Thermal Degradation of Organophosphorus Flame Retardants
Abstract
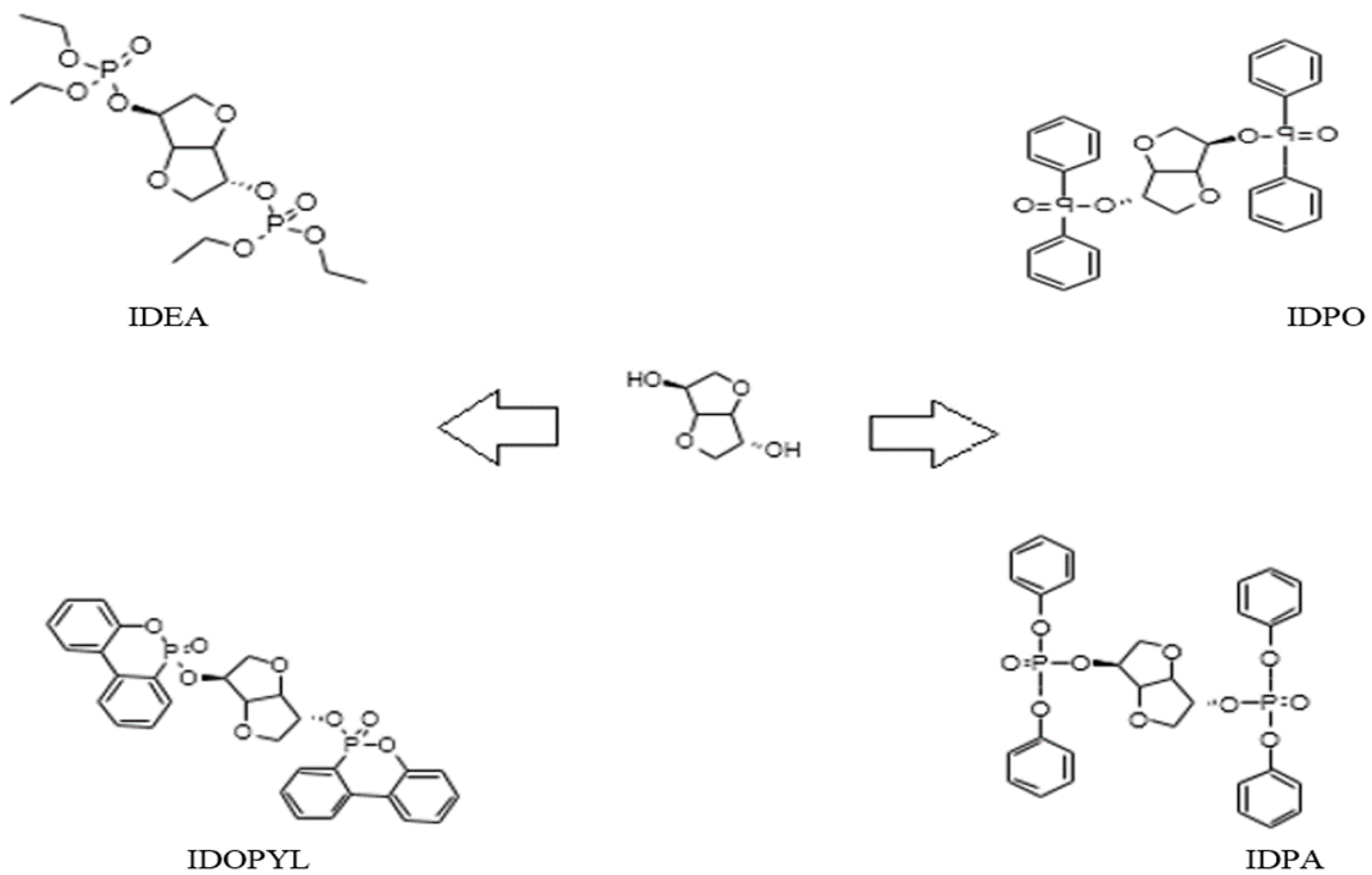
:1. Introduction
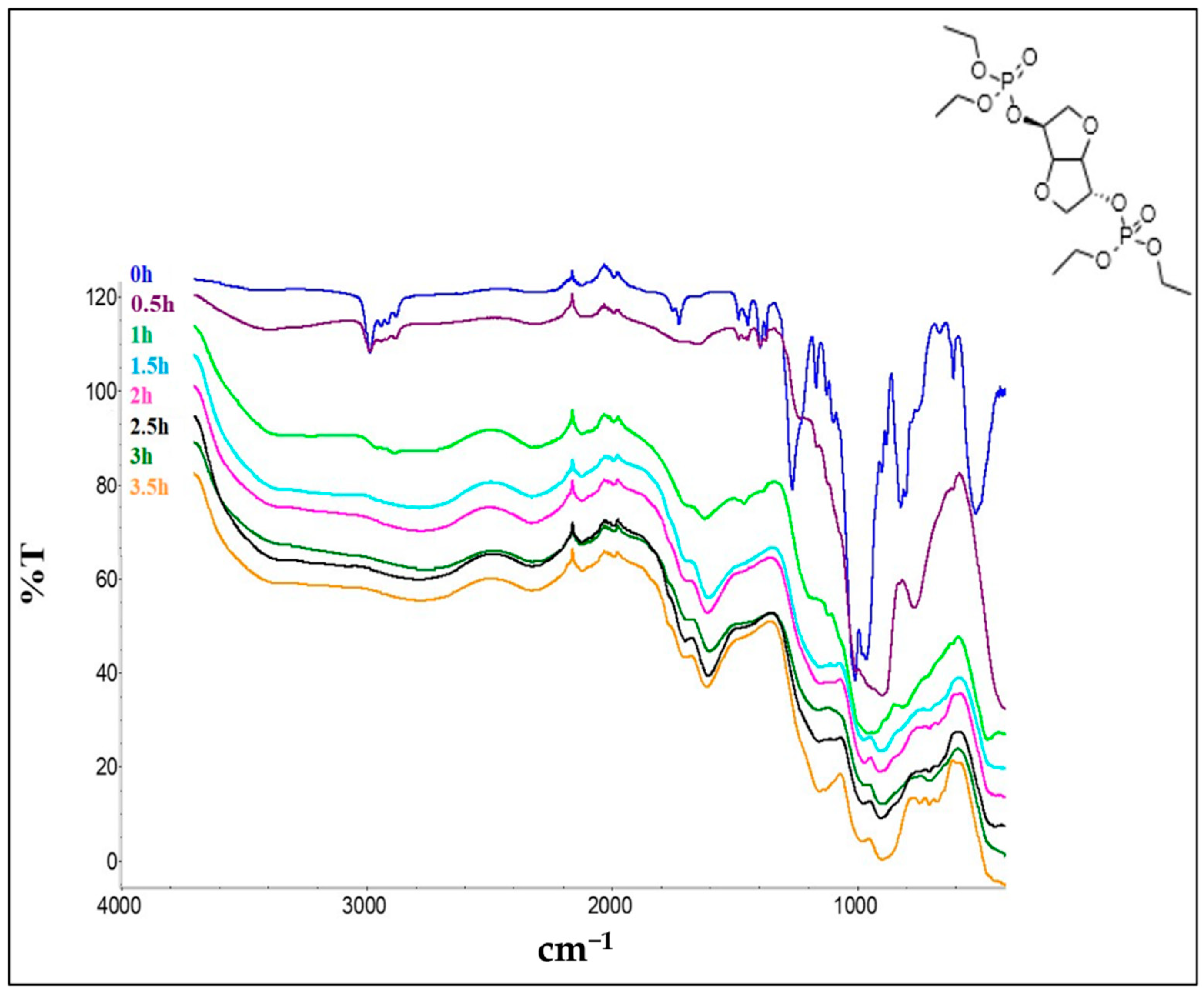
2. Experimental
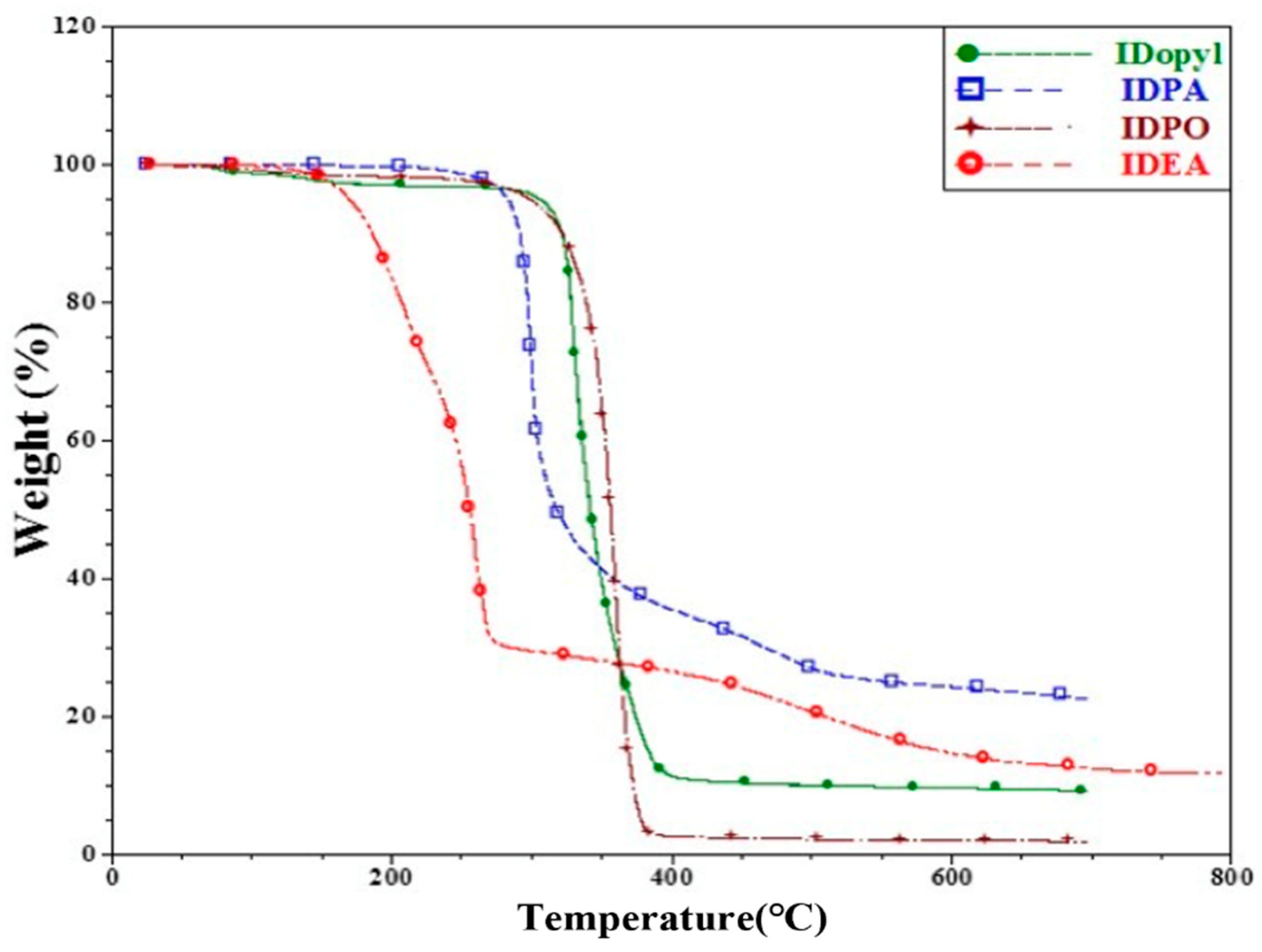
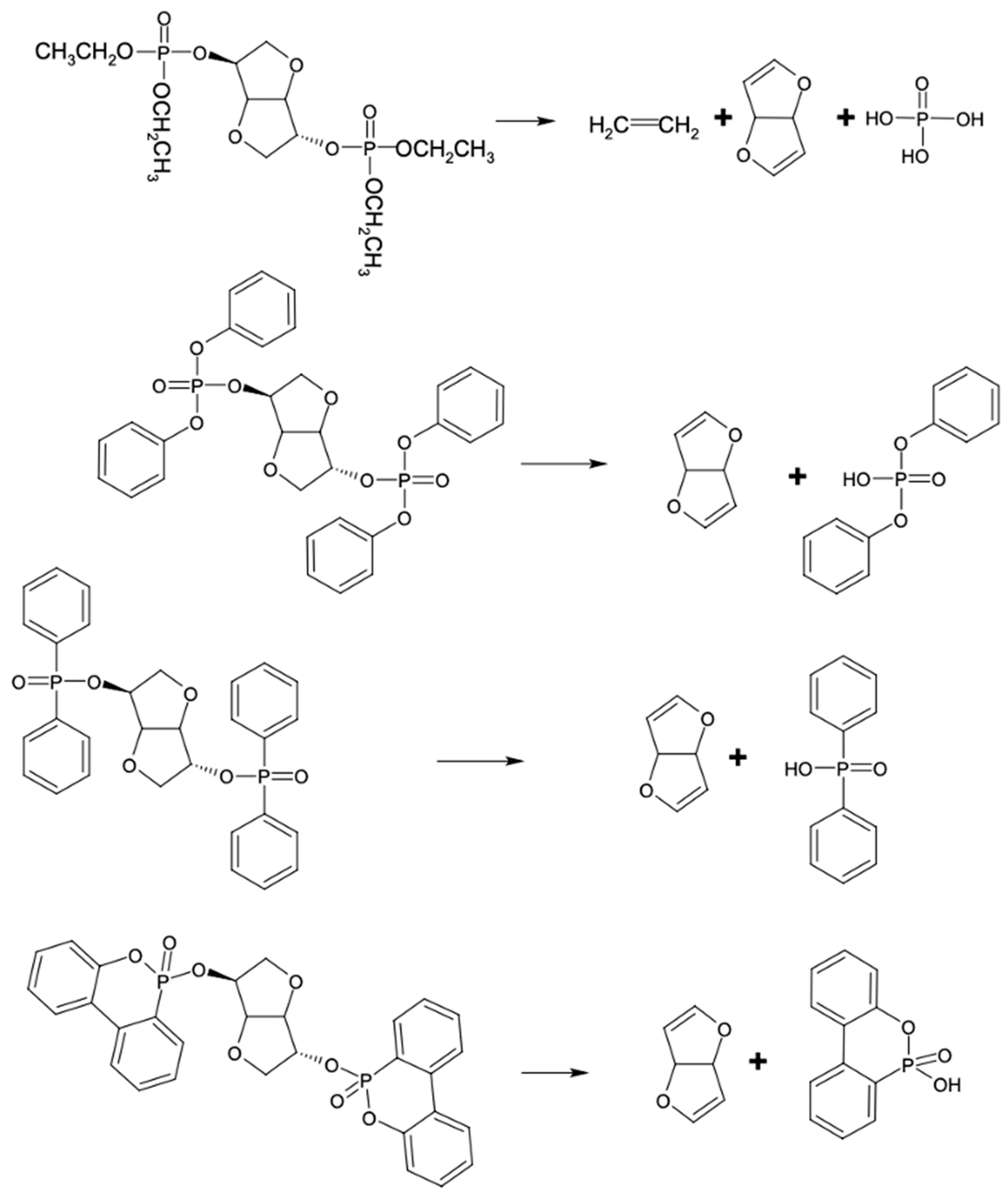
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staudinger, H. Uber Polymerization. Chem. Ber. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Carothers, W.A. An Introduction to the General Theory of Condensation Polymers. J. Am. Chem. Soc. 1929, 51, 2548–2559. [Google Scholar] [CrossRef]
- Furukawa, Y. Inventing Polymer Science; University of Pennsylvania Press: Philadelphia, PA, USA, 1998. [Google Scholar]
- Hermes, M.E. Enough for One Lifetime: Wallace Carothers, Inventor of Nylon; Chemical Heritage Foundation: Philadelphia, PA, USA, 1996. [Google Scholar]
- Hounshell, D.A.; Smith, J.K., Jr. Science and Corporate Strategy: Dupont R&D, 1920–1980; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Lyons, J.W. The Chemistry and Uses of Fire Retardants; Wiley Interscience, Inc.: New York, NY, USA, 1970. [Google Scholar]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An Overview of Commercially Used Brominated Flame Retardants, their Application, their Use Patterns in Different Countries/Regions and Possible