Polymorphic Crystallization Behavior of a Poly(butylene adipate) Midblock within a Poly(L-lactide-butylene adipate-L-lactide) Triblock Copolymer
Abstract
:1. Introduction
2. Materials, Instruments, and Methods
2.1. Materials
2.2. Instruments
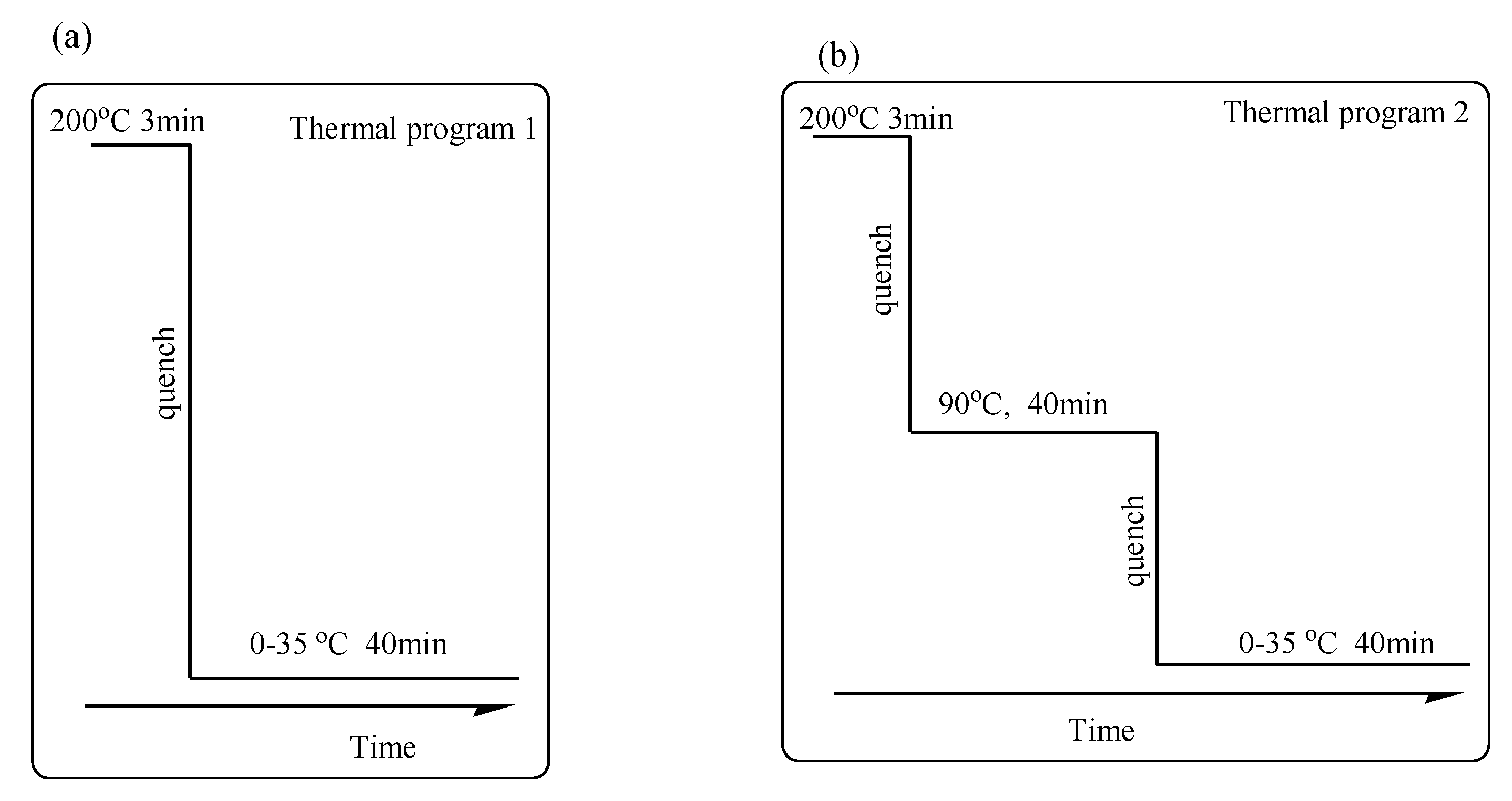
2.3. Methods
3. Results
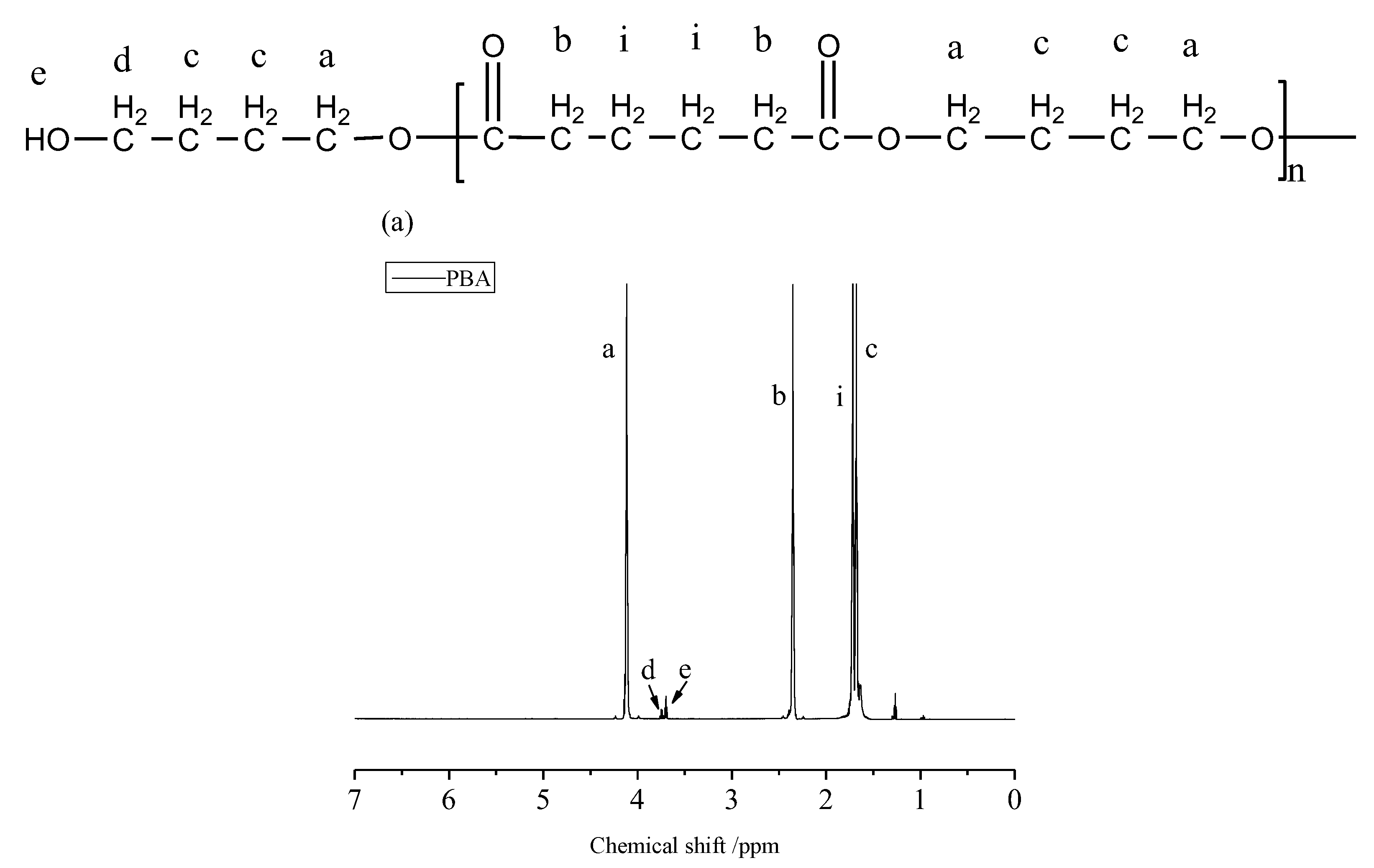
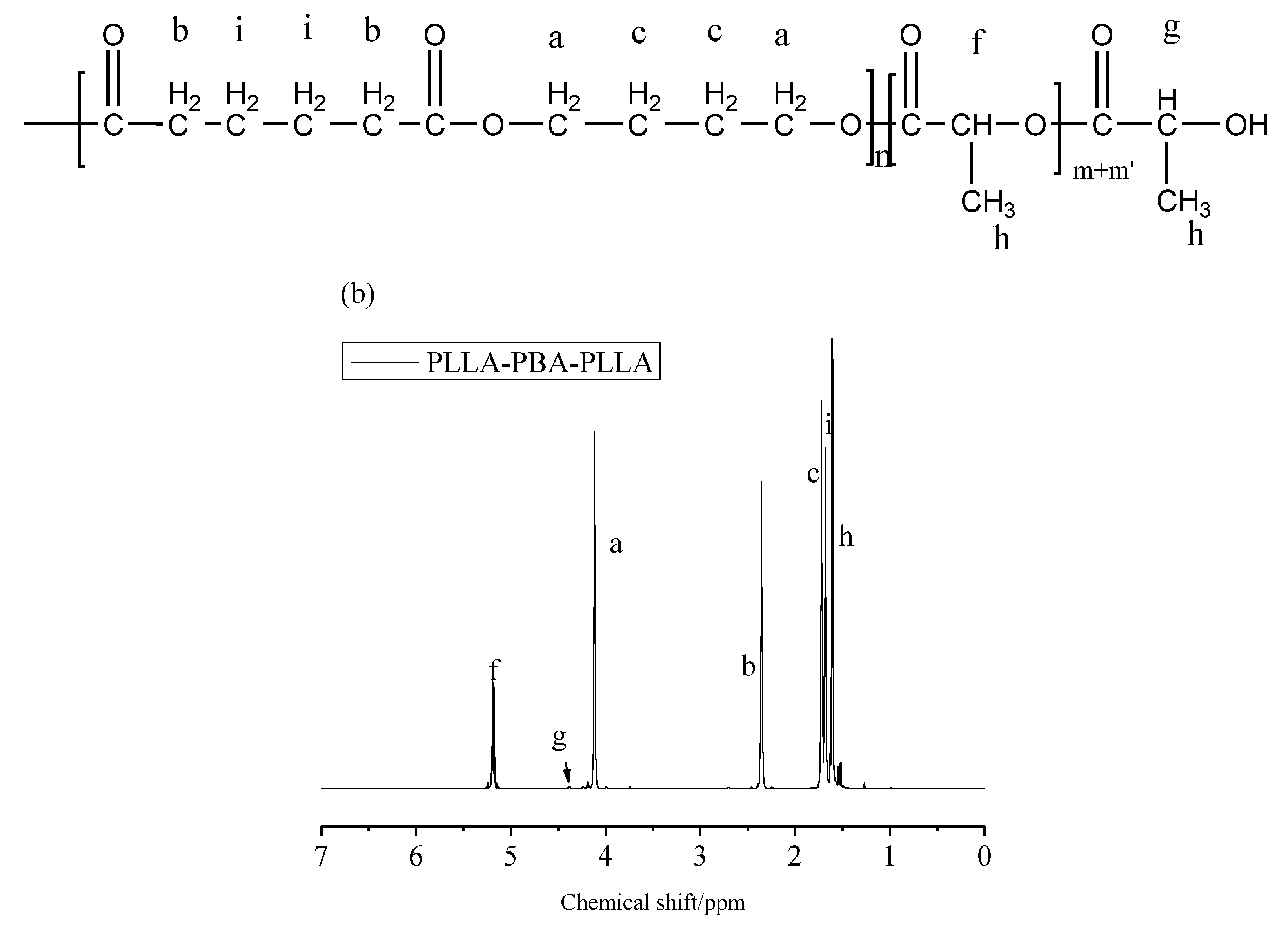
3.1. Chemical Properties and Compositions
3.2. Nonisothermal Crystallization Analysis by DSC
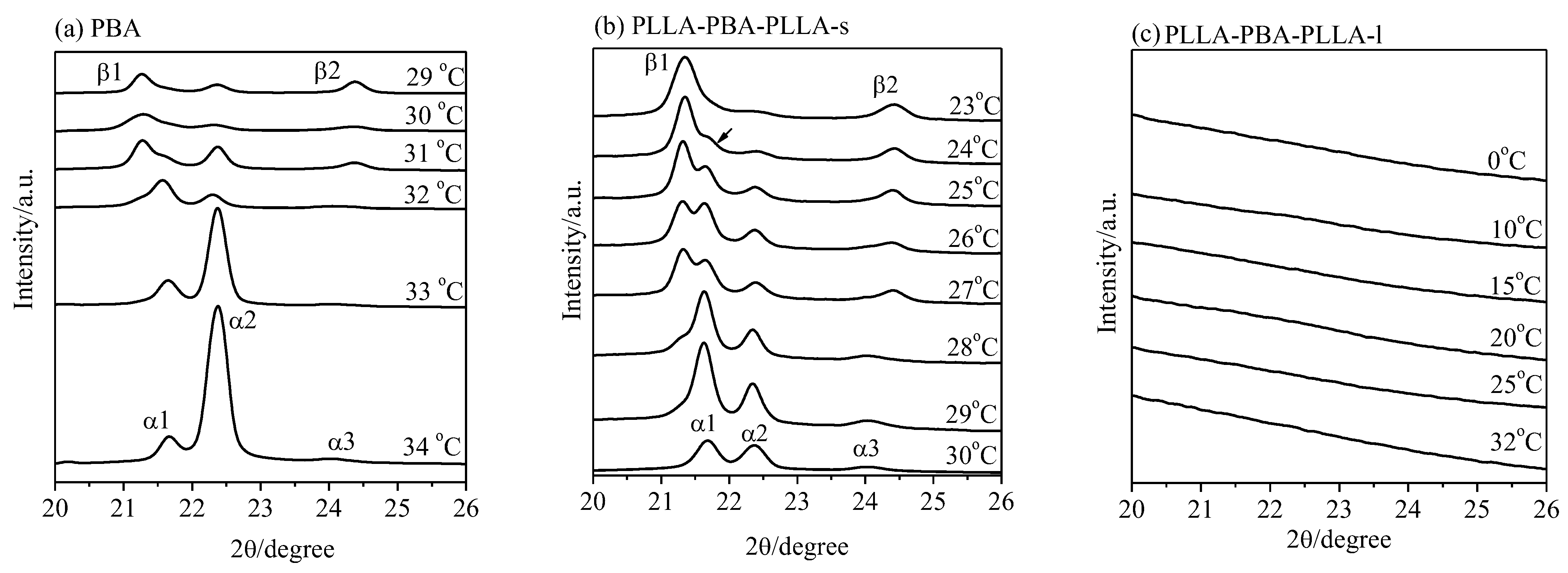
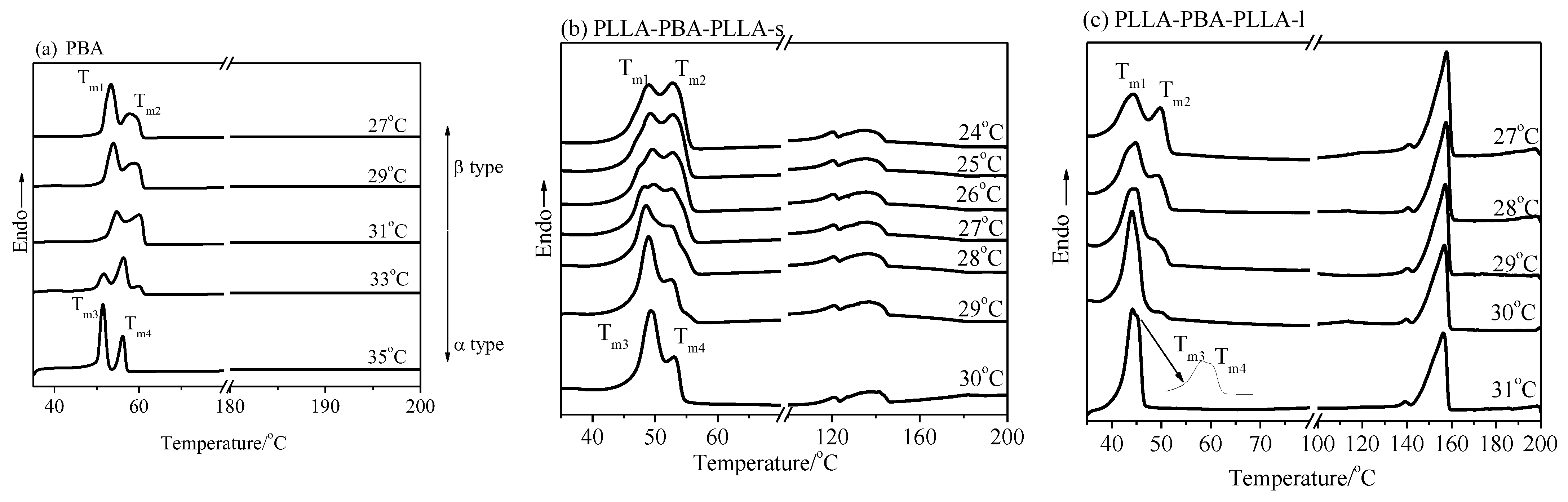
3.3. Crystalline Structure of Neat PBA and PBA-mid Analyzed by WAXD and DSC
3.3.1. Crystalline Structure of Neat PBA and PBA-mid Analyzed by WAXD
3.3.2. Crystalline Structure of Neat PBA and PBA-mid Analyzed by DSC
3.3.3. Compared Results of WAXD and DSC
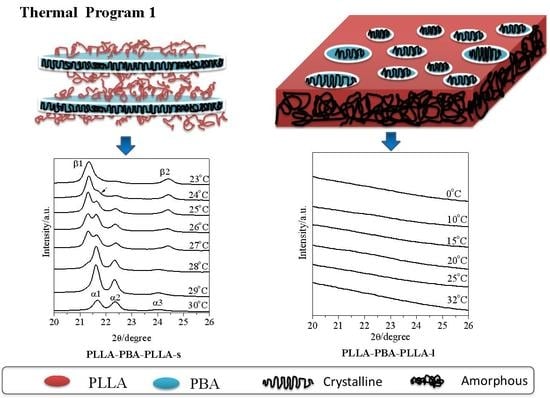
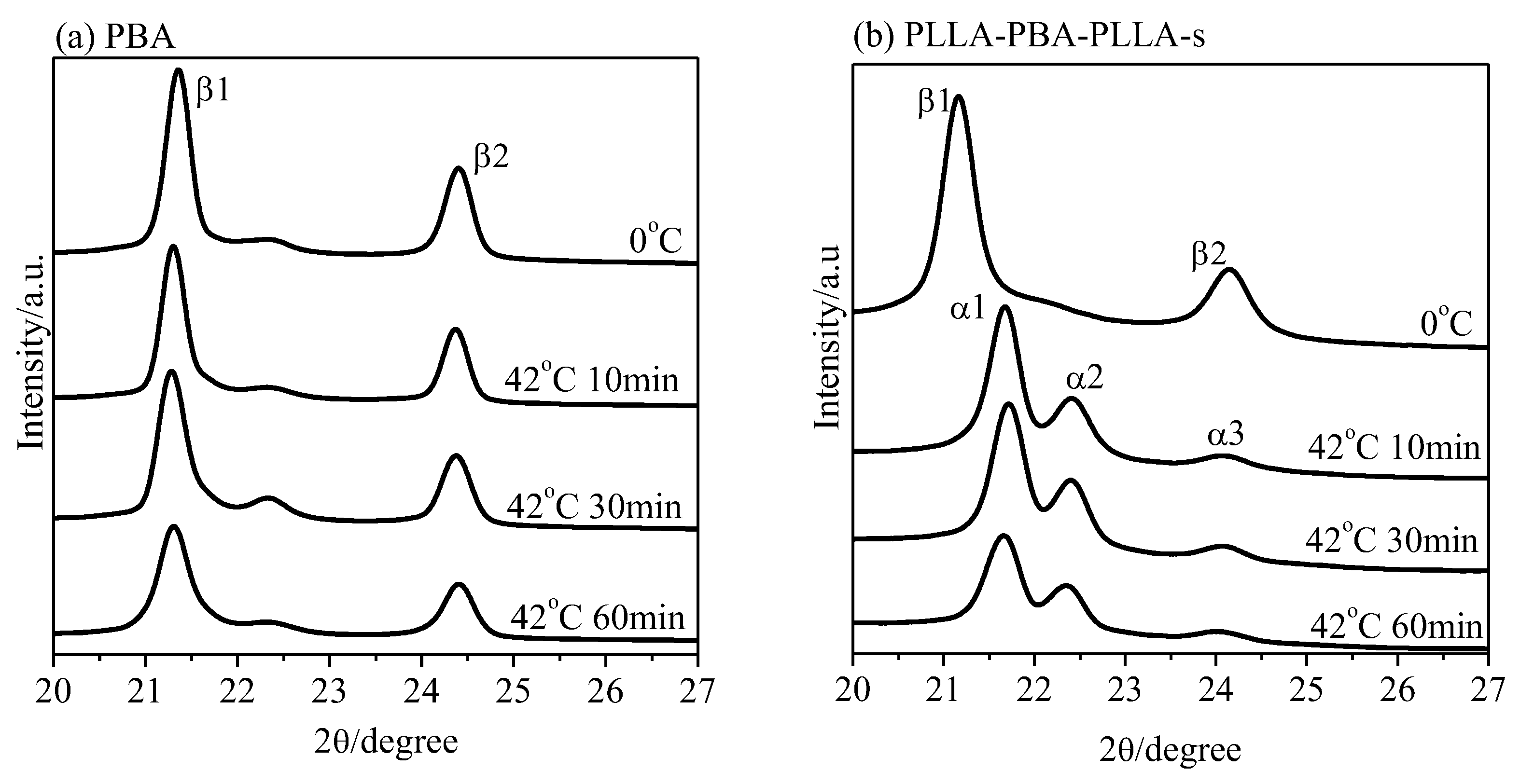
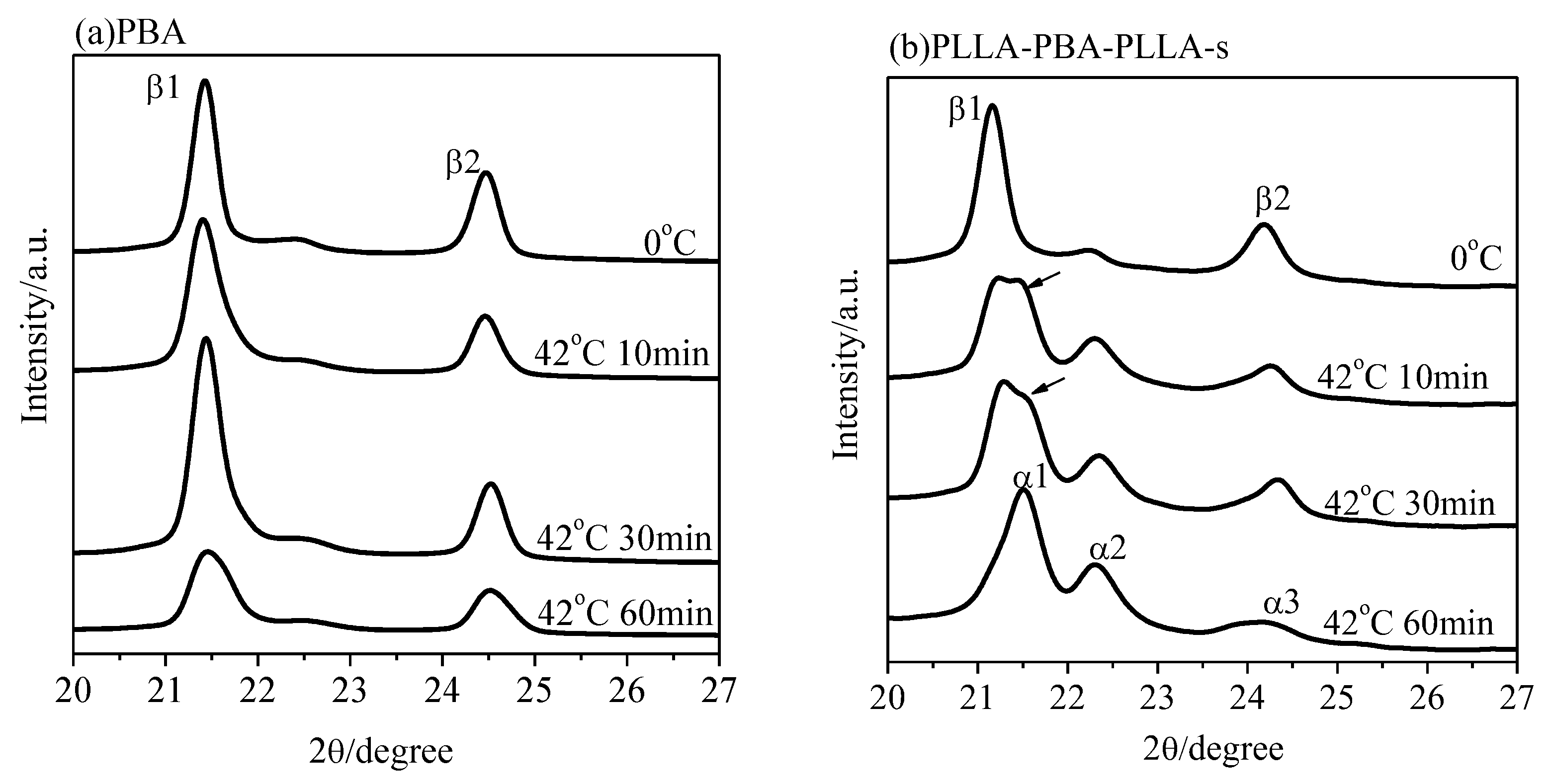
3.4. Phase Transition of PBA-mid upon Annealing Analyzed by WAXD
3.5. Spherulitic Morphology Observation by POM
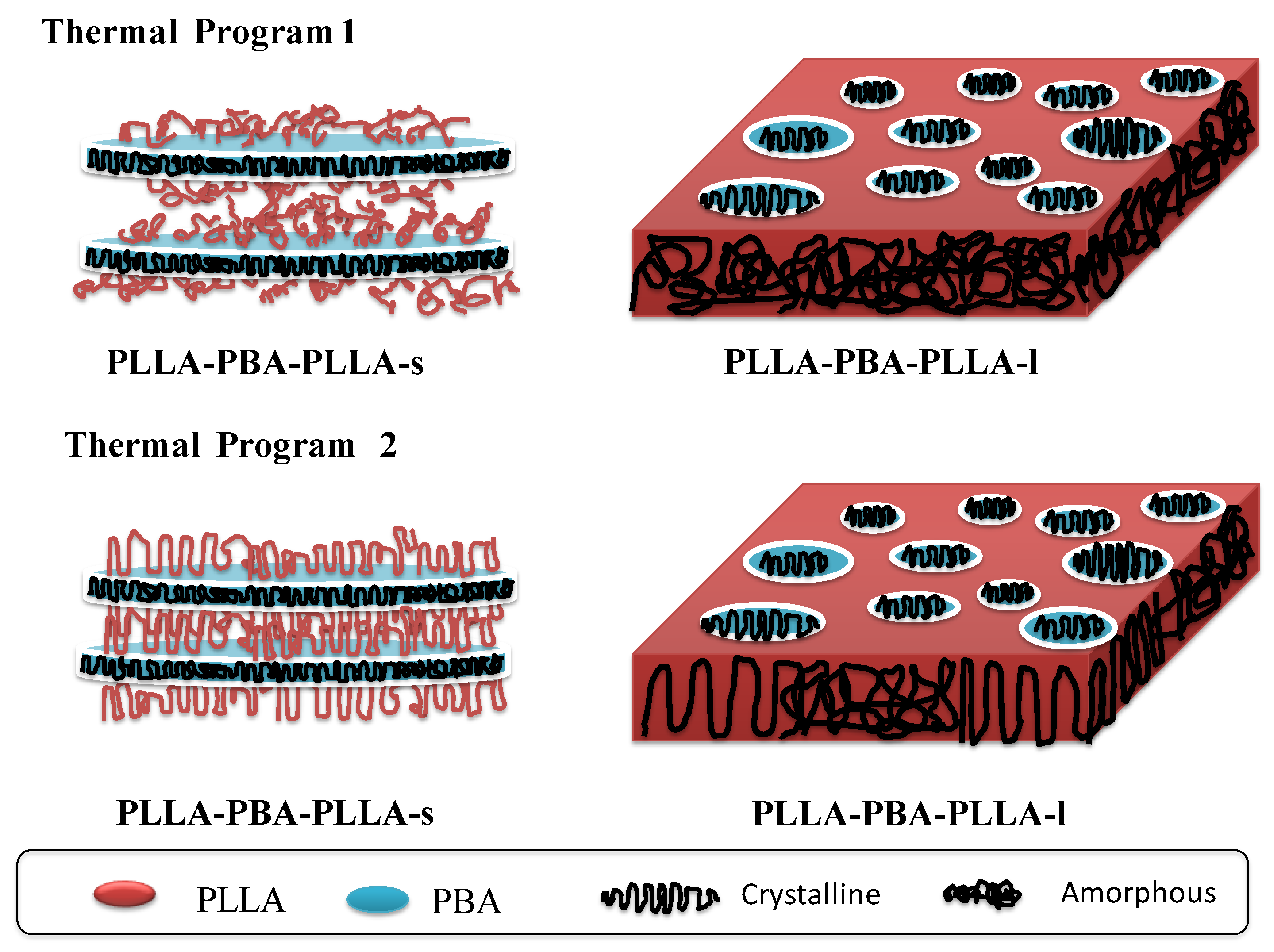
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, Z.; Kuwabara, K.; Abe, H.; Iwata, T.; Doi, Y. Metastability and Transformation of Polymorphic Crystals in Biodegradable Poly(butylene adipate). Biomacromolecules 2004, 5, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Hu, Y.; Ozaki, Y.; Shen, D.Y.; Gan, Z.; Yan, S.K.; Takahashi, I. Melt crystallization and Crystal Transition of Poly(butylene adipate) Revealed by Infrared Spectroscopy. J. Phys. Chem. B 2008, 112, 3311–3314. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, P.; Hua, L.; Xie, Y.; Dong, T.; Zhu, B.; Inoue, Y.; Feng, X. Fractionated crystallization, polymorphic crystalline structure, and spherulite morphology of poly(butylene adipate) in its miscible blend with poly(butylene succinate). Polymer 2011, 52, 3460–3468. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. Polymorphic structures of poly(tetramethylene adipate). J. Macromol. Sci. B 1979, 16, 407–417. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. Single crystals of poly(tetramethylene adipate). J. Macromol. Sci. B 1980, 18, 233–255. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwabara, K.; Abe, H.; Iwata, T.; Doi, Y. The role of polymorphic crystal structure and morphology in enzymatic degradation of melt-crystallized poly(butylene adipate) films. Polym. Degrad. Stab. 2005, 87, 191–199. [Google Scholar] [CrossRef]
- Dechet, M.A.; Demina, A.; Römlinga, L.; Bonilla, J.S.; Lanyi, F.J.; Schubert, D.W.; Bück, A.; Peukert, W.; Schmidt, J. Development of poly(L-lactide) (PLLA) microspheres precipitated from triacetin for application in powder bed fusion of polymers. Addit. Manuf. 2020, 32, 100966. [Google Scholar] [CrossRef]
