Porphyrin Polymers Bearing N,N′-Ethylene Crosslinkers as Photosensitizers against Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PSs
2.2. Spectroscopic Determinations
2.3. Photooxidation of 9,10-Dimethylanthracene (DMA)
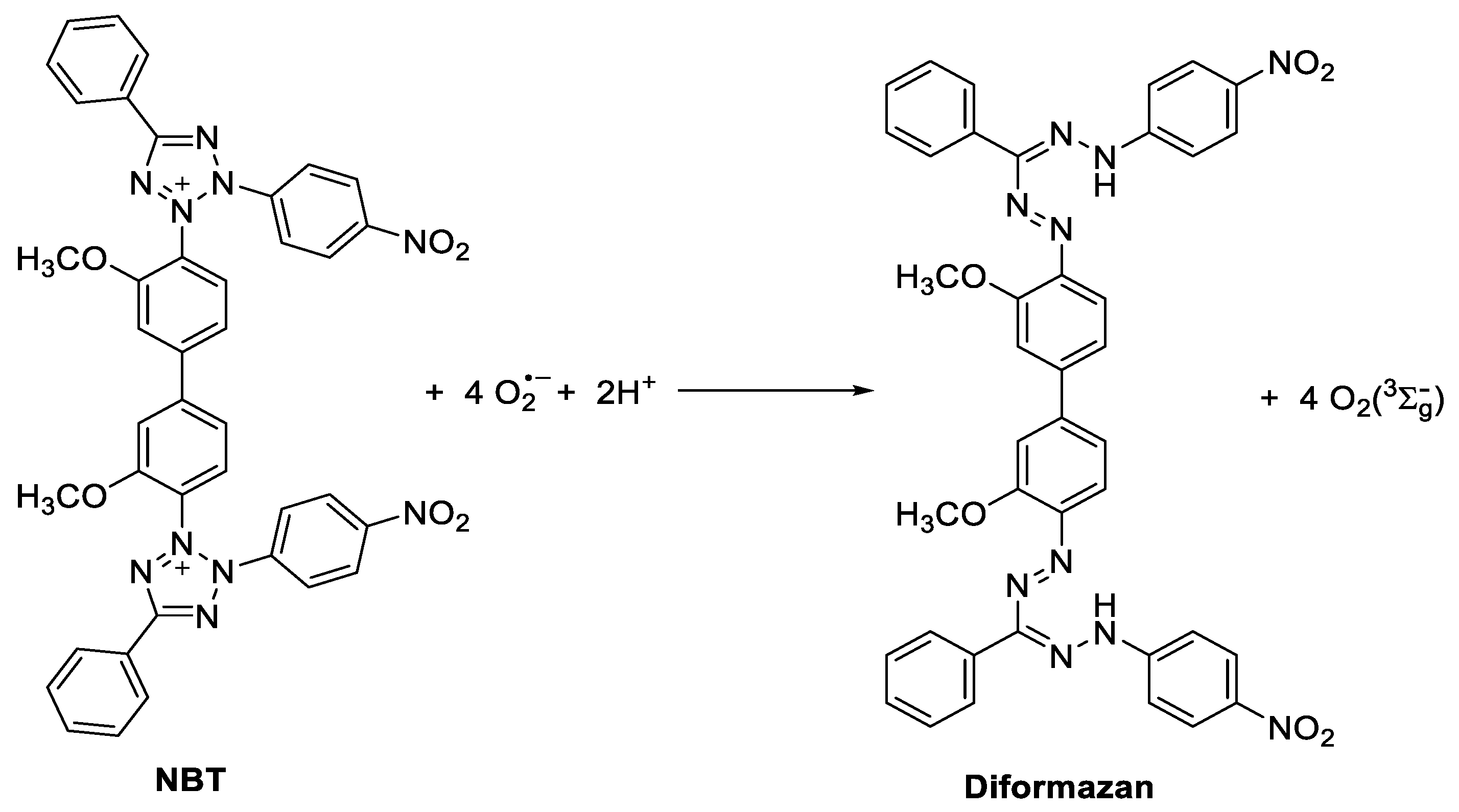
2.4. Photoreduction of Nitrotetrazolium Blue (NBT)
2.5. Bacterial Strains and Growth Conditions
2.6. Photosensitized Inactivation of Bacterial Suspensions
2.7. Formation of Triiodine (I3−)
2.8. Controls and Statistical Analysis
3. Results and Discussion
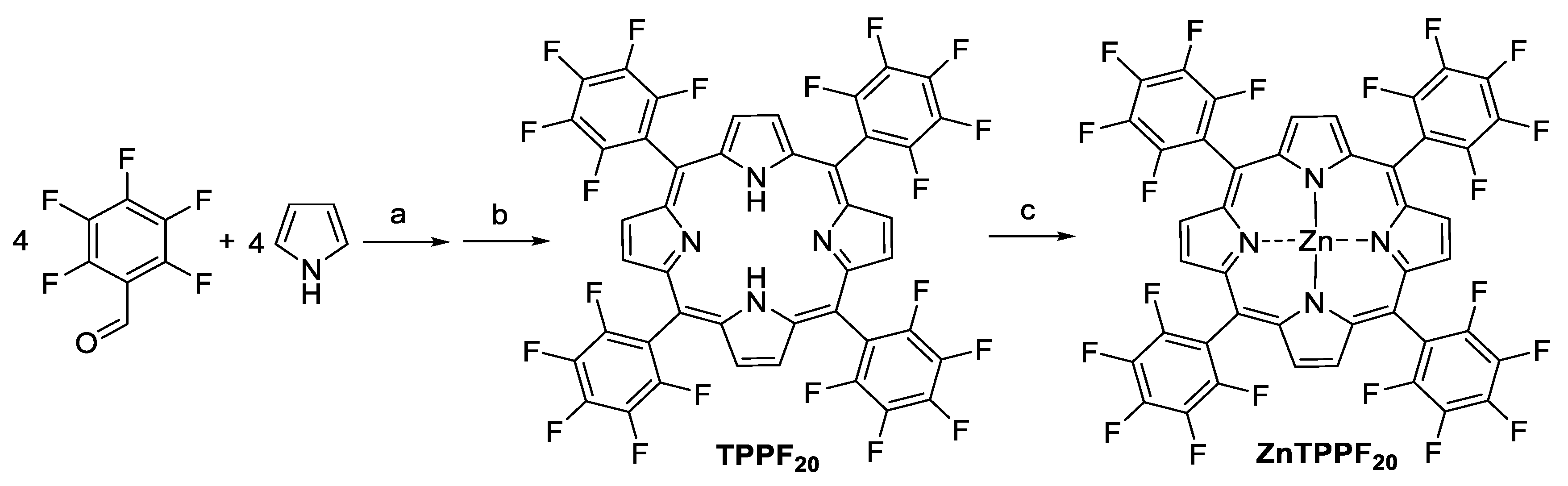
3.1. Synthesis of TPPF20 and ZnTPPF20
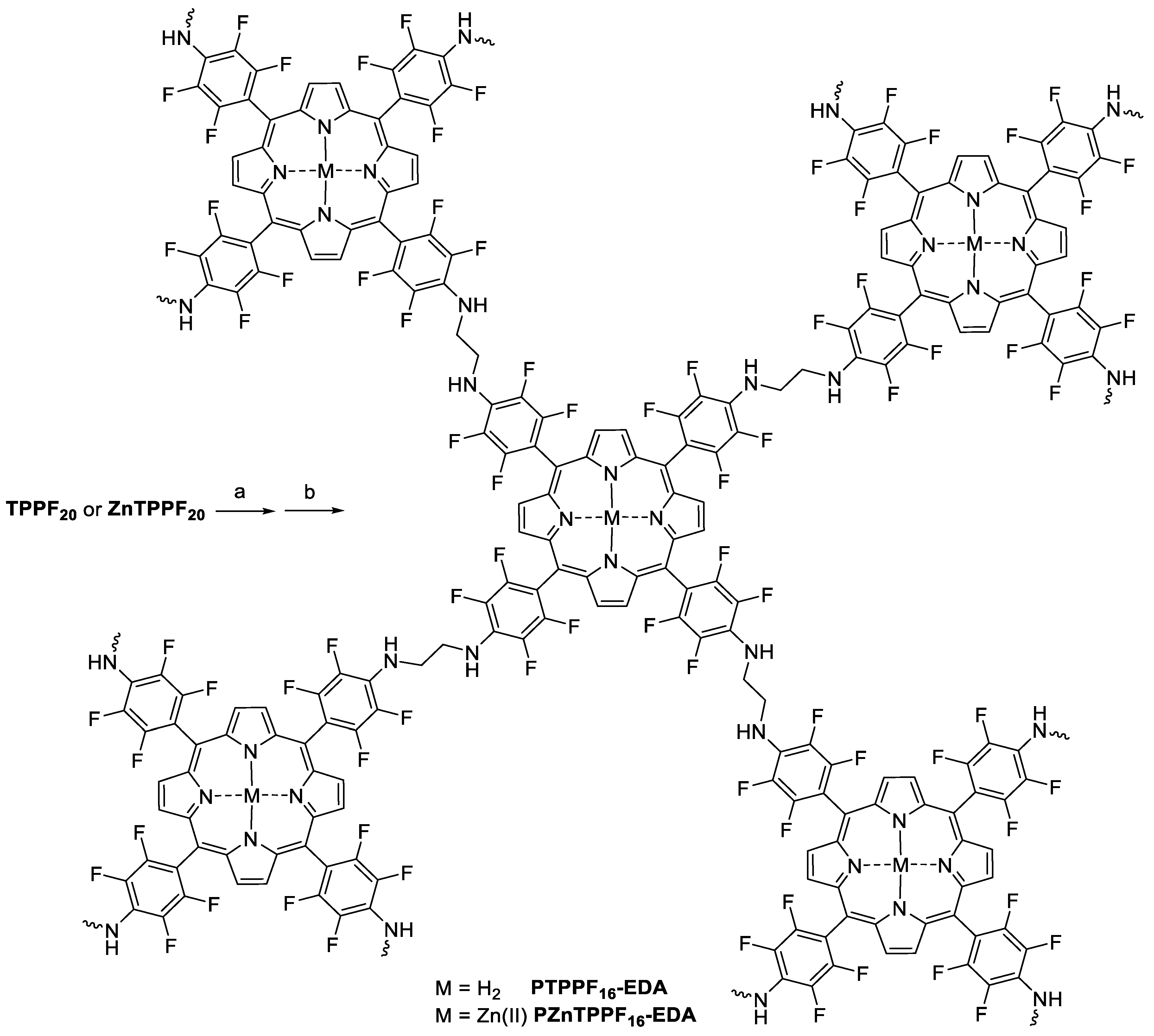
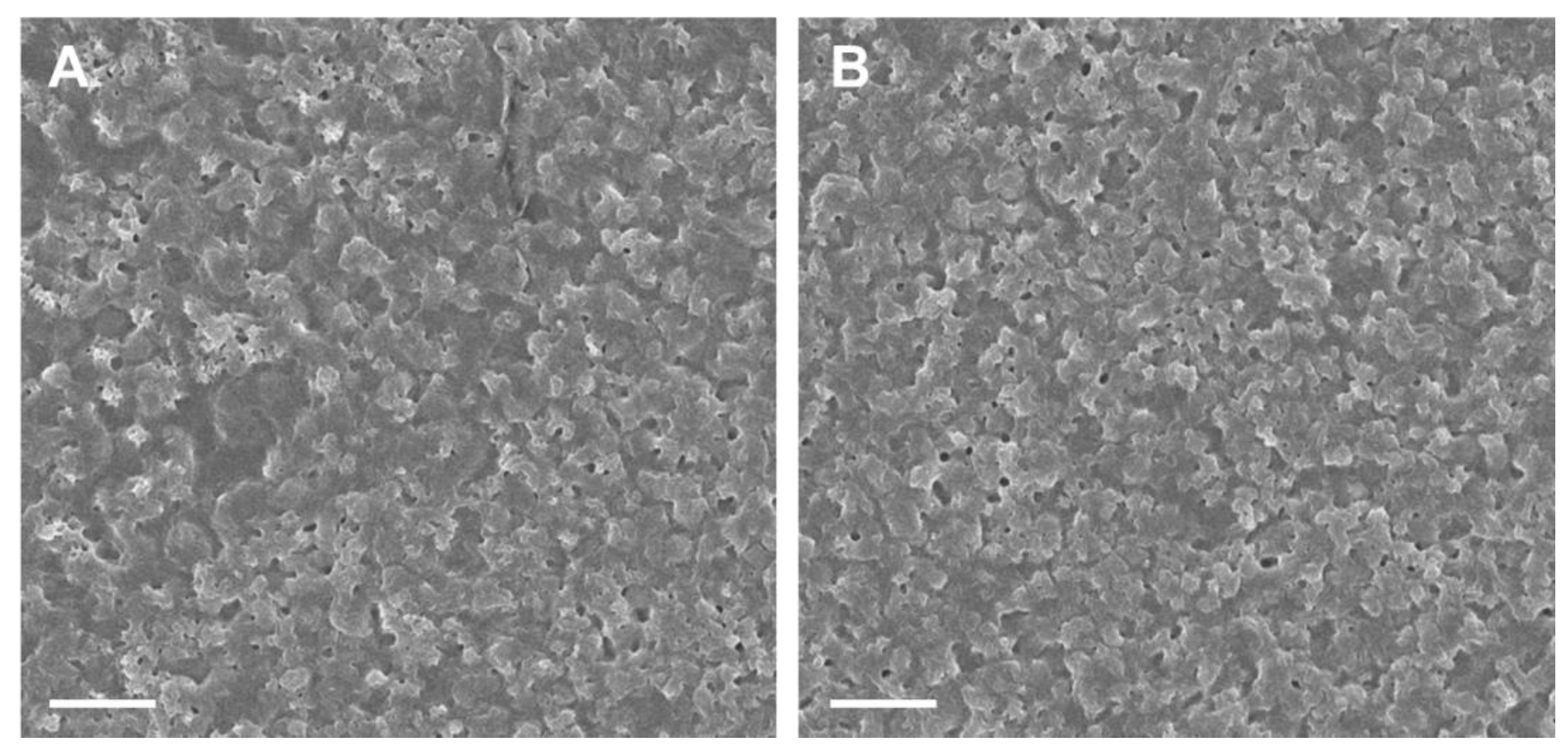
3.2. Synthesis of PTPPF16-EDA and PZnTPPF16-EDA
3.3. UV-Visible Spectroscopic Characterization
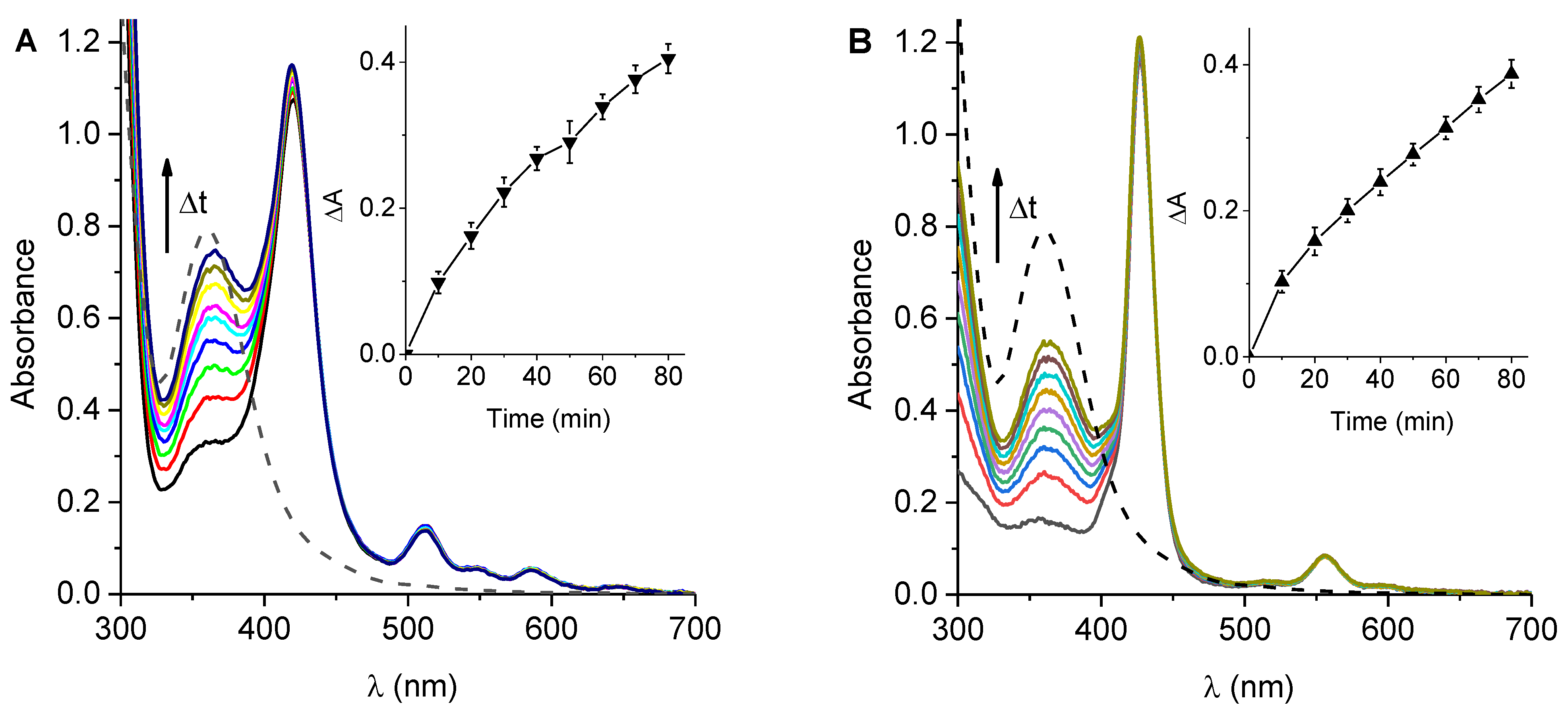
3.4. Production of O2(1Δg)
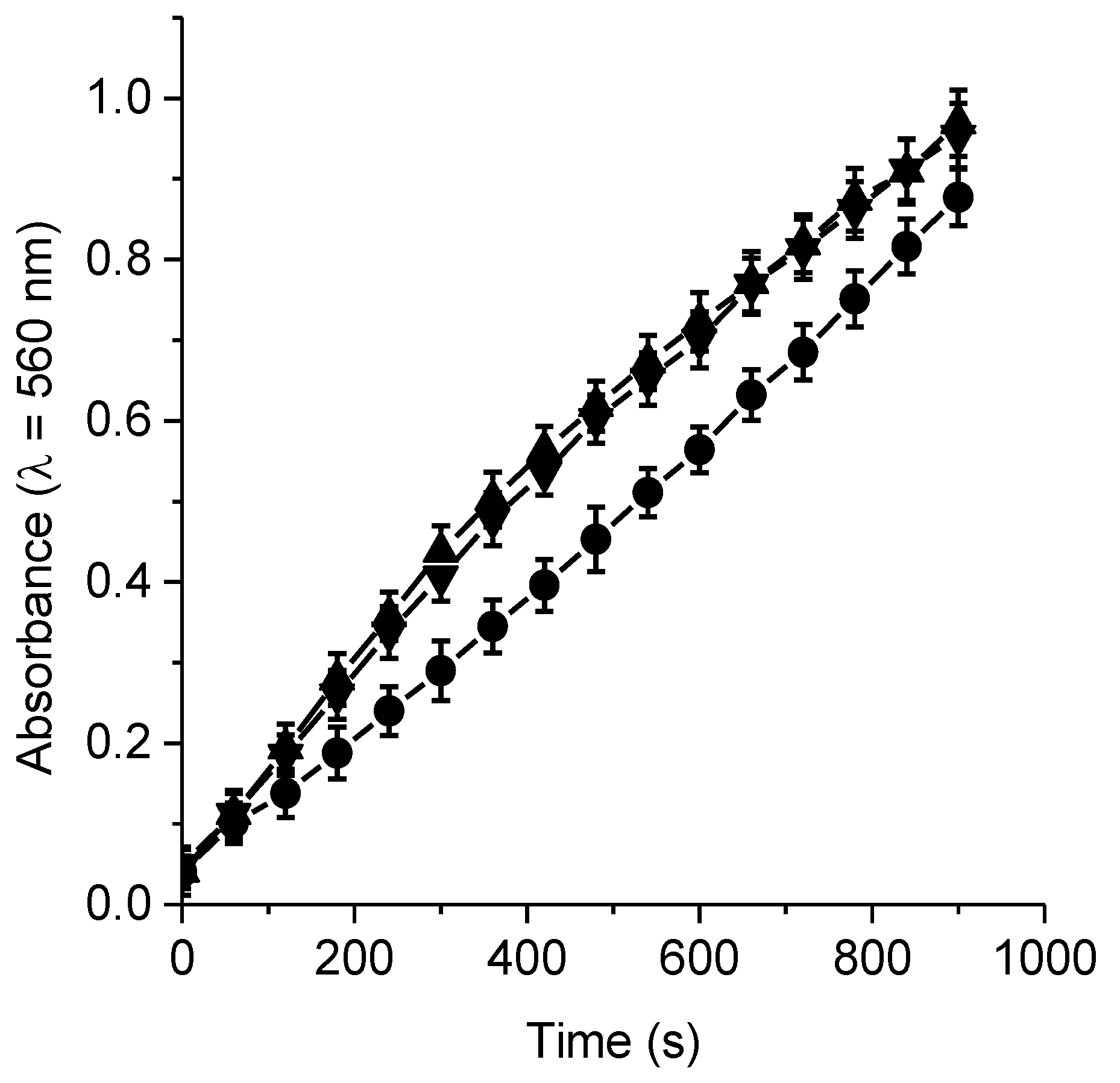
3.5. Formation of O2•−
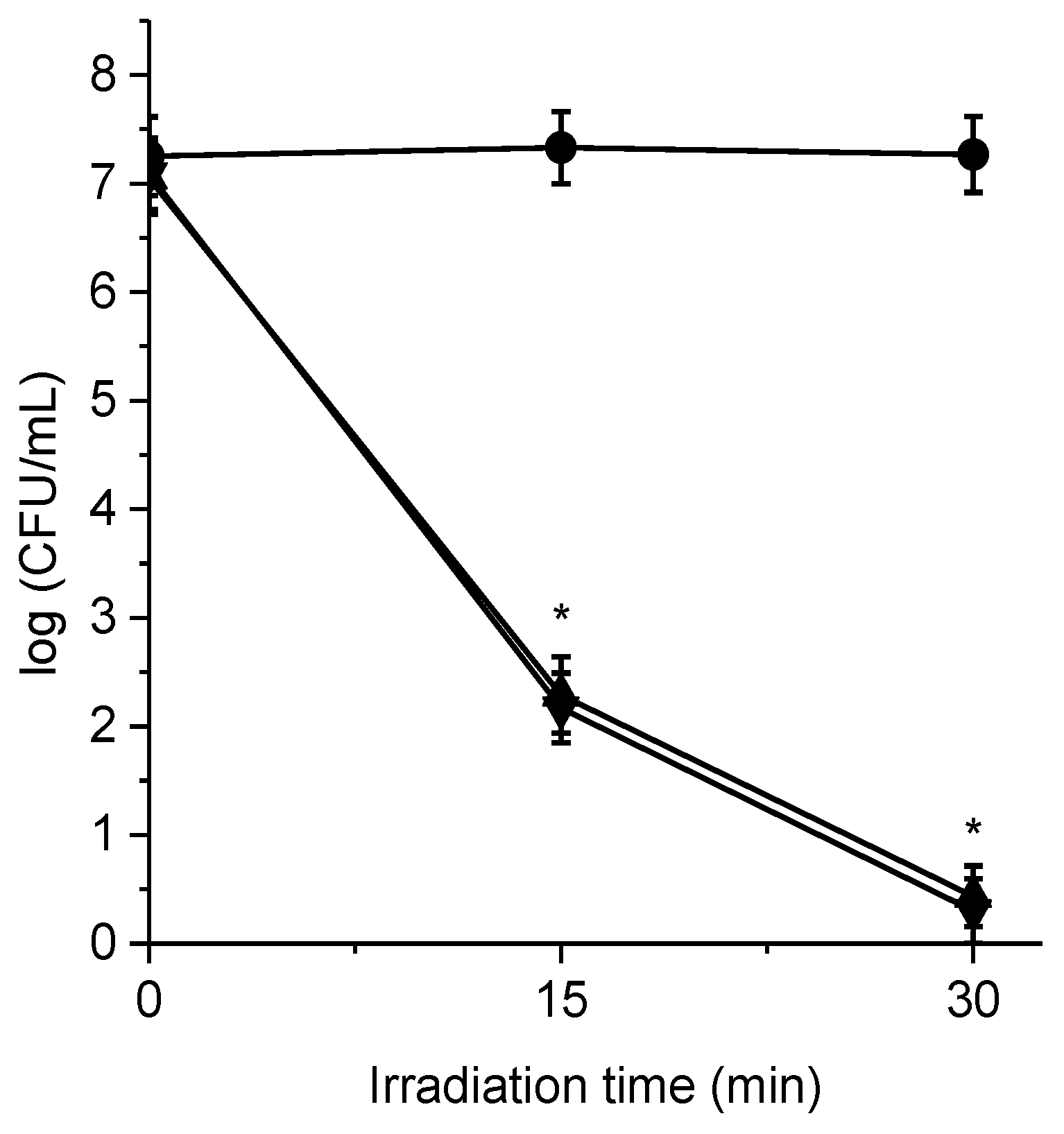
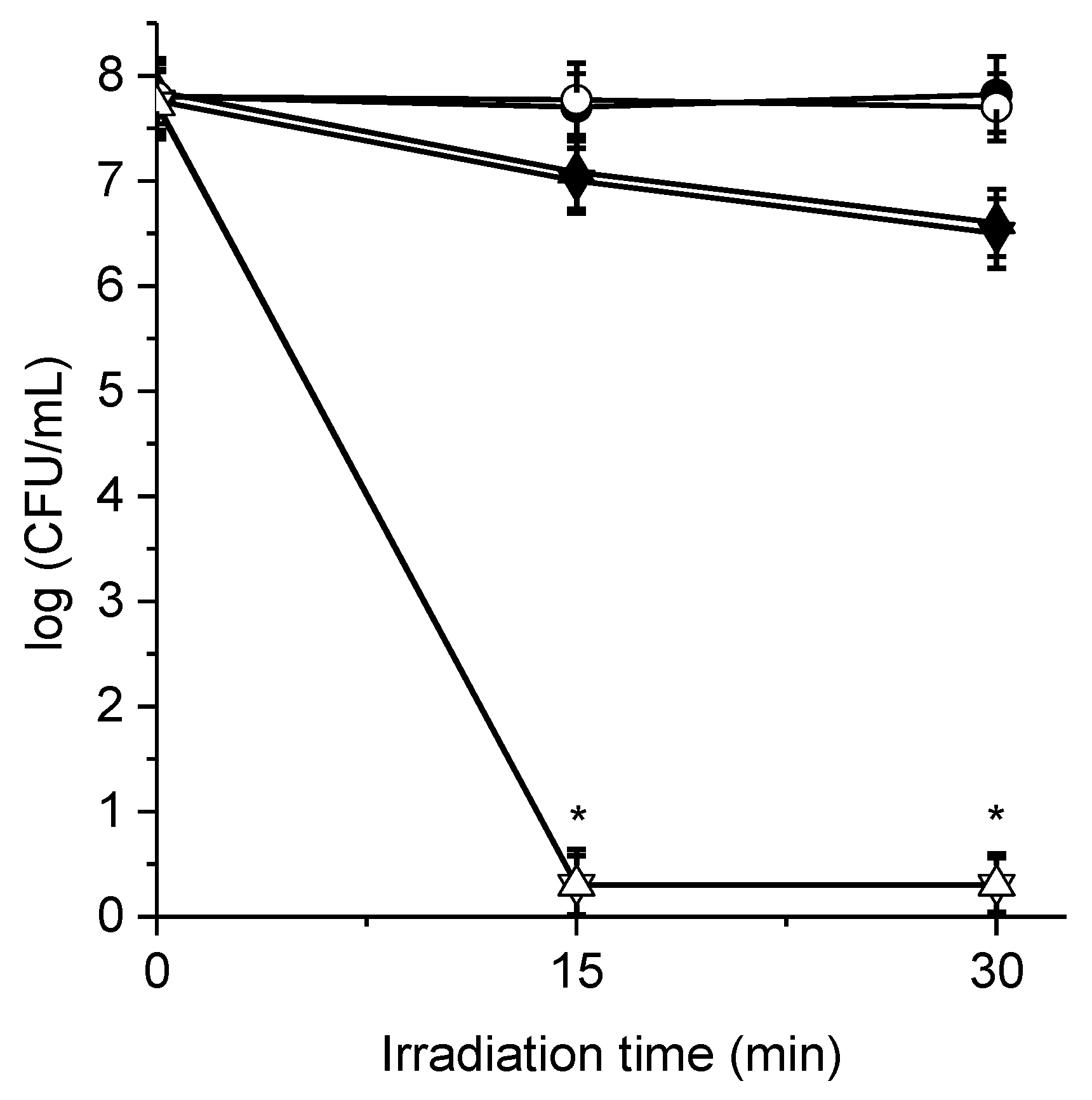
3.6. Photoinactivation of Bacterial Cell Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioannou, P.; Karakonstantis, S.; Schouten, J.; Kostyanev, T.; Charani, E.; Vlahovic-Palcevski, V.; Kofteridis, D.P. Indications for medical antibiotic prophylaxis and potential targets for antimicrobial stewardship intervention: A narrative review. Clin. Microbiol. Infect. 2021, 28, 362–370. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Puvača, N.; Frutos, R.D.L. Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals. Antibiotics 2021, 10, 69. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.-K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Bentham Science Publisher Hemlata; Jan, A.T.; Tiwari, A. The Ever Changing Face of Antibiotic Resistance: Prevailing Problems and Preventive Measures. Curr. Drug Metab. 2017, 18, 69–77. [Google Scholar] [CrossRef]
- Aslam, A.; Gajdács, M.; Zin, C.S.; Ab Rahman, N.S.; Ahmed, S.I.; Zafar, M.Z.; Jamshed, S. Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review. Antibiotics 2020, 9, 597. [Google Scholar] [CrossRef]