Modes of Release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact During their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Bele, T.G.A.; Lacorte, S.; de Marchi, M.R.R. Occurence of Flame Retardants in Landfills: A Case Study in Brazil. Environ. Res. 2019, 168, 420–427. [Google Scholar] [CrossRef]
- Osako, M.; Kim, Y.-J.; Sakai, S.-I. Leaching of Brominated Flame Retardants in Leachate from Landfills in Japan. Chemosphere 2004, 57, 1571–1579. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Brominated Flame Retardants and the Formation of Dioxins and Furans in Fires. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef]
- Behnisch, P.A.; Hosoe, K.; Sakai, S. Brominated Dioxin-like Compounds: In Vitro Assessment in Comparison to Classical Dioxin-like Compounds and Other Polyaromatic Compounds. Environ. Int. 2003, 29, 861–877. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated Flame Retardants as Possible Endocrine Disruptors. Int. J. Androl. 2008, 31, 152. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonnevelt, E.; Murk, A.J.; Kester, M.H.A.; Anderson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Merkwart, J.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, B.A.; Daniel, Y.G. Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants. Insi. Chem. Biochem. 2020, 1. [Google Scholar] [CrossRef]
- Factor, A. The Chemistry of Polymer Burning and Flame Retardance. J. Chem. Enduc. 1974, 51, 453–456. [Google Scholar] [CrossRef]
- Granzow, A. Flame Retardation by Phosphorus Compounds. Accounts Chem. Res. 1978, 11, 177–183. [Google Scholar] [CrossRef]
- Braun, U.; Balbanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doering, M.; Perez, R.; Sandler, J.K.W.; Alstadt, V.; et al. Influence of the Oxidation State of PHosphorus on the Decomposition and Fire Behaviorof Flame-retarded Epoxy Resin Composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of Phosphorus Valency on the Thermal Behavior of Flame Retarded Polyurethane Foam. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Sonnier, R.; Negrell-Guirao, C.; Vahabi, H.; Otazaghine, B.; David, G.; Lopez-Cuesta, J.M. Relationship Between the Molecular Structure and Flammability of Polymers: Study of Phosphonate Functions Using a Microscale Combustion Calorimeter. Polymer 2012, 53, 1258–1266. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Kramer, J.; Mueller, P.; Alstadt, V.; Zhang, L.; Doering, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar]
