Polyurethane Foam Waste Upcycling into an Efficient and Low Pollutant Gasification Syngas
Abstract
:1. Introduction
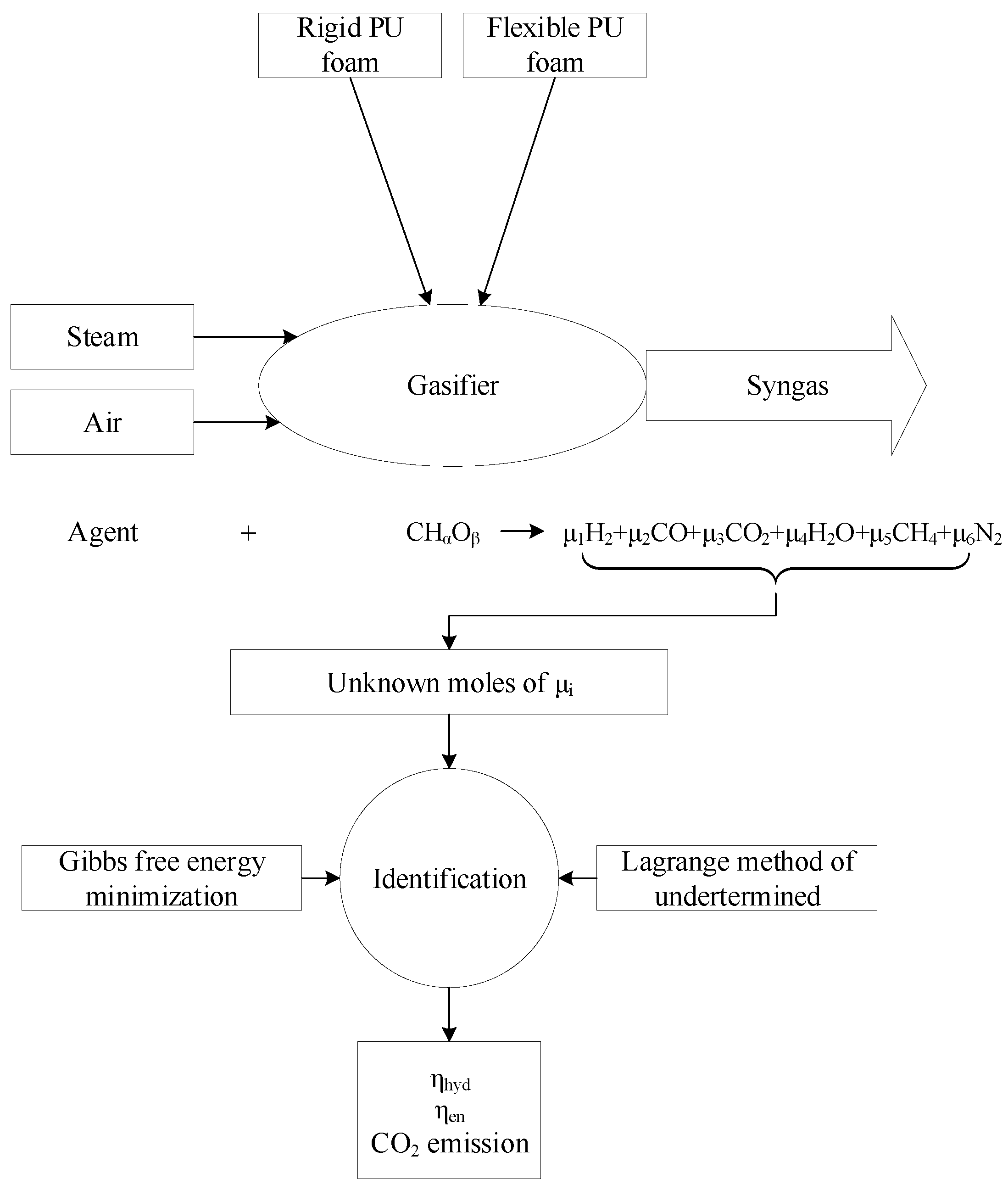
2. Gasification Modeling
3. Results and Discussion
3.1. Calculation of Higher Heating Value
3.2. Model Validation
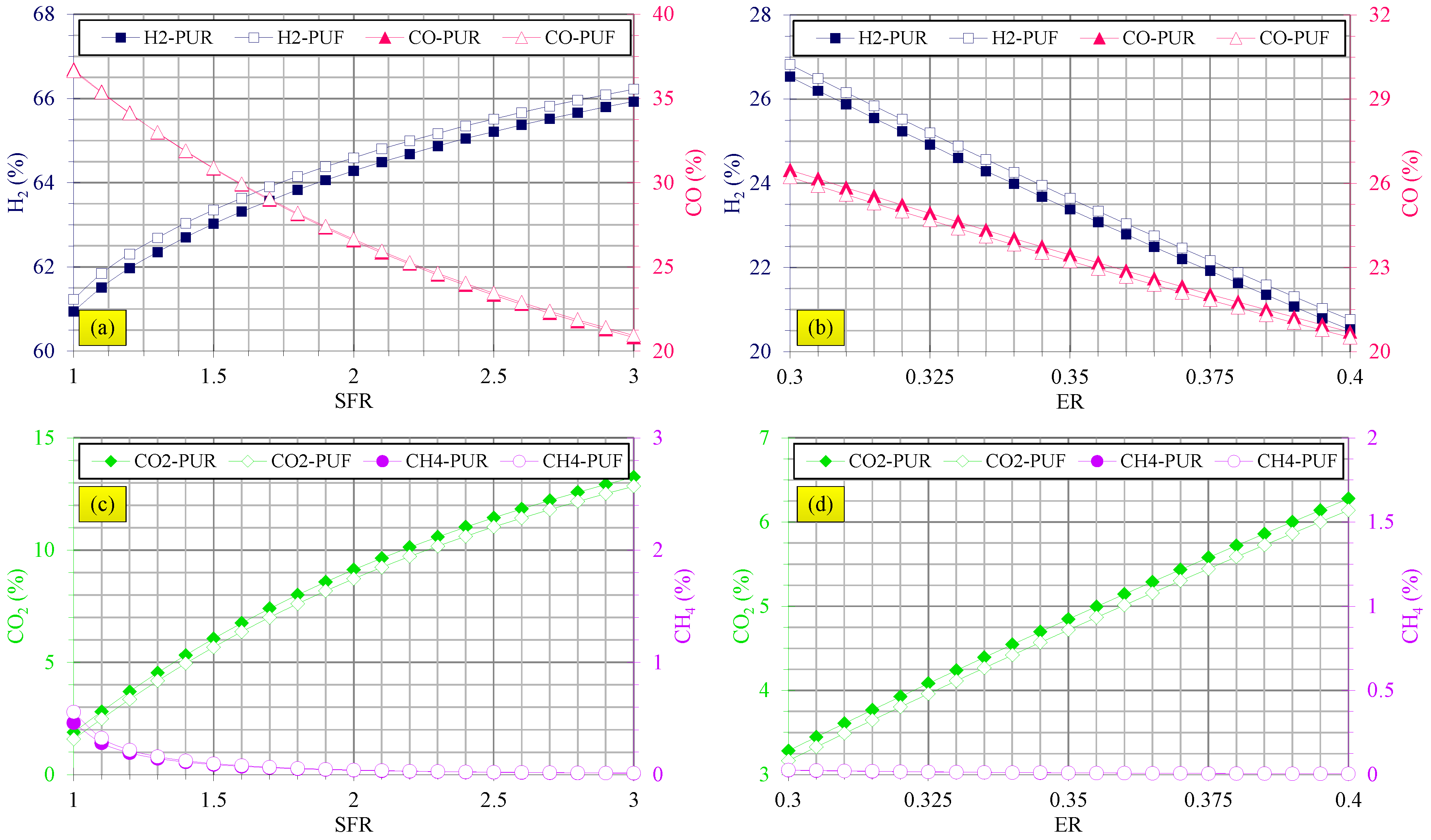
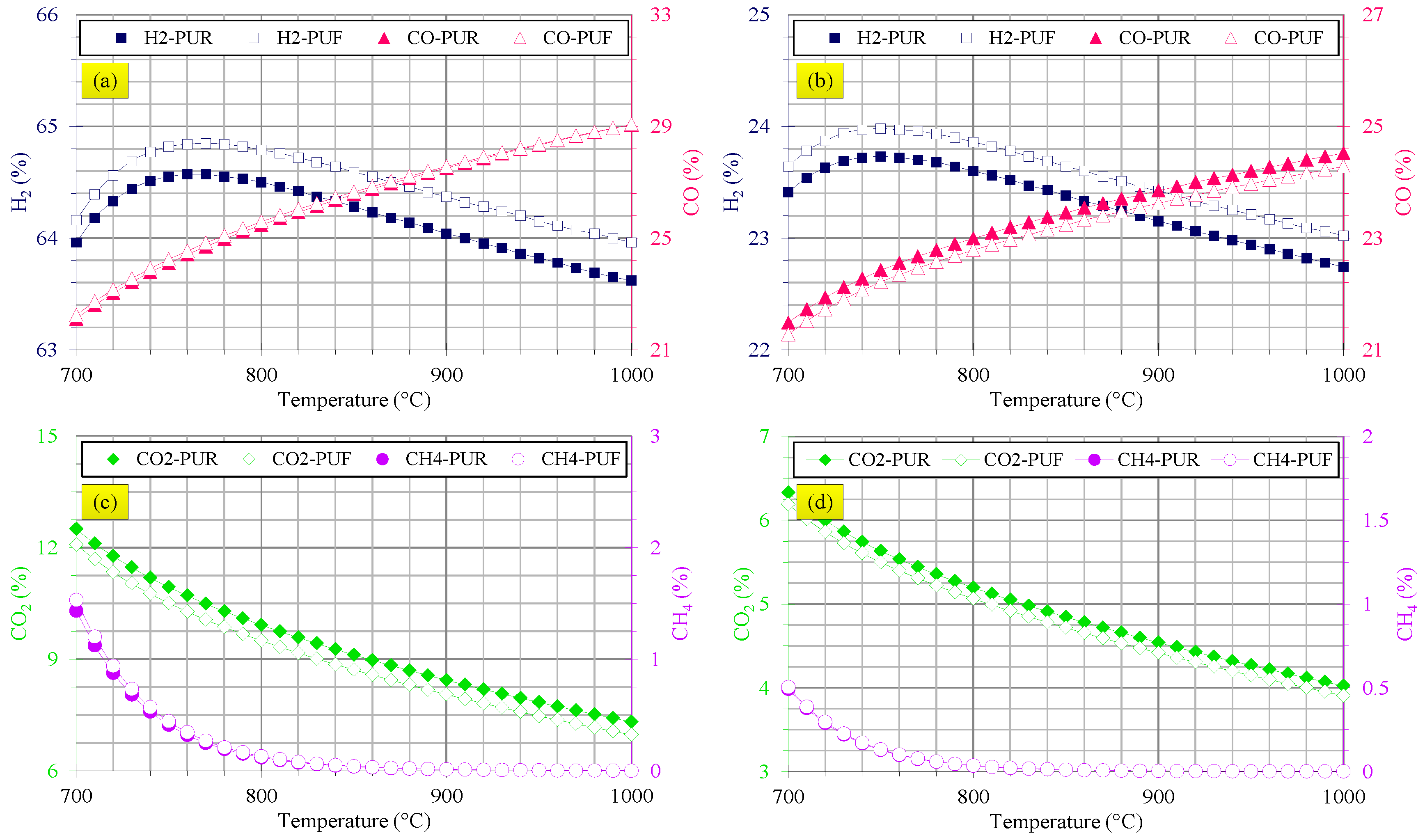
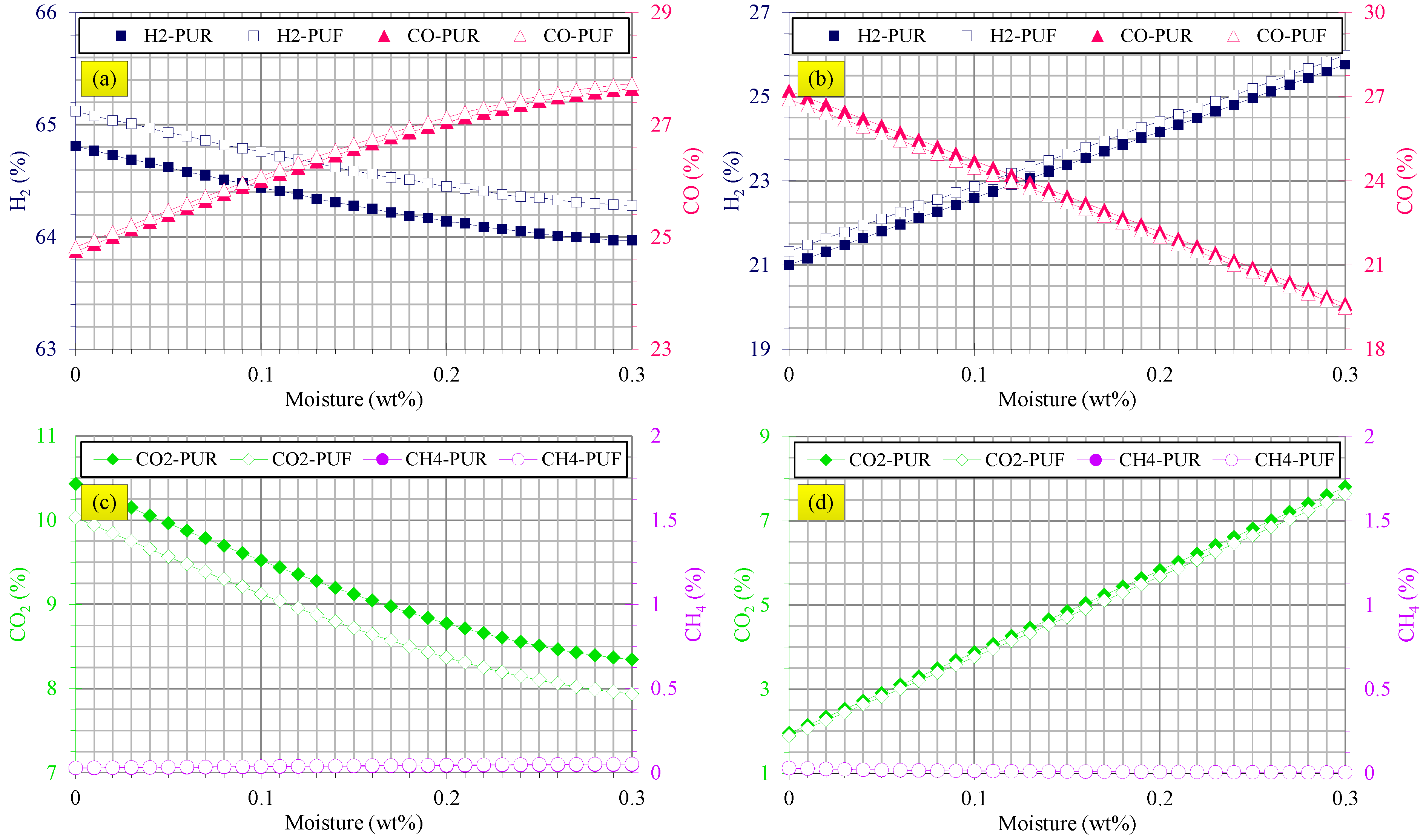
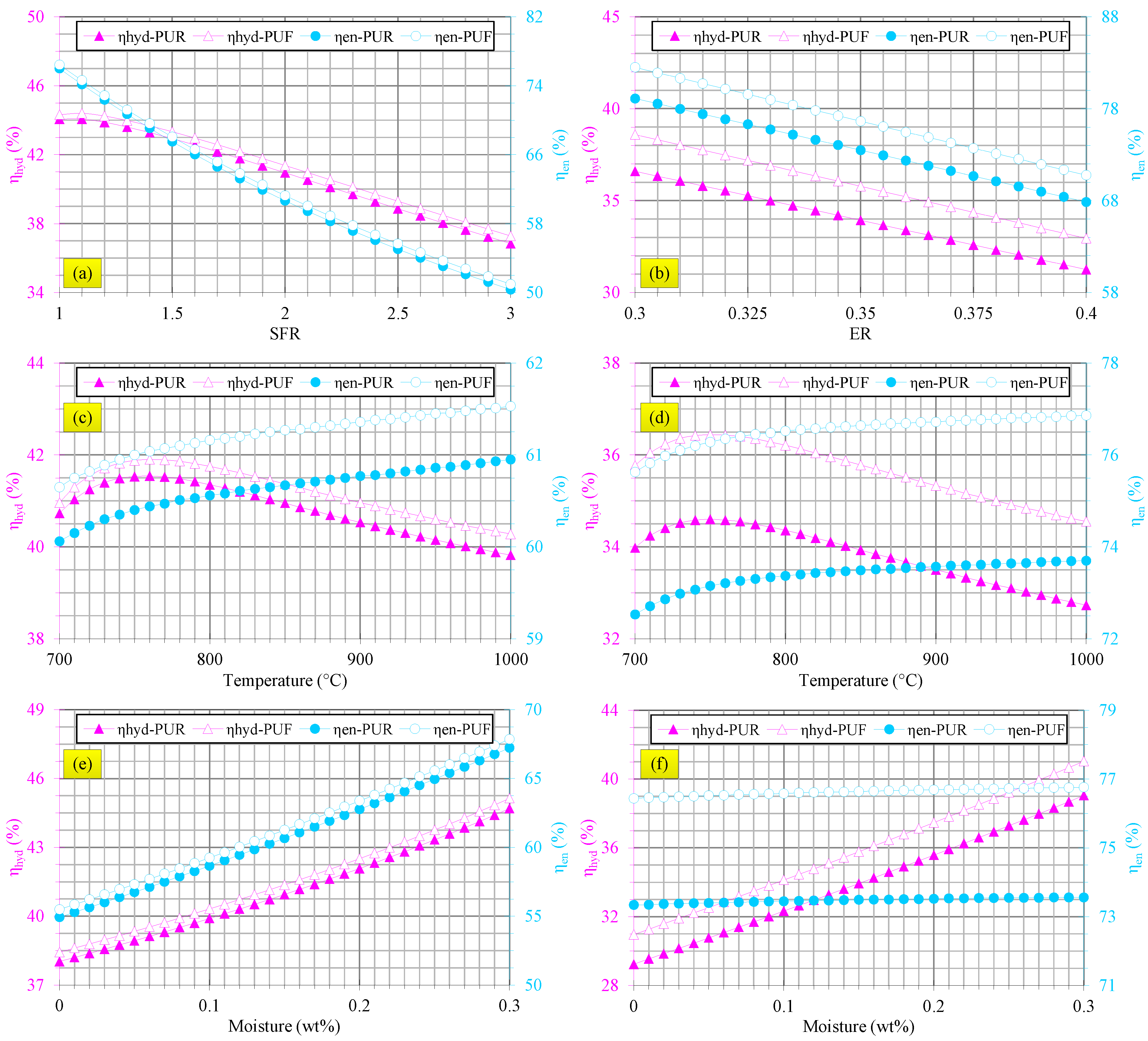
3.3. Gasification Assessments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Input energy (J) | |
| Molar numbers of products | |
| Hydrogen efficiency (%) | |
| Energy efficiency (%) | |
| Energy of ith component (J) | |
| Lower heating value of ith component (J/g) | |
| C | Carbon |
| H | Hydrogen |
| N | Nitrogen |
| O | Oxygen |
| T | Temperature (K) |
| Hydrogen/carbon molar ratio | |
| Oxygen/carbon molar ratio | |
| Moisture content of feedstock (mol) | |
| Feeding steam as the gasifying medium (mol) | |
| Air content per mole of feedstock (mol) |
References
- Pang, Y.; Cao, Y.; Zheng, W.; Park, C.B. A comprehensive review of cell structure variation and general rules for polymer microcellular foams. Chem. Eng. J. 2022, 430, 132662. [Google Scholar] [CrossRef]
- Azdast, T.; Hasanzadeh, R.; Lee, R.E.; Lee, P.C.; Wang, G.; Park, C.B. High-Pressure Foam Injection Molding of Polylactide/Nano-Fibril Composites with Mold Opening. In Polymeric Foams; CRC Press: Boca Raton, FL, USA, 2022; pp. 129–139. [Google Scholar]
- Rostami-Tapeh-Esmaeil, E.; Shojaei, S.; Rodrigue, D. Mechanical and Thermal Properties of Functionally Graded Polyolefin Elastomer Foams. Polymers 2022, 14, 4124. [Google Scholar] [CrossRef] [PubMed]
- Azdast, T.; Hasanzadeh, R. Increasing cell density/decreasing cell size to produce microcellular and nanocellular thermoplastic foams: A review. J. Cell. Plast. 2020, 57, 769–797. [Google Scholar] [CrossRef]