- Jamshed, S.; Padzil, F.; Shamsuddin, S.H.; Bux, S.H.; Jamaluddin, A.A.; Bhagavathula, A.S.; Azhar, S.; Hassali, M.A.A. Antibiotic Stewardship in Community Pharmacies: A Scoping Review. Pharmacy 2018, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Gajdács, M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Puvača, N.; Lika, E.; Tufarelli, V.; Bursić, V.; Pelić, D.L.; Nikolova, N.; Petrović, A.; Prodanović, R.; Vuković, G.; Lević, J.; et al. Influence of different tetracycline antimicrobial therapy of mycoplasma (Mycoplasma synoviae) in laying hens compared to tea tree essential oil on table egg quality and antibiotics residues. Foods 2020, 9, 612. [Google Scholar] [CrossRef]
- Shankar, N.; Soe, P.-M.; Tam, C.C. Prevalence and risk of acquisition of methicillin-resistant Staphylococcus aureus among households: A systematic review. Int. J. Infect. Dis. 2020, 92, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Rao, Y.; Li, J.; Huang, Q.; Rao, X. Staphylococcus aureus small-colony variants: Formation, infection, and treatment. Microbiol. Res. 2022, 260, 127040. [Google Scholar] [CrossRef]
- Montealegre, M.C.; Roy, S.; Böni, F.; Hossain, M.I.; Navab-Daneshmand, T.; Caduff, L.; Faruque, A.S.G.; Islam, M.A.; Julian, T.R. Risk Factors for Detection, Survival, and Growth of Antibiotic-Resistant and Pathogenic Escherichia coli in Household Soils in Rural Bangladesh. Appl. Environ. Microbiol. 2018, 84, e01978-18. [Google Scholar] [CrossRef] [Green Version]
- Aijuka, M.; Buys, E.M. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: Implications for food safety and public health. Food Microbiol. 2019, 82, 363–370. [Google Scholar] [CrossRef]
- Dunn, S.J.; Connor, C.; McNally, A. The evolution and transmission of multi-drug resistant Escherichia coli and Klebsiella pneumoniae: The complexity of clones and plasmids. Curr. Opin. Microbiol. 2019, 51, 51–56. [Google Scholar] [CrossRef]
- Petrocchi-Rilo, M.; Gutiérrez-Martín, C.-B.; Pérez-Fernández, E.; Vilaró, A.; Fraile, L.; Martínez-Martínez, S. Antimicrobial Resistance Genes in Porcine Pasteurella multocida Are Not Associated with Its Antimicrobial Susceptibility Pattern. Antibiotics 2020, 9, 614. [Google Scholar] [CrossRef]
