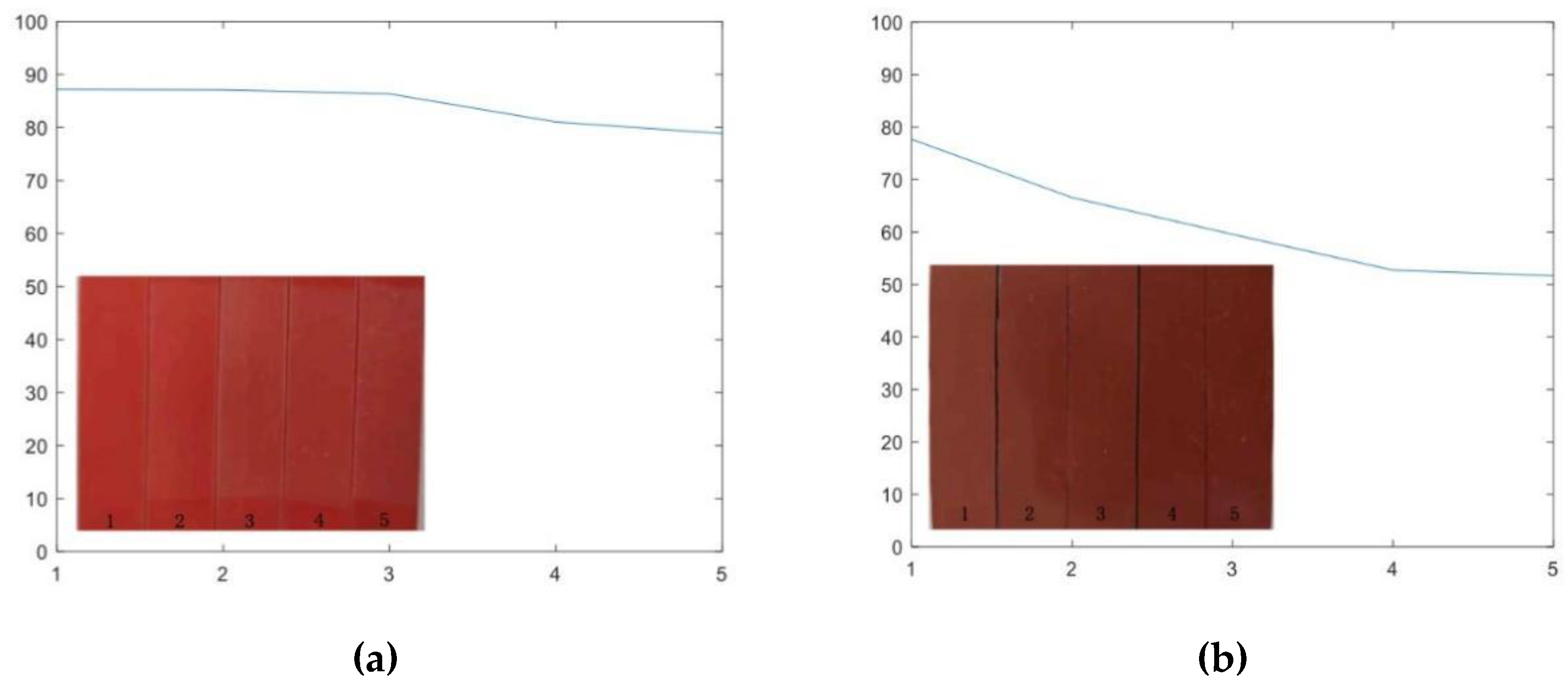
3.3. Hydrophobicity Analysis with UV-A Aging Time
3.4. FTIR Analysis of Samples with UV-A Aging Time
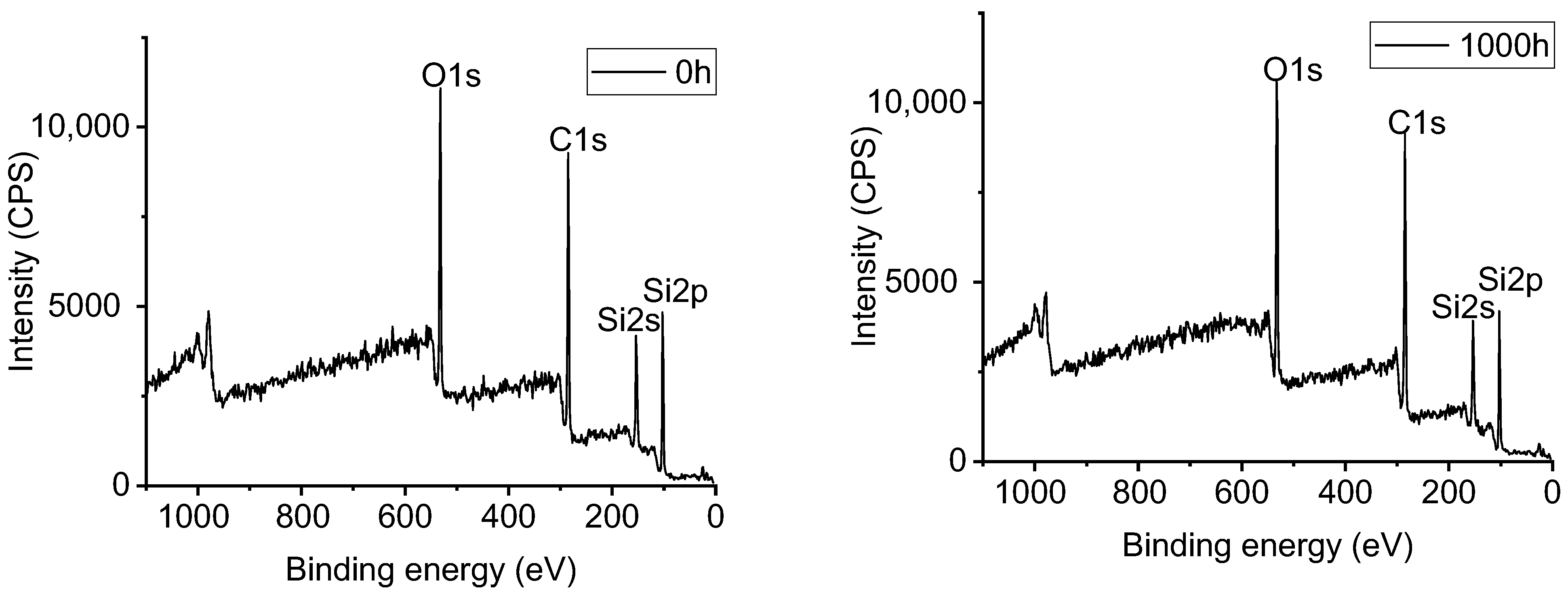
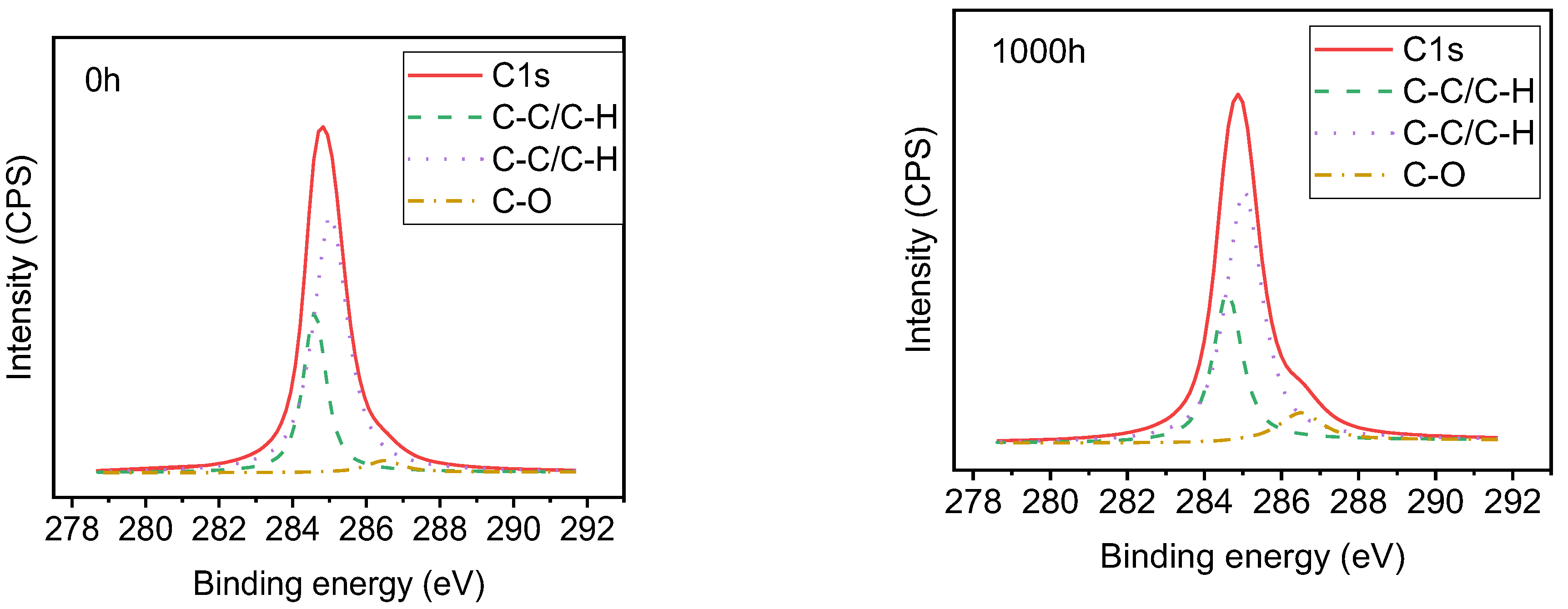
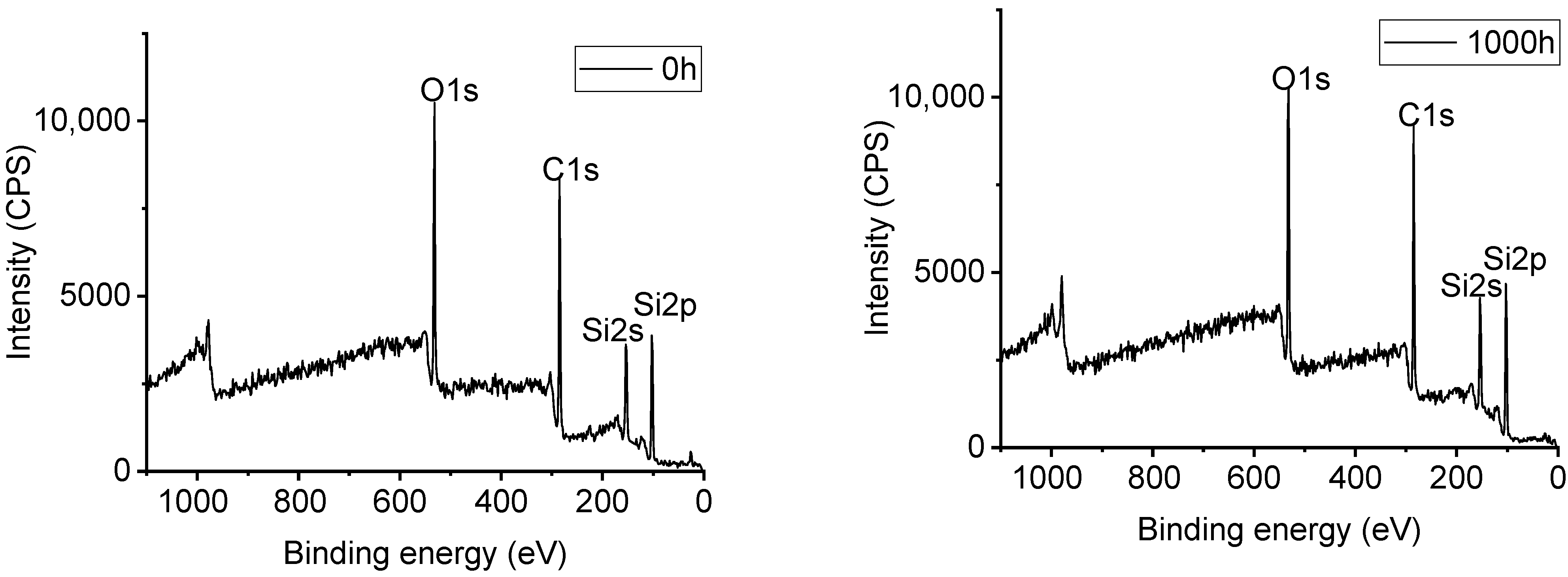
3.5. XPS Analysis of Samples with UV-A Aging Time
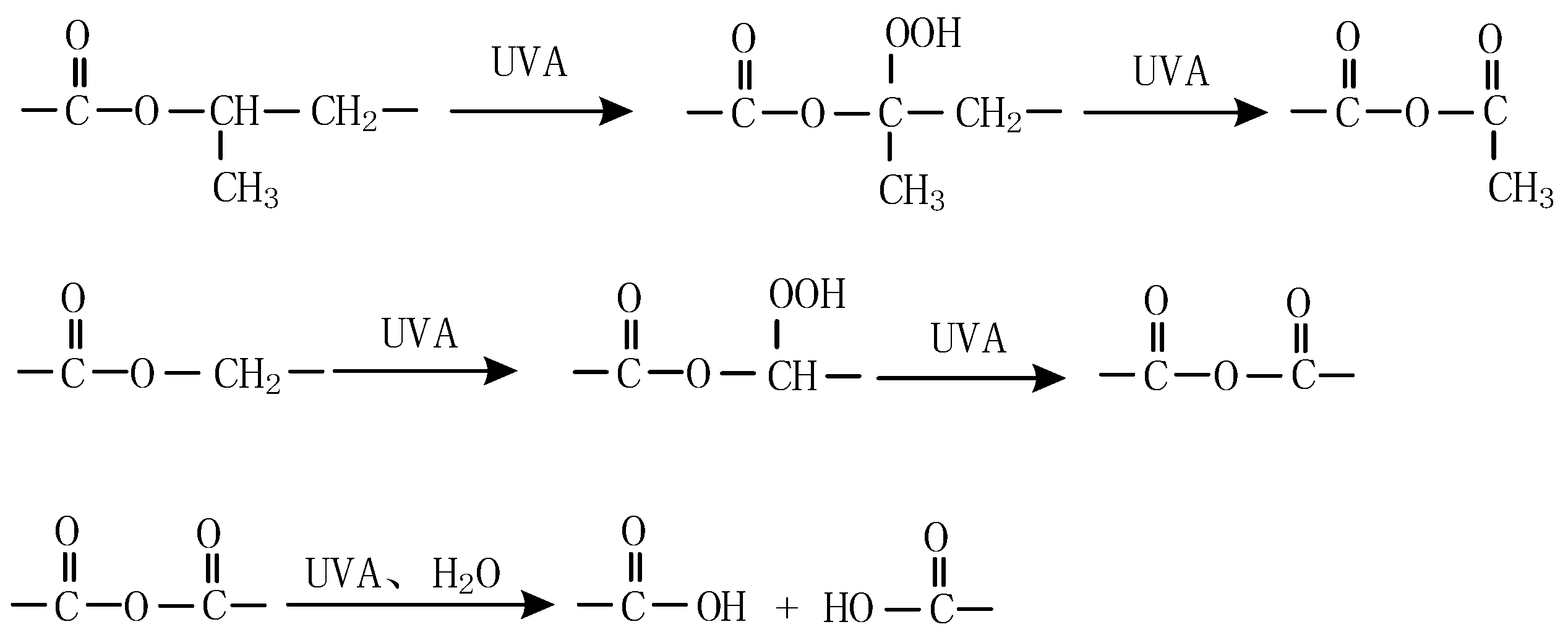
3.6. Possible UV-A Degradation Mechanism with UV-A Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Yang, H.; Shentu, B.; Chen, S.; Chen, M. Effect of titanium dioxide on the UV-C ageing behavior of silicone rubber. J. Appl. Polym. Sci. 2018, 135, 46099. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, H.; Chen, A.; Guan, H.; Kong, J.; Liu, S.; He, C. Effect of surface chemistry and morphology of silica on the thermal and mechanical properties of silicone elastomers. J. Appl. Polym. Sci. 2018, 135, 46646. [Google Scholar] [CrossRef]