- Schafer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Doering, M. Synthesis and Properties of Flame-retardant Epoxy Resins Based on DOPO and One of Its Analogs DPPO. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisins, N.M.; Bodi, A.; Grutzmacher, H.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, a Key Species in Flame-retardant Mechanisms. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef]
- Howell, B.A.; Lienhart, G.W. Phosphorus Esters of 1-Dopyl-1,2-(4-hydroxyphenyl)ethene as Effective Flame Retardants for Polymeric Materials. Fire Mater. 2020, 44, 1127–1134. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Synthesis and Characterization of Isosorbide bis-Phosphorus Esters. Heteroat. Chem. 2017, 28, e21369. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame Retardant Properties of Isosorbide bis-Phosphorus Esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Thermal Degradation of bis-Phosphorus Esters of Isosorbide. J. Therm. Anal. Calorim. 2018, 131, 363–369. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Incorporation of Comonomer exo-5-(Diphenylphosphato)isorsorbide-2-endo-acrylate to Generate Flame Retardant Poly(styrene). Polymers 2019, 11, 2033. [Google Scholar] [CrossRef] [Green Version]
- Howell, B.A.; Ostrander, E.A. Thermal Degradation of Flame-retardant Compounds Derived from Castor Oil. J. Therm. Anal. Calorim. 2019, 138, 3961–3975. [Google Scholar] [CrossRef]