- Torban, A.S.; Venezia, S.N.; Kelmer, E.; Cohen, A.; Paitan, Y.; Arielly, H.; Steinman, A. Extended-spectrum β-lactamase-producing Enterobacterles shedding by dogs and cats hospitalized in an emergency and critical care department of a veterinary teaching hospital. Antibiotics 2020, 9, 545. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Schwengers, O.; Schmiedel, J.; Baars, C.; Lambrecht, O.; Heß, S.; Berendonk, T.U.; Falgenhauer, J.; Chakraborty, T.; Imirzalioglu, C. Multidrug-Resistant and Clinically Relevant Gram-Negative Bacteria Are Present in German Surface Waters. Front. Microbiol. 2019, 10, 2779. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Nadais, H.; Almeida, A. Potencial applications of porphyrins un photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C. Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef] [Green Version]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, W. Synthesis, self-assembly and applications of functional polymers based on porphyrins. Prog. Polym. Sci. 2019, 95, 65–117. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.-T. Recent advances in engineered polymeric materials for efficient photodynamic inactivation of bacterial pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef]
- Souza, T.H.; Sarmento-Neto, J.F.; Souza, S.O.; Raposo, B.L.; Silva, B.P.; Borges, C.P.; Santos, B.S.; Filho, P.E.C.; Rebouças, J.S.; Fontes, A. Advances on antimicrobial photodynamic inactivation mediated by Zn(II) porphyrins. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100454. [Google Scholar] [CrossRef]
- Wu, K.; Guo, J.; Wang, C. Gelation of Metalloporphyrin-Based Conjugated Microporous Polymers by Oxidative Homocoupling of Terminal Alkynes. Chem. Mater. 2014, 26, 6241–6250. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Co-Ord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Bhupathiraju, N.V.S.D.K.; Rizvi, W.; Batteas, J.D.; Drain, C.M. Fluorinated porphyrinoids as efficient platforms for new photonic materials, sensors, and therapeutics. Org. Biomol. Chem. 2015, 14, 389–408. [Google Scholar] [CrossRef]