- Li, Z.; Yin, F.; Cao, B.; Wang, L.; Shao, S.; Farzaneh, M. Pollution flashover performance of RTV coatings with Partial Damage. Int. J. Electr. Power Energy Syst. 2020, 121, 106102. [Google Scholar] [CrossRef]
- Yin, F.; Liu, P.; Mei, H.; Wang, L.; Li, L.; Farzaneh, M. The shed hole influence on the electrical performance of composite insulator. IEEE Access 2020, 8, 217447–217455. [Google Scholar] [CrossRef]
- Liang, X.; Gao, Y.; Wang, J.; Li, S. Rapid development of silicone rubber composite insulator in China. High Volt. Eng. 2016, 42, 2888–2896. [Google Scholar]
- Jia, Z.; Ma, G.; Zhu, Z.; Wang, X.; Guan, Z. Deformation and stress concentration of composite insulator in strong wind. High Volt. Technol. 2015, 41, 602–607. [Google Scholar]
- Meng, X.; Mei, H.; Zhu, B.; Yin, F.; Wang, L. Influence of pollution on surface streamer discharge. Electr. Power Syst. Res. 2022, 212, 108638. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, L.; Liao, R.; Liu, Y.; Wang, X.; Wang, T.; Fu, J. Process Improvement to Restrain Emergency Heating Defect of Composite Insulator. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 446–453. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Y.; Chen, R.; Zhang, S.; Liao, R.; Yang, L.; Wang, T. Method for predicting the water absorption of external insulating silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1242–1250. [Google Scholar] [CrossRef]
- Shang, R.; Hao, N.; Wang, L.; Yin, F.; Farzaneh, M. Analysis of High-Temperature Wide Band Dielectric Properties of ATH Filled Silicone Rubber Used for On-site Insulation of Bare Overhead Conductors. IEEE Trans. Dielectr. Electr. Insul. 2022; in press. [Google Scholar]
- Sun, Z.; Li, Z.; Zhang, J.; Fu, M. Analysis on bird pest of composite insulators for 1000 kv AC transmission line and its counter measures. Power Syst. Technol. 2009, 33, 52–54. [Google Scholar]
- Shi, Q. Study on the UV Aging Characteristics of HTV Silicone Rubber Material Under Different Humidity. Master’s Thesis, North China Electric Power University, Beijing, China, 2014. [Google Scholar]
- Li, G. Study on the Influence of UV Aging on Mechanical and Electrical Properties of HTV Silicone Rubber. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2015. [Google Scholar]
- Gao, H. Study on Aging Properties of Polymer Composites Insulators. Master’s Thesis, Xi’an Polytechnic University, Xi’an, China, 2019. [Google Scholar]
- Andersson, J.; Gubanski, S.M.; Hillborg, H. Properties of interfaces in silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 137–145. [Google Scholar] [CrossRef]
- Hedir, A.; Moudoud, M.; Lamrous, O.; Rondot, S.; Jbara, O.; Dony, P. Ultraviolet radiation aging impact on physicochemical properties of crosslinked polyethylene cable insulation. J. Appl. Polym. Sci. 2020, 137, 48575. [Google Scholar] [CrossRef]
- Qin, Y. Study on UV Irradiation Characteristics and Aging Mechanism of HTV Silicone Rubber for UHV Power Transmission. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2013. [Google Scholar]