- Howell, B.A.; Zhang, T.; Smith, P.B. Biobased Hyperbranched Poly(ester)s of Precise Structure for the Release of Therapeutics. Med. Res. Arch. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Smith, P.B. Rational Synthesis of Hyperbranched Poly(ester)s. Ind. Eng. Chem. Res. 2017, 56, 1661–1670. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and Characterization of Glycerol-Adipic Acid Hyperbranched Poly(ester)s. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Howell, B.A.; Lazar, S.T. Biobased Plasticizers from Glycerol/Adipic Acid Hyperbranched Poly(ester)s. Ind. Eng. Chem. Res. 2019, 58, 17227–17234. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, B.A. Thermal Degradation of Organophosphorus Flame Retardants. Polymers 2022, 14, 4929. https://doi.org/10.3390/polym14224929
Howell BA. Thermal Degradation of Organophosphorus Flame Retardants. Polymers. 2022; 14(22):4929. https://doi.org/10.3390/polym14224929
Chicago/Turabian StyleHowell, Bob A. 2022. "Thermal Degradation of Organophosphorus Flame Retardants" Polymers 14, no. 22: 4929. https://doi.org/10.3390/polym14224929
APA StyleHowell, B. A. (2022). Thermal Degradation of Organophosphorus Flame Retardants. Polymers, 14(22), 4929. https://doi.org/10.3390/polym14224929