- Lobos, J.; Thirumuruganandham, S.P.; Rodríguez-Pérez, M.A. Density Gradients, Cellular Structure and Thermal Conductivity of High-Density Polyethylene Foams by Different Amounts of Chemical Blowing Agent. Polymers 2022, 14, 4082. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, R.; Azdast, T.; Doniavi, A. Thermal Conductivity of Low-Density Polyethylene Foams Part II: Deep Investigation using Response Surface Methodology. J. Therm. Sci. 2020, 29, 159–168. [Google Scholar] [CrossRef]
- Albozahid, M.; Naji, H.Z.; Alobad, Z.K.; Wychowaniec, J.K.; Saiani, A. Thermal, Mechanical, and Morphological Characterisations of Graphene Nanoplatelet/Graphene Oxide/High-Hard-Segment Polyurethane Nanocomposite: A Comparative Study. Polymers 2022, 14, 4224. [Google Scholar] [CrossRef]
- Graul, L.M.; Horn, S.J.; Nash, L.D.; Cheung, T.B.; Clubb, F.J.; Maitland, D.J. Image-Based Evaluation of In Vivo Degradation for Shape-Memory Polymer Polyurethane Foam. Polymers 2022, 14, 4122. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Mojaver, M.; Azdast, T.; Park, C.B. A novel systematic multi-objective optimization to achieve high-efficiency and low-emission waste polymeric foam gasification using response surface methodology and TOPSIS method. Chem. Eng. J. 2021, 430, 132958. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M. Influence of Interactions among Polymeric Components of Automobile Shredder Residue on the Pyrolysis Temperature and Characterization of Pyrolytic Products. Polymers 2020, 12, 1682. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Mojaver, P.; Azdast, T.; Chitsaz, A.; Park, C.B. Low-emission and energetically efficient co-gasification of coal by incorporating plastic waste: A modeling study. Chemosphere 2022, 299, 134408. [Google Scholar] [CrossRef]
- Dang, Q.; Zhang, X.; Zhou, Y.; Jia, X. Prediction and optimization of syngas production from a kinetic-based biomass gasification process model. Fuel Process. Technol. 2021, 212, 106604. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A comparison of steam and oxygen fed biomass gasification through a techno-economic-environmental study. Energy Convers. Manag. 2020, 208, 112612. [Google Scholar] [CrossRef]
