Investigation on Some Algal Extracts as Appropriate Stabilizers for Radiation-Processed Polymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
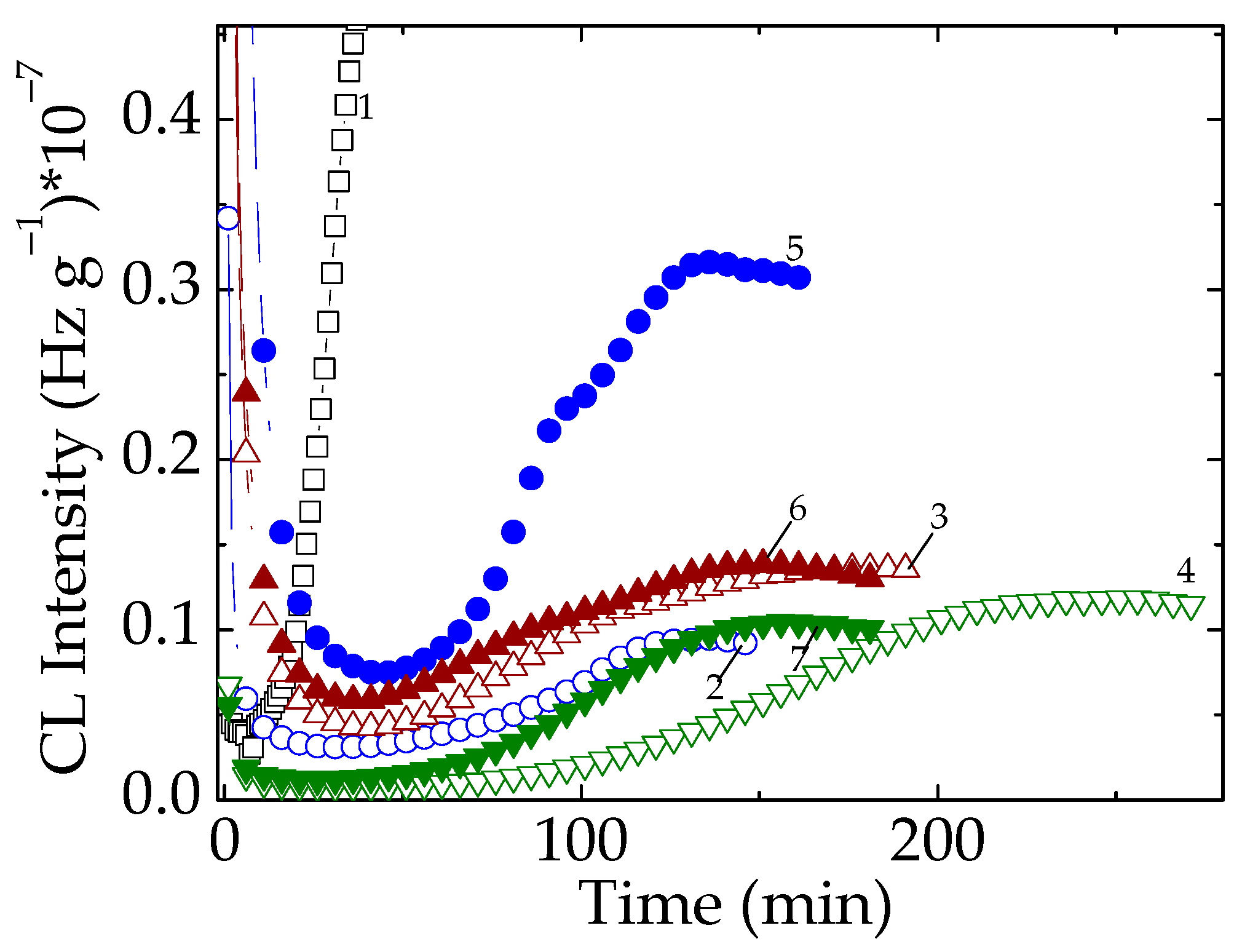
3.1. Isothermal Chemiluminescence
3.2. Nonisothermal Chemiluminescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xua, L.Q.; Neohd, K.-G.; Kang, E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, B.; Gu, X.; Lin, T.; Li, Y. Determining the optimal molecular architecture for reactive splicing compatibilization: Toward a better understanding of reactive polymer processing. Polymer 2020, 208, 122948. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of polymers by controlling of ionizing radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Ferreira Soares, D.C.; Poletto, F.; Eberhardt, M.J.; Calazans Domingues, S.; De Sousa, F.B.; Tebaldi, M.L. Polymer-hybrid nanosystems for antiviral applications: Current advances. Biomed. Pharmacother. 2022, 146, 112249. [Google Scholar] [CrossRef]
- Zaharescu, T.; Blanco, I. Stabilization Effects of Natural Compounds and Polyhedral Oligomeric Silsesquioxane Nanoparticles on the Accelerated Degradation of Ethylene-Propylene-Diene Monomer. Molecules 2021, 26, 4390. [Google Scholar] [CrossRef]
- Scott, G. (Ed.) Mechanisms of Segradation and Stabilization of Polymers; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Blivet, C.; Larché, J.-F.; Israëli, Y.; Bussière, P.-O. Non-Arrhenius behavior: Influence of the crystallinity on lifetime predictions of polymer materials used in the cable and wire industries. Polym. Degrad. Stab. 2022, 199, 109890. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Brunet, C. Promises and challenges of microalgal antioxidant production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, L.M.A. Screening microalgae as potential sources of antioxidants. J. Appl. Phycol. 2017, 29, 865–877. [Google Scholar] [CrossRef]
- Li, H.; Cheng, K.; Wong, C.; Fan, K.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Liu, X.; Luo, G.; Wang, L.; Yuan, W. Optimization of antioxidant extraction from edible brown algae Ascophyllum nodosum using response surface methodology. Food Bioprod. Proc. 2019, 114, 205–215. [Google Scholar] [CrossRef]
- Delfan, P.; Mortazavi, A.; Rad, A.H.E.; Zenoozian, M.S. Measurement of phenolic content and antioxidant capacity of Pennyroyal (Mentha pulegium L.) and microalgae Spirulina platensis extracted by steeping, ultrasonic and microwave methods. J. Food Process. Technol. 2019, 9, 712. [Google Scholar]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant compounds from microalgae: A review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.P.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological Applications of Microalgal Oleaginous Compounds: Current Trends on microalgal bioproprocessing of products. Front. Energy Res. 2020, 8, 598803. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal Biotechnology. In The Algae World. Cellular Origin, Life in Extreme Habitats and Astrobiology; Sahoo, D., Sechbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 26, pp. 319–338. [Google Scholar]
- Mateescu, C.; Zaharescu, T. Comprehensive overview of biomethane production potential of algal biomass cultivated in wastewater. In Application of Microalgae in Wastewater Treatment; Kumar Gupta, S., Bux, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 427–443. [Google Scholar]
- Lafarga, T.; Acién, G. (Eds.) Cultured Microalgae for the Food Industry. Current and Potential Applications, 1st ed.; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Shen, J.; Costa, L.; Xu, Y.; Cong, Y.; Cheng, Y.; Liu, X.; Fu, J. Stabilization of highly crosslinked ultra high molecular weight polyethylene with natural polyphenols. Polym. Degrad. Stab. 2014, 105, 197–205. [Google Scholar] [CrossRef]