- Bobel, A.C.; Lohfeld, S.; Shirazi, R.N.; McHugh, P.E. Experimental mechanical testing of Poly (l-Lactide) (PLLA) to facilitate pre-degradation characteristics for application in cardiovascular stenting. Polym. Test. 2016, 54, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Sakshi, P.; Unnati, B.; Samshrith, R.N.; Sudhanshu, S.; Akash, C.; Gautam, S. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: A review. Int. J. Biol. Macromol. 2022, 218, 209–224. [Google Scholar]
- Jikei, M.; Yamadoi, Y.; Suga, T.; Matsumoto, K. Stereocomplex formation of poly(l-lactide)-poly(ε-caprolactone) multiblock copolymers with Poly(d-lactide). Polymer 2017, 123, 73–80. [Google Scholar] [CrossRef]
- Tien, N.D.; Prud’homme, R.E. Crystallization and morphology of ultrathin films of poly(d-lactide) with BAB block copolymers in which the A block is made of poly(l-lactide). Polymer 2017, 117, 25–29. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Oligo(lactic acid)-grafted starch: A compatibilizer for poly(lactic acid)/thermoplastic starch blend. Int. J. Biol. Macromol. 2020, 160, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.L.; Tian, P.; Liu, X.; Zhou, B. Hemocompatibility and selective cell fate of polydopamine-assisted heparinized PEO/PLLA composite coating on biodegradable AZ31 alloy. Colloids Surf. B Biointerfaces 2014, 121, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chevalia, V.S.; Song, P.; Yu, B.; Yang, Y.; Wang, H. Enhanced toughness of PLLA/PCL blends using poly(d-lactide)-poly(ε-caprolactone)-poly(d-lactide) as compatibilizer. Compos. Commun. 2020, 21, 100385. [Google Scholar] [CrossRef]
- Lin, K.-W.; Lan, C.-H.; Sun, Y.-M. Poly[(R)3-hydroxybutyrate] (PHB)/poly(l-lactic acid) (PLLA) blends with poly(PHB/PLLA urethane) as a compatibilizer. Polym. Degrad. Stab. 2016, 134, 30–40. [Google Scholar] [CrossRef]
- Kervran, K.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal degradation of polylactic acid (PLA)/polyhydroxybutyrate (PHB) blends: A systematic review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Lay, M.; Thajudin, N.L.N.; Hamid, Z.A.A.; Rusli, A.; Abdullah, M.K.; Shuib, R.K. Comparison of physical and mechanical properties of PLA, ABS and nylon 6 fabricated using fused deposition modeling and injection molding. Compos. Part B Eng. 2019, 1761, 107341. [Google Scholar] [CrossRef]
- Ahmadzadeh, Y.; Babaei, A.; Goudarzi, A. Assessment of localization and degradation of ZnO nano-particles in the PLA/PCL biocompatible blend through a comprehensive rheological characterization. Polym. Degrad. Stab. 2018, 158, 136–147. [Google Scholar] [CrossRef]