- Policella, M.; Wang, Z.; Burra, K.G.; Gupta, A.K. Characteristics of syngas from pyrolysis and CO2-assisted gasification of waste tires. Appl. Energy 2019, 254, 113678. [Google Scholar] [CrossRef]
- Mojaver, P.; Jafarmadar, S.; Khalilarya, S.; Chitsaz, A. Study of synthesis gas composition, exergy assessment, and multi-criteria decision-making analysis of fluidized bed gasifier. Int. J. Hydrog. Energy 2019, 44, 27726–27740. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.; McKay, G. Techno-economic and sensitivity analysis of coconut coir pith-biomass gasification using ASPEN PLUS. Appl. Energy 2020, 261, 114350. [Google Scholar] [CrossRef]
- Singh, P.; Déparrois, N.; Burra, K.; Bhattacharya, S.; Gupta, A. Energy recovery from cross-linked polyethylene wastes using pyrolysis and CO2 assisted gasification. Appl. Energy 2019, 254, 113722. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 2020, 262, 116630. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Park, K.-B.; Kim, J.-S. Hydrogen production from steam gasification of polyethylene using a two-stage gasifier and active carbon. Appl. Energy 2020, 262, 114495. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Mojaver, M.; Azdast, T.; Park, C.B. Polyethylene waste gasification syngas analysis and multi-objective optimization using central composite design for simultaneous minimization of required heat and maximization of exergy efficiency. Energy Convers. Manag. 2021, 247, 114713. [Google Scholar] [CrossRef]
- Mojaver, M.; Azdast, T.; Hasanzadeh, R. Assessments of key features and Taguchi analysis on hydrogen rich syngas production via gasification of polyethylene, polypropylene, polycarbonate and polyethylene terephthalate wastes. Int. J. Hydrog. Energy 2021, 46, 29846–29857. [Google Scholar] [CrossRef]
- Mojaver, M.; Hasanzadeh, R.; Azdast, T.; Park, C.B. Comparative study on air gasification of plastic waste and conventional biomass based on coupling of AHP/TOPSIS multi-criteria decision analysis. Chemosphere 2022, 286, 131867. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, N.; Zhang, T. Preparation of hydrogen-rich gas from waste polyurethane foam by steam gasification and catalytic reforming in a two-stage fixed bed reactor. J. Mater. Cycles Waste Manag. 2021, 23, 1955–1963. [Google Scholar] [CrossRef]