- Kvíčala, J.; Beneš, M.; Paleta, O.; Král, V. Regiospecific nucleophilic substitution in 2,3,4,5,6-pentafluorobiphenyl as model compound for supramolecular systems. Theoretical study of transition states and energy profiles, evidence for tetrahedral SN2 mechanism. J. Fluor. Chem. 2010, 131, 1327–1337. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, J.E.; Ferreyra, D.D.; Reynoso, E.; Lopez, E.J.G.; Durantini, A.M.; Milanesio, M.E.; Durantini, E.N. Charge density distribution effect in pyrrolidine-fused chlorins on microbial uptake and antimicrobial photoinactivation of microbial pathogens. J. Photochem. Photobiol. B Biol. 2021, 225, 112321. [Google Scholar] [CrossRef]
- Golf, H.R.A.; Reissig, H.-U.; Wiehe, A. Regioselective nucleophilic aromatic substitution reaction of meso-pentafluorophenyl-substituted porphyrinoids with alcohols. Eur. J. Org. Chem. 2015, 2015, 1548–1568. [Google Scholar] [CrossRef]
- Costa, D.C.; Pais, V.F.; Silva, A.M.; Cavaleiro, J.A.; Pischel, U.; Tomé, J.P. Cationic porphyrins with inverted pyridinium groups and their fluorescence properties. Tetrahedron Lett. 2014, 55, 4156–4159. [Google Scholar] [CrossRef]
- Milanesio, M.E.; Alvarez, M.G.; Yslas, E.I.; Borsarelli, C.D.; Silber, J.J.; Rivarola, V.; Durantini, E.N. Photodynamic studies of metallo 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin: Photochemical characterization and biological consequences in a human carcinoma cell line. Photochem. Photobiol. 2001, 74, 14–21. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.; Görner, H.; Lacerda, P.S.; MacDonald, J.; Mark, G.; Neves, M.G.; Nohr, R.S.; Schuchmann, H.-P.; von Sonntag, C.; Tomé, A.C. Singlet oxygen formation and photostability of meso-tetraarylporphyrin derivatives and their copper complexes. J. Photochem. Photobiol. A Chem. 2001, 144, 131–140. [Google Scholar] [CrossRef]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl] chlorin as potential broad-spectrum antimicrobial photosensitizers. J. Photochem. Photobiol. B. Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin–Schiff Base Conjugates Bearing Basic Amino Groups as Antimicrobial Phototherapeutic Agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef]