- Rigoussen, A.; Raquez, J.M.; Dubois, P.; Verge, P. A dual approach to compatibilize PLA/ABS immiscible blends with epoxidized cardanol derivatives. Eur. Polym. J. 2019, 114, 118–126. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.-J.; Lu, Z.-Y.; Shi, A.-C. Enhancing composition window of bicontinuous structures by designed polydispersity distribution of ABA triblock copolymers. Polymer 2013, 54, 6253–6260. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S.; Deepthi, K.; Gowd, E.B. Structural evolution of poly(l-lactide) block upon heating of the glassy ABA triblock copolymers containing poly(l-lactide) A blocks. Polymer 2016, 105, 422–430. [Google Scholar] [CrossRef]
- Sheng, Y.; An, J.; Zhu, Y. Self-assembly of ABA triblock copolymers under soft confinement. Chem. Phys. 2015, 452, 46–52. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Jin, Q.; Ding, D.; Shi, A.-C. Self-Assembly of Cylinder-Forming ABA Triblock Copolymers under Cylindrical Confinement. Macromol. Theory Simul. 2008, 17, 301–312. [Google Scholar] [CrossRef]
- Konwar, D.B.; Sethy, S.; Satapathy, B.K.; Jacob, J. Effect of poly(l-lactide) chain length on microstructural and thermo-mechanical properties of poly(l-lactide)-b-poly(butylene carbonate)-b-poly(l-lactide) triblock copolymers. Polymer 2017, 123, 87–99. [Google Scholar] [CrossRef]