- Guo, X.; Song, Z.; Zhang, W. Production of hydrogen-rich gas from waste rigid polyurethane foam via catalytic steam gasification. Waste Manag. Res. 2020, 38, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Li, S.; Tang, X.; Hao, J. Gasification of waste rigid polyurethane foam: Optimizing operational conditions. J. Mater. Cycles Waste Manag. 2015, 17, 560–565. [Google Scholar] [CrossRef]
- Mojaver, P.; Jafarmadar, S.; Khalilarya, S.; Chitsaz, A. Investigation and optimization of a Co-Generation plant integrated of gasifier, gas turbine and heat pipes using minimization of Gibbs free energy, Lagrange method and response surface methodology. Int. J. Hydrog. Energy 2020, 45, 19027–19044. [Google Scholar] [CrossRef]
- Özdenkçi, K.; Prestipino, M.; Björklund-Sänkiaho, M.; Galvagno, A.; De Blasio, C. Alternative energy valorization routes of black liquor by stepwise supercritical water gasification: Effect of process parameters on hydrogen yield and energy efficiency. Renew. Sustain. Energy Rev. 2020, 134, 110146. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Cheng, C.-M.; Huang, H.-C. Glycolysis of waste flexible polyurethane foam. Polym. Degrad. Stab. 2003, 80, 103–111. [Google Scholar] [CrossRef]
- Vargas-Moreno, J.; Callejón-Ferre, A.; Pérez-Alonso, J.; Velázquez-Martí, B. A review of the mathematical models for predicting the heating value of biomass materials. Renew. Sustain. Energy Rev. 2012, 16, 3065–3083. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass-Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Thipkhunthod, P.; Meeyoo, V.; Rangsunvigit, P.; Kitiyanan, B.; Siemanond, K.; Rirksomboon, T. Predicting the heating value of sewage sludges in Thailand from proximate and ultimate analyses. Fuel 2005, 84, 849–857. [Google Scholar] [CrossRef]