- Gardner, J.M.; Abrahamsson, M.; Farnum, B.H.; Meyer, G.J. Visible light generation of iodine atoms and I-I bonds: Sensitized I−oxidation and I3−photodissociation. J. Am. Chem. Soc. 2009, 131, 16206–16214. [Google Scholar] [CrossRef] [Green Version]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef]
- Dommaschk, M.; Gutzeit, F.; Boretius, S.; Haag, R.; Herges, R. Coordination-Induced Spin-State-Switch (CISSS) in water. Chem. Commun. 2014, 50, 12476–12478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.I.; Tomé, A.; Neves, M.D.G.P.M.S.; Cavaleiro, J. 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Banfi, S.; Alberton, A.S.; Caruso, E. Photodynamic activity of new photosensitizers obtained from 5,10,15,20-tetrapentafluorophenylporphyrin. J. Porphyr. Phthalocyanines 2019, 23, 1047–1056. [Google Scholar] [CrossRef]
- Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins. Antibiotics 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad-spectrum antimicrobial agents. Photodiagnosis Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef]
- Baigorria, E.; Milanesio, M.E.; Durantini, E.N. Synthesis, spectroscopic properties and photodynamic activity of Zn(II) phthalocyanine-polymer conjugates as antimicrobial agents. Eur. Polym. J. 2020, 134, 109816. [Google Scholar] [CrossRef]
- Çetinkaya, A.; Sadak, A.E.; Ayhan, M.M.; Zorlu, Y.; Kahveci, M.U. Porphyrin-based covalent organic polymer by inverse electron demand Diels-Alder reaction. Eur. Polym. J. 2021, 157, 110664. [Google Scholar] [CrossRef]
- Guo, D.; Li, C.; Liu, G.; Luo, X.; Wu, F. Oxidase Mimetic Activity of a Metalloporphyrin-Containing Porous Organic Polymer and Its Applications for Colorimetric Detection of Both Ascorbic Acid and Glutathione. ACS Sustain. Chem. Eng. 2021, 9, 5412–5421. [Google Scholar] [CrossRef]
- Grancho, J.C.P.; Pereira, M.M.; Miguel, M.D.G.; Gonsalves, A.M.R.; Burrows, H.D. Synthesis, Spectra and Photophysics of some Free Base Tetrafluoroalkyl and Tetrafluoroaryl Porphyrins with Potential Applications in Imaging. Photochem. Photobiol. 2007, 75, 249–256. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Sarotti, A.M.; Gsponer, N.S.; Ferreyra, D.D.; Bertolotti, S.G.; Milanesio, M.E.; Durantini, E.N. Proton-Dependent Switching of a Novel Amino Chlorin Derivative as a Fluorescent Probe and Photosensitizer for Acidic Media. Chem. Eur. J. 2018, 24, 5950–5961. [Google Scholar] [CrossRef]