- He, X.; Qiu, Z. Influence of high molecular weight poly(ethylene adipate) on the crystallization behavior and mechanical properties of biodegradable poly(l-lactide) in their immiscible polymer blend. Polym. Test. 2018, 67, 421–427. [Google Scholar] [CrossRef]
- Ye, H.-M.; Wang, C.-S.; Zhang, Z.-Z.; Yao, S.F. Effect of cellulose nanocrystals on the crystallization behavior and enzymatic degradation of poly(butylene adipate). Carbohydr. Polym. 2018, 189, 99–106. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Woo, E.M. Cracks and Ring Bands of Poly(3-hydroxybutyrate) on Precrystallized Poly(l-lactic acid) Template. Ind. Eng. Chem. Res. 2011, 50, 4494–4503. [Google Scholar] [CrossRef]
- Seretoudi, G.; Bikiaris, D.; Panayiotou, C. Synthesis, characterization and biodegradability of poly(ethylene succinate)/poly(ε-caprolactone) block copolymers. Polymer 2002, 43, 5405–5415. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Meier-Haack, J. ABA triblock copolymers of L-lactide and poly(ethylene glycol). Makromol. Chem. 1993, 194, 715–725. [Google Scholar] [CrossRef]
- Ba, C.; Yang, J.; Hao, Q.; Liu, X.; Cao, A. Syntheses and Physical Characterization of New Aliphatic Triblock Poly(L-lactide-b-butylene succinate-b-L-lactide)s Bearing Soft and Hard Biodegradable Building Blocks. Biomacromolecules 2003, 4, 1827–1834. [Google Scholar] [CrossRef]