- Yin, C.-Y. Prediction of higher heating values of biomass from proximate and ultimate analyses. Fuel 2011, 90, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Callejón-Ferre, A.; Velázquez-Martí, B.; López-Martínez, J.; Manzano-Agugliaro, F. Greenhouse crop residues: Energy potential and models for the prediction of their higher heating value. Renew. Sustain. Energy Rev. 2011, 15, 5224, Erratum in Renew. Sustain. Energy Rev. 2011, 15, 948–955. [Google Scholar] [CrossRef]
- Callejón-Ferre, A.; Velázquez-Martí, B.; López-Martínez, J.; Manzano-Agugliaro, F. Greenhouse crop residues: Energy potential and models for the prediction of their higher heating value. Renew. Sustain. Energy Rev. 2011, 15, 948–955. [Google Scholar] [CrossRef]
- Huang, C.; Han, L.; Liu, X.; Yang, Z. Models Predicting Calorific Value of Straw from the Ash Content. Int. J. Green Energy 2008, 5, 533–539. [Google Scholar] [CrossRef]
- Graboski, M.; Bain, R. Properties of biomass relevant to gasification [Fuel gas production]. In Biomass Gasification; Principles and Technology (USA); Noyes Data Corporation: New Vernon, NJ, USA, 1981; pp. 41–69. [Google Scholar]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Van Nguyen, Q.; Choi, Y.S.; Choi, S.K.; Jeong, Y.W.; Kwon, Y.S. Improvement of bio-crude oil properties via co-pyrolysis of pine sawdust and waste polystyrene foam. J. Environ. Manag. 2019, 237, 24–29. [Google Scholar] [CrossRef]
- Van Nguyen, Q.; Choi, Y.-S.; Choi, S.-K.; Jeong, Y.-W.; Han, S.-Y. Co-pyrolysis of coffee-grounds and waste polystyrene foam: Synergistic effect and product characteristics analysis. Fuel 2021, 292, 120375. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P. Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl. Catal. B Environ. 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Cho, M.-H.; Mun, T.-Y.; Kim, J.-S. Production of low-tar producer gas from air gasification of mixed plastic waste in a two-stage gasifier using olivine combined with activated carbon. Energy 2013, 58, 688–694. [Google Scholar] [CrossRef]