- Spellane, P.J.; Gouterman, M.; Antipas, A.; Kim, S.; Liu, Y.C. Porphyrins. 40. Electronic spectra and four-orbital energies of free-base, zinc, copper, and palladium tetrakis(perfluorophenyl)porphyrins. Inorg. Chem. 1980, 19, 386–391. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis, spectroscopic properties and photodynamic activity of porphyrin–fullerene C60 dyads with application in the photodynamic inactivation of Staphylococcus aureus. Eur. J. Med. Chem. 2014, 83, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Sampaio, R.; Ito, A.; Batista, A.; Machado, A.; Araujo, P.; Neto, N.B. Evolution of electronic and vibronic transitions in metal(II) meso-tetra(4-pyridyl)porphyrins. Biomol. Spectrosc. 2019, 215, 327–333. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Scalise, I.; Durantini, E.N. Photodynamic effect of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl)porphyrins in biomimetic AOT reverse micelles containing urease. J. Photochem. Photobiol. A Chem. 2004, 162, 105–113. [Google Scholar] [CrossRef]
- Bartosz, G. Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta 2006, 368, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, D.D.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis and photodynamic properties of 5,10,15,20-tetrakis[3-(N-ethyl-N-methylcarbazoyl)]chlorin and its analogous porphyrin in solution and in human red blood cells. J. Photochem. Photobiol. A Chem. 2014, 282, 16–24. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Mechanistic insight into the photodynamic effect mediated by porphyrin-fullerene C60 dyads in solution and in Staphylococcus Aureus Cells. RSC Adv. 2018, 8, 22876–22886. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Futur. Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Hynek, J.; Zelenka, J.; Rathouský, J.; Kubát, P.; Ruml, T.; Demel, J.; Lang, K. Designing Porphyrinic Covalent Organic Frameworks for the Photodynamic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8527–8535. [Google Scholar] [CrossRef]