- Witzke, D.R.; Narayan, R.; Kolstad, J.J. Reversible Kinetics and Thermodynamics of the Homopolymerization of L-Lactide with 2-Ethylhexanoic Acid Tin(II) Salt. Macromolecules 1997, 30, 7075–7085. [Google Scholar] [CrossRef]
- Schmitt, A.K.; Mahanthappa, M.K. Characteristics of Lamellar Mesophases in Strongly Segregated Broad Dispersity ABA Triblock Copolymers. Macromolecules 2014, 47, 4346–4356. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pan, P.; Hua, L.; Feng, X.; Yue, J.; Ge, Y.; Inoue, Y. Effects of Crystallization Temperature of Poly (vinylidene fluoride) on Crystal Modification and Phase Transition of Poly(butylene adipate) in Their Blends: A Novel Approach for Polymorphic Control. J. Phys. Chem. B 2012, 116, 1265–1272. [Google Scholar] [CrossRef] [PubMed]




| Sample | fPBA a | Mn GPC | Mw GPC | Mw/Mn GPC | Mn NMR | Tc e | Tm f | ||
|---|---|---|---|---|---|---|---|---|---|
| % | (g/mol) | (g/mol) | /°C | /°C | |||||
| PBA | 100 | 8219 | 13,235 | 1.61 | 7920 | 31.9 | 55.6 | ||
| PLLA-PBA-PLLA-s b | 68 | 13,479 | 23,511 | 1.74 | 13,200 | 31.1 | 76 | 50 | 137 |
| PLLA-PBA-PLLA-l c | 35 | 29,818 | 54.073 | 1.81 | 28,512 | - | 90 | - | 160 |
| PLLA d | 0 | 25,052 | 48,625 | 1.89 | - | 103 | 167 | ||
| Sample | Instrument | Thermal Program 1 | Thermal Program 2 | ||||
|---|---|---|---|---|---|---|---|
| α | α + β | β | α | α + β | β | ||