- Jayathilake, R.; Rudra, S. Numerical and Experimental Investigation of Equivalence Ratio (ER) and Feedstock Particle Size on Birchwood Gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef] [Green Version]
- Marcantonio, V.; Ferrario, A.M.; Di Carlo, A.; Del Zotto, L.; Monarca, D.; Bocci, E. Biomass Steam Gasification: A Comparison of Syngas Composition between a 1-D MATLAB Kinetic Model and a 0-D Aspen Plus Quasi-Equilibrium Model. Computation 2020, 8, 86. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.; Yao, X.; Chen, S. Prediction and optimization of syngas production from steam gasification: Numerical study of operating conditions and biomass composition. Energy Convers. Manag. 2021, 236, 114077. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Yan, F.; Luo, S.-Y.; Hu, Z.-Q.; Xiao, B.; Cheng, G. Hydrogen-rich gas production by steam gasification of char from biomass fast pyrolysis in a fixed-bed reactor: Influence of temperature and steam on hydrogen yield and syngas composition. Bioresour. Technol. 2010, 101, 5633–5637. [Google Scholar] [CrossRef]
- Herguido, J.; Corella, J.; Gonzalez-Saiz, J. Steam gasification of lignocellulosic residues in a fluidized bed at a small pilot scale. Effect of the type of feedstock. Ind. Eng. Chem. Res. 1992, 31, 1274–1282. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam gasification of various feedstocks at a dual fluidised bed gasifier: Impacts of operation conditions and bed materials. Biomass-Convers. Biorefinery 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Gerun, L.; Paraschiv, M.; Vîjeu, R.; Bellettre, J.; Tazerout, M.; Gøbel, B.; Henriksen, U. Numerical investigation of the partial oxidation in a two-stage downdraft gasifier. Fuel 2008, 87, 1383–1393. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.D.; Hanna, M.A. Steam–air fluidized bed gasification of distillers grains: Effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ye, J.; Zhao, C.; Zhang, Q. Gasification of Rice Husk in a Downdraft Gasifier: The Effect of Equivalence Ratio on the Gasification Performance, Properties, and Utilization Analysis of Byproducts of Char and Tar. BioResources 2015, 10, 2888–2902. [Google Scholar] [CrossRef] [Green Version]