- Ehsani, M.; Borsi, H.; Gockenbach, E.; Bakhshandeh, G.R.; Morshedian, J. Modified silicone rubber for use as high voltage outdoor insulators. Adv. Polym. Technol. 2005, 24, 51–61. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Lv, F.; Liang, Y. Influence of UV radiation on HTV silicon rubber performance. High Volt. Eng. 2010, 36, 2634–2638. [Google Scholar]
- Liu, Y.; Lin, Y.; Wu, K.; Fan, H.; Wang, L. Analysis and Optimization on Non-uniformity of Temperature Distribution in Hydrophobic Cycloaliphatic Epoxy Resin Insulators during the Curing Process. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1810–1818. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Wang, Y.; Wu, K.; Cao, B.; Wang, L. Simultaneously improving toughness and hydrophobic properties of cycloaliphatic epoxy resin through silicone prepolymer. J. Appl. Polym. Sci. 2022, 139, e52478. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Cao, B.; Wu, K.; Wang, L. Enhancement of polysiloxane/epoxy resin compatibility through an electrostatic and van der Waals potential design strategy. Polym. Test. 2022, 117, 107820. [Google Scholar] [CrossRef]
- ASTM D 2240–81; Test for Rubber Property—Durometer Hardness, Annual Book of ASTM Standards. ASTM: West Conshohocken, PA, USA, 1982.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ISO 34–1; Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength—Part 1: Trouser, Angle, and Crescent Test Pieces. ISO: Geneva, Switzerland, 2015.
- IEC 61109; Insulators for Overhead Lines—Composite Suspension and Tension Insulators for A.C. Systems with a Nominal Voltage Greater than 1000 V—Definitions, Test Methods, and Acceptance Criteria. ISO: Geneva, Switzerland, 2008.
- Guang, X. Modern Material Analysis and Testing Technology; China University of Mining and Technology Press: Xuzhou, China, 2013. [Google Scholar]