- Meng, F.-L.; Qian, H.-L.; Yan, X.-P. Conjugation-regulating synthesis of high photosensitizing activity porphyrin-based covalent organic frameworks for photodynamic inactivation of bacteria. Talanta 2021, 233, 122536. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Moura, N.M.M.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, Â.; Almeida Paz, F.A.; Mendes, R.F.; Almeida, A.; Freire, C.S.R.; et al. Synthesis and characterization of photoactive porphyrin and poly(2-hydroxyethyl methacrylate) based materials with bactericidal properties. Appl. Mater. Today 2019, 16, 332–341. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Agazzi, M.L.; Spesia, M.B.; Durantini, E.N. Approaches to unravel pathways of reactive oxygen species in the photoinactivation of bacteria induced by a dicationic fulleropyrrolidinium derivative. Methods 2016, 109, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A novel tricationic fullerene C60 as broad-spectrum antimicrobial photosensitizer: Mechanisms of action and potentiation with potassium iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic inactivation of microorganisms sensitized by cationic BODIPY derivatives potentiated by potassium iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; El-Hussein, A.; Xuan, W.; Hamblin, M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B Biol. 2017, 178, 277–286. [Google Scholar] [CrossRef]
- Ladeira, B.; Dias, C.; Gomes, A.; Tomé, A.; Neves, M.; Moura, N.; Almeida, A.; Faustino, M. Cationic Pyrrolidine/Pyrroline-Substituted Porphyrins as Efficient Photosensitizers against E. coli. Molecules 2021, 26, 464. [Google Scholar] [CrossRef]
- Baigorria, E.; Durantini, J.E.; Martínez, S.R.; Milanesio, M.E.; Palacios, Y.B.; Durantini, A.M. Potentiation Effect of Iodine Species on the Antimicrobial Capability of Surfaces Coated with Electroactive Phthalocyanines. ACS Appl. Bio Mater 2021, 4, 8559–8570. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.G.; Farnum, B.H.; Ardo, S.; Meyer, G.J. Iodide Chemistry in Dye-Sensitized Solar Cells: Making and Breaking I−I Bonds for Solar Energy Conversion. J. Phys. Chem. Lett. 2010, 1, 3132–3140. [Google Scholar] [CrossRef]