- Han, S.W.; Lee, J.J.; Tokmurzin, D.; Lee, S.H.; Nam, J.Y.; Park, S.J.; Ra, H.W.; Mun, T.-Y.; Yoon, S.J.; Yoon, S.M.; et al. Gasification characteristics of waste plastics (SRF) in a bubbling fluidized bed: Effects of temperature and equivalence ratio. Energy 2021, 238, 121944. [Google Scholar] [CrossRef]
- Hwang, I.S.; Sohn, J.; Lee, U.D.; Hwang, J. CFD-DEM simulation of air-blown gasification of biomass in a bubbling fluidized bed gasifier: Effects of equivalence ratio and fluidization number. Energy 2021, 219, 119533. [Google Scholar] [CrossRef]
- Parrillo, F.; Ardolino, F.; Calì, G.; Marotto, D.; Pettinau, A.; Arena, U. Fluidized bed gasification of eucalyptus chips: Axial profiles of syngas composition in a pilot scale reactor. Energy 2021, 219, 119604. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrog. Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Syngas production by catalytic steam gasification of municipal solid waste in fixed-bed reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Fagbemi, L.; Khezami, L.; Capart, R. Pyrolysis products from different biomasses: Application to the thermal cracking of tar. Appl. Energy 2001, 69, 293–306. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Cao, L.; Ma, L.; You, S.; Li, W. Co-gasification of digestate and lignite in a downdraft fixed bed gasifier: Effect of temperature. Energy Convers. Manag. 2020, 213, 112798. [Google Scholar] [CrossRef]
- Xie, L.-P.; Li, T.; Gao, J.-D.; Fei, X.-N.; Wu, X.; Jiang, Y.-G. Effect of moisture content in sewage sludge on air gasification. J. Fuel Chem. Technol. 2010, 38, 615–620. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Effect of Operating Parameters and Moisture Content on Municipal Solid Waste Pyrolysis and Gasification. Energy Fuels 2016, 30, 3994–4001. [Google Scholar] [CrossRef]
| Model | Error (%) | ||
|---|---|---|---|
| Experimental [40,41] | Theoretical | ||
| Equation (6) [31] | 41.46 | 49.306 | 18.92 |
| Equation (7) [32] | 41.46 | 33.087 | 20.20 |