- Chen, C.; Jia, Z.; Wang, X.; Lu, H.; Guan, Z.; Yang, C. Micro characterization and degradation mechanism of liquid silicone rubber used for external insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 313–321. [Google Scholar] [CrossRef]



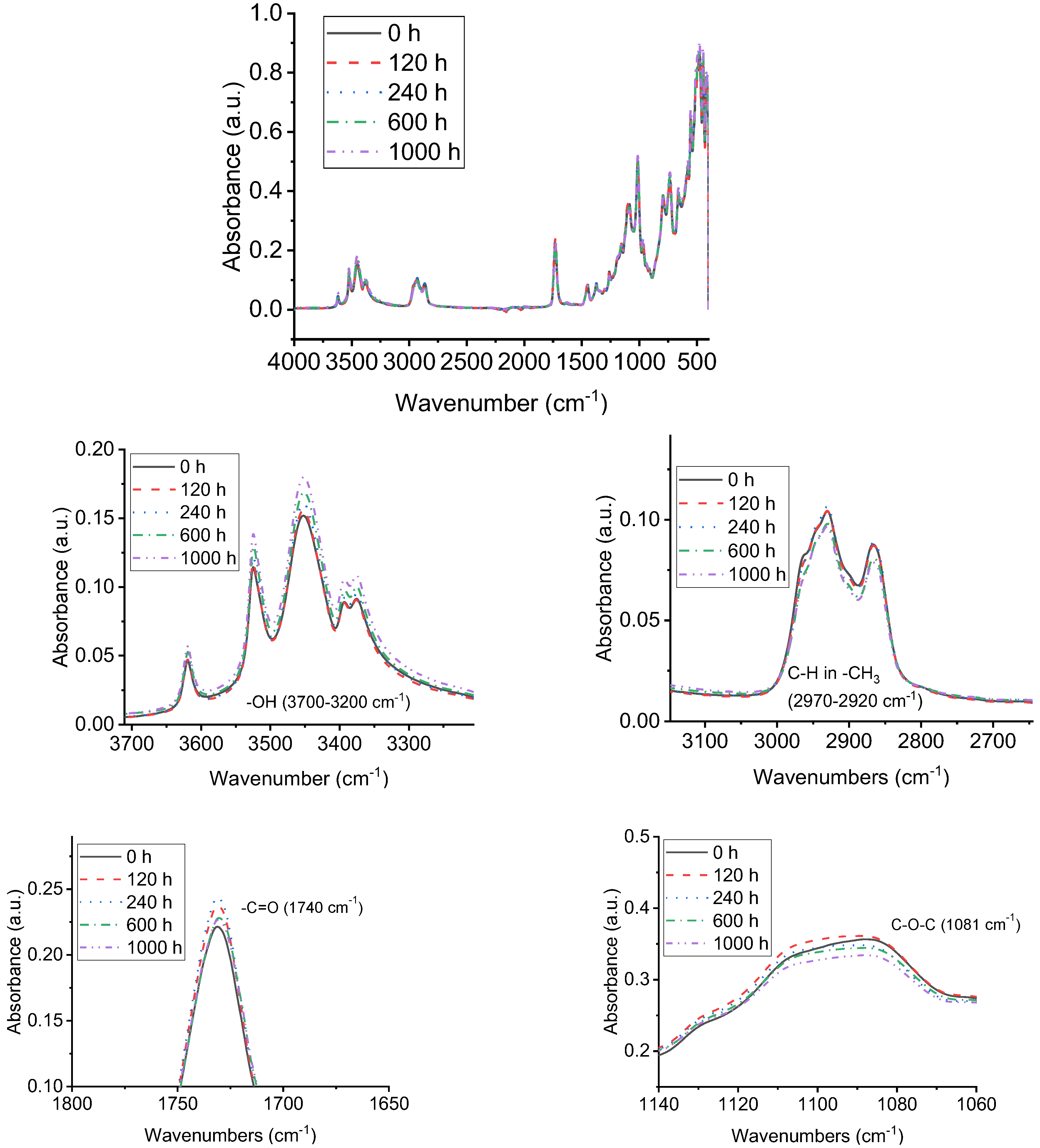
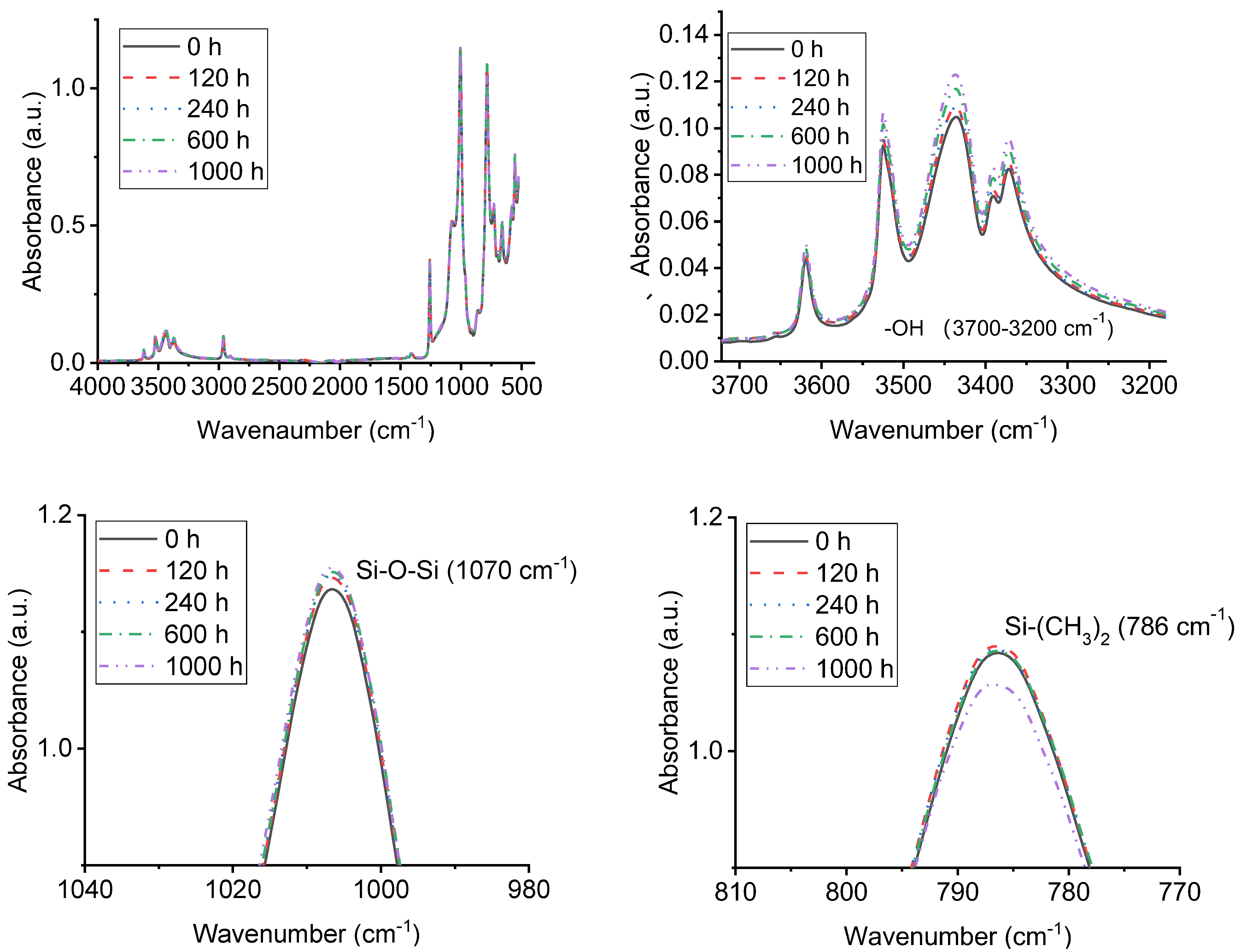


| Chemical Bond | Wavenumbers (cm−1) |
|---|---|
| –OH | 3700–3200 |
| C–H (in –CH3) | 2970–2920 |
| C=O | 1770–1680 |
| C–O–C | 1081 |
| Chemical Bond | Wavenumbers (cm−1) |
|---|---|
| –OH | 3700–3200 |
| Si–O (in Si–O–Si) | 1100–1000 |
| Si–(CH3)2 | 840–790 |
| Sample | Aging Time(h) | Position of Peak Binding Energy (eV) | Content of Element (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Si | C | O | Si | C | O | C/O | ||
| Alicyclic epoxy resin | 0 | 102.70 | 285.10 | 533.10 | 19.8 | 54.9 | 25.3 | 2.17 |
| 1000 | 102.62 | 285.02 | 531.42 | 18.9 | 55.0 | 26.1 | 2.12 | |
| HTV silicone rubber | 0 | 101.16 | 285.16 | 531.56 | 21.5 | 53.2 | 25.3 | 2.10 |
| 1000 | 101.22 | 285.22 | 531.62 | 23.3 | 50.2 | 26.5 | 1.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fan, H.; Li, W.; Zhang, Y.; Shang, R.; Yin, F.; Wang, L. Effect of Ultraviolet—A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators. Polymers 2022, 14, 4889. https://doi.org/10.3390/polym14224889
Wang X, Fan H, Li W, Zhang Y, Shang R, Yin F, Wang L. Effect of Ultraviolet—A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators. Polymers. 2022; 14(22):4889. https://doi.org/10.3390/polym14224889
Chicago/Turabian StyleWang, Xiaoqing, Haonan Fan, Wenrong Li, Yuyang Zhang, Ruiqi Shang, Fanghui Yin, and Liming Wang. 2022. "Effect of Ultraviolet—A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators" Polymers 14, no. 22: 4889. https://doi.org/10.3390/polym14224889
APA StyleWang, X., Fan, H., Li, W., Zhang, Y., Shang, R., Yin, F., & Wang, L. (2022). Effect of Ultraviolet—A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators. Polymers, 14(22), 4889. https://doi.org/10.3390/polym14224889