- Mosinger, J.; Janošková, M.; Lang, K.; Kubát, P. Light-induced aggregation of cationic porphyrins. J. Photochem. Photobiol. A. Chem. 2006, 181, 283–289. [Google Scholar] [CrossRef]
- Felgenträger, A.; Maisch, T.; Späth, A.; Schröder, J.A.; Bäumler, W. Singlet oxygen generation in porphyrin-doped polymeric surface coating enables antimicrobial effects on Staphylococcus aureus. Phys. Chem. Chem. Phys. 2014, 16, 20598–20607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, C.; Gomes, A.T.P.D.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed]

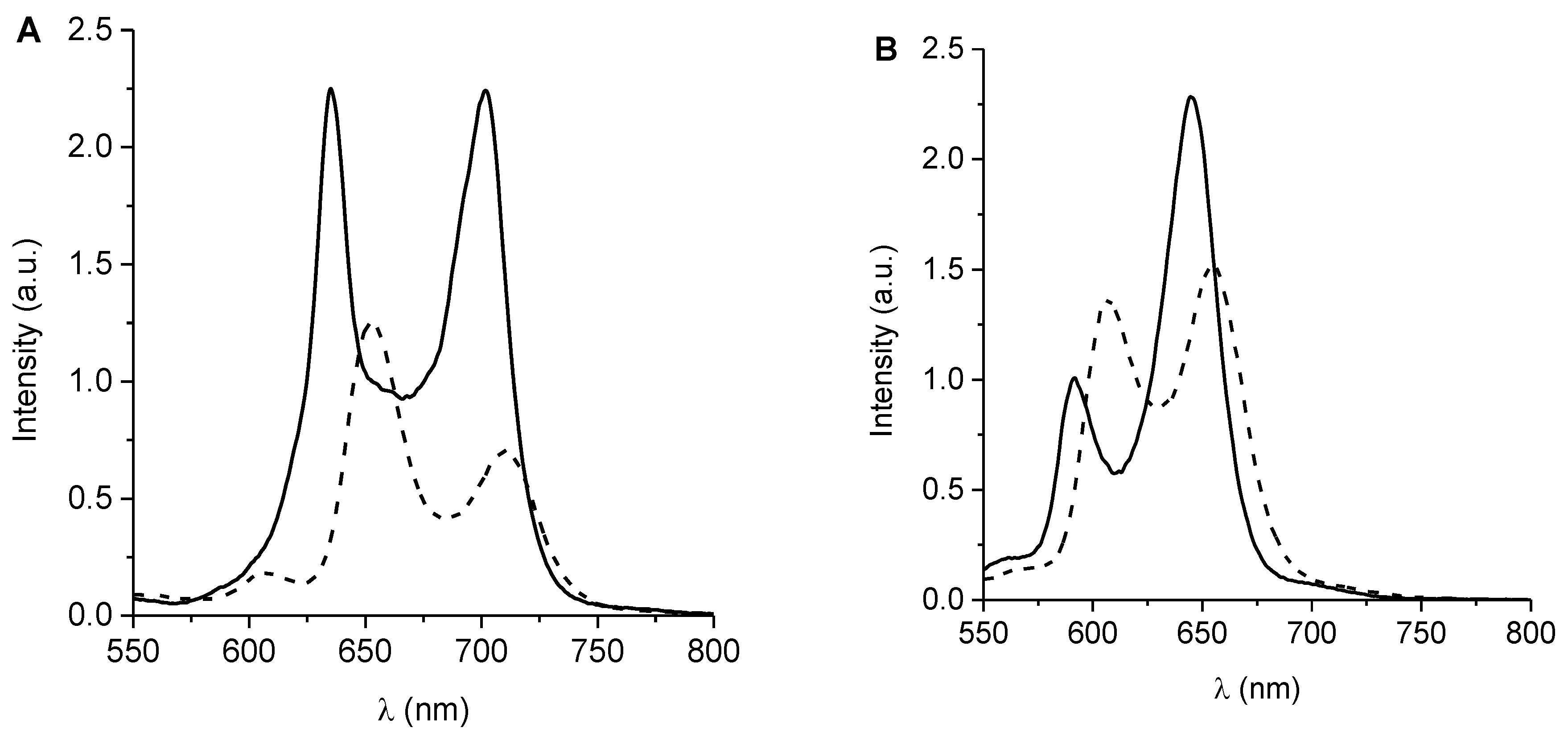


| PS | λSoret (nm) | λem (nm) | ΦF a | kobsDMA (s−1) | ΦΔ d |
|---|---|---|---|---|---|
| TPPF20 | 410 | 635 | 0.039 ± 0.003 | (2.23 ± 0.04) × 10−4 b | 0.80 ± 0.03 e |
| ZnTPPF20 | 419 | 592 | 0.025 ± 0.002 | (2.83 ± 0.05) × 10−4 b | 0.92 ± 0.04 e |
| PTPPF16-EDA | 421 | 653 | 0.030 ± 0.003 | (1.84 ± 0.03) × 10−4 c | 0.75 ± 0.03 f |
| PZnTPPF16-EDA | 428 | 607 | 0.018 ± 0.002 | (2.14 ± 0.04) × 10−4 c | 0.87 ± 0.04 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Porphyrin Polymers Bearing N,N′-Ethylene Crosslinkers as Photosensitizers against Bacteria. Polymers 2022, 14, 4936. https://doi.org/10.3390/polym14224936
Santamarina SC, Heredia DA, Durantini AM, Durantini EN. Porphyrin Polymers Bearing N,N′-Ethylene Crosslinkers as Photosensitizers against Bacteria. Polymers. 2022; 14(22):4936. https://doi.org/10.3390/polym14224936
Chicago/Turabian StyleSantamarina, Sofía C., Daniel A. Heredia, Andrés M. Durantini, and Edgardo N. Durantini. 2022. "Porphyrin Polymers Bearing N,N′-Ethylene Crosslinkers as Photosensitizers against Bacteria" Polymers 14, no. 22: 4936. https://doi.org/10.3390/polym14224936
APA StyleSantamarina, S. C., Heredia, D. A., Durantini, A. M., & Durantini, E. N. (2022). Porphyrin Polymers Bearing N,N′-Ethylene Crosslinkers as Photosensitizers against Bacteria. Polymers, 14(22), 4936. https://doi.org/10.3390/polym14224936