| Equation (8) [33] | 41.46 | 35.946 | 13.30 |
| Equation (9) [34] | 41.46 | 33.758 | 18.58 |
| Equation (10) [35,36] | 41.46 | 40.115 | 3.24 |
| Equation (11) [32] | 41.46 | 19.900 | 52.00 |
| Equation (12) [37] | 41.46 | 18.947 | 54.30 |
| Equation (13) [35,36] | 41.46 | 20.070 | 51.59 |
| Equation (14) [38] | 41.46 | 38.151 | 7.98 |
| Equation (15) [39] | 41.46 | 41.618 | 0.38 |
| Equation (16) [33] | 41.46 | 35.280 | 14.91 |
| Equation (17) [33] | 41.46 | 28.557 | 31.12 |
| Equation (18) [35,36] | 41.46 | 38.567 | 6.98 |
| Equation (19) [35,36] | 41.46 | 38.562 | 6.99 |
| Component | Steam Gasification | Air Gasification | |||
|---|---|---|---|---|---|
| Exp. [42] | Model [43] | This Model | Exp. [44] | This Model | |
| H2 | 64 | 67.25 | 67.19 | 18.61 | 16.59 |
| CO | 25.7 | 25.24 | 26.51 | 12.11 | 13.99 |
| CO2 | 6.4 | 7.33 | 6.027 | 8.88 | 7.269 |
| CH4 | 3.3 | 0.18 | 0.2731 | 2.24 | 0.006 |
| N2 | - | - | - | 58.17 | 62.14 |
| Output | Steam Gasification | Air Gasification | ||
|---|---|---|---|---|
| Waste Rigid PU Foam | Waste Flexible PU Foam | Waste Rigid PU Foam | Waste Flexible PU Foam | |
| (%) | 40.95 | 41.37 | 33.93 | 35.78 |
| (%) | 60.67 | 61.27 | 73.49 | 76.64 |
| CO2 emission (g) | 11.24 | 10.84 | 7.53 | 7.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanzadeh, R.; Mojaver, P.; Khalilarya, S.; Azdast, T.; Chitsaz, A.; Mojaver, M. Polyurethane Foam Waste Upcycling into an Efficient and Low Pollutant Gasification Syngas. Polymers 2022, 14, 4938. https://doi.org/10.3390/polym14224938
Hasanzadeh R, Mojaver P, Khalilarya S, Azdast T, Chitsaz A, Mojaver M. Polyurethane Foam Waste Upcycling into an Efficient and Low Pollutant Gasification Syngas. Polymers. 2022; 14(22):4938. https://doi.org/10.3390/polym14224938
Chicago/Turabian StyleHasanzadeh, Rezgar, Parisa Mojaver, Shahram Khalilarya, Taher Azdast, Ata Chitsaz, and Mehran Mojaver. 2022. "Polyurethane Foam Waste Upcycling into an Efficient and Low Pollutant Gasification Syngas" Polymers 14, no. 22: 4938. https://doi.org/10.3390/polym14224938