| /°C | /°C | /°C | /°C | /°C | /°C | ||
| PBA | DSC | 34 | 33~31 | 30 | 35 | 34~30 | 29 |
| WAXD | 34 | 33~30 | 29 | 34 | 33~30 | 29 | |
| PLLA-PBA-PLLA-s | DSC | 30 | 29~26 | 25 | 30 | 29~25 | 24 |
| WAXD | 30 | 29~24 | 23 | 29 | 28~25 | 24 | |
| PLLA-PBA-PLLA-l | DSC | 0 | − | − | 31 | 30~28 | 27 |
| WAXD | − | − | − | + | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, L.; Wang, X. Polymorphic Crystallization Behavior of a Poly(butylene adipate) Midblock within a Poly(L-lactide-butylene adipate-L-lactide) Triblock Copolymer. Polymers 2022, 14, 4902. https://doi.org/10.3390/polym14224902
Hua L, Wang X. Polymorphic Crystallization Behavior of a Poly(butylene adipate) Midblock within a Poly(L-lactide-butylene adipate-L-lactide) Triblock Copolymer. Polymers. 2022; 14(22):4902. https://doi.org/10.3390/polym14224902
Chicago/Turabian StyleHua, Lei, and Xiaodong Wang. 2022. "Polymorphic Crystallization Behavior of a Poly(butylene adipate) Midblock within a Poly(L-lactide-butylene adipate-L-lactide) Triblock Copolymer" Polymers 14, no. 22: 4902. https://doi.org/10.3390/polym14224902
APA StyleHua, L., & Wang, X. (2022). Polymorphic Crystallization Behavior of a Poly(butylene adipate) Midblock within a Poly(L-lactide-butylene adipate-L-lactide) Triblock Copolymer. Polymers, 14(22), 4902. https://doi.org/10.3390/polym14224902