- Parameswari, R.P.; Lakshmi, T. Microalgae as a potential therapeutic drug candidate for neurodegenerative diseases. J. Biotechnol. 2022, 358, 128–139. [Google Scholar] [CrossRef]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Kumar Nayak, A.; Hasnain, M.S.; Aminabhavi, T.M. Drug delivery using interpenetrating polymeric networks of natural polymers: A recent update. J. Drug. Deliv. Sci. Tec. 2021, 66, 102915. [Google Scholar] [CrossRef]
- Khan, B.M.; Zheng, L.-X.; Khan, W.; Shah, A.A.; Liu, Y.; Cheong, K.-L. Antioxidant potential of physicochemically characterized Gracilaria blodgettii sulfated polysaccharides. Polymers 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Brunet, C. Marine Algal Antioxidants. Antioxidants 2020, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Zaharescu, T.; Mateescu, C.; Dima, A.; Varca, G.H.C. Evaluation of thermal and radiation stability of EPDM in the presence of some algal powders. J. Therm. Anal. Calorim. 2022, 147, 327–336. [Google Scholar] [CrossRef]
- Frazzini, S.; Scaglia, E.; Dell’Anno, M.; Reggi, S.; Panseri, S.; Giríomini, C.; Lanzoni, D.; Sgoifo Rossi, C.A.; Ross, L. Antioxidant and antimicrobial activity of algal and cyanobacterial extracts: An in vitro study. Antioxidants 2022, 11, 992. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Lamanen, C.A.; Desjardins, S.M.; Senhorinho, G.N.A.; Scott, J.A. Harvesting microalgae for health beneficial dietary supplements. Algal Res. 2021, 54, 102189. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdotti, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Food 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Singh, R.; Kaushik Bhardwaj, S.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the therapeutic applications of algal polysaccharides. J. Polym. Environ. 2022, 30, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Sharif, N.; Naz, S.; Manzoor, F. Algae: A potent antioxidant source. Sky J. Microbiol. Res. 2013, 1, 22–31. [Google Scholar]
- Pospišil, J. Mechanistic action of phenolic antioxidants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Rychla, L.; Rychly, J.; Krivosik, I. Chemiluminescence and inhibited oxidation of polypropylene. Polym. Degrad. Stab. 1988, 20, 325–335. [Google Scholar] [CrossRef]
- Du, C.; Fikhman, D.A.; Browning Monroe, M.B. Shape memory polymer foams with phenolic acid-based antioxidant properties. Antioxidants 2022, 11, 1105. [Google Scholar] [CrossRef]
- Kwon, T.; Lim, Y.; Cho, J.; Lawler, R.; Min, B.J.; Goddard, W.A.; Jang, S.S.; Kim, J.Y. Antioxidant technology for durability enhancement in polymer electrolyte membranes for fuel cell applications. Mater. Today 2022, 58, 135–163. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible materials based on plasticized poly(lactic acid), chitosan and rosemary ethanolic extract I. Effect of chitosan on the properties of plasticized poly(lactic acid) materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef] [Green Version]
- Richaud, E.; Audouin, L.; Fayolle, B.; Verdu, J.; Matisová-Rychlá, L.; Rychlý, J. Rate constants of oxidation of unsaturated fatty esters studied by chemiluminescence. Chem. Phys. Lipids 2012, 165, 753–759. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LCMS (APCI). Mol. Cell. Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, P. Review on the thermo-oxidative degradation of polymers during processing and in service. e-Polymers 2008, 8, 65. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in natural antioxidants for food improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef] [PubMed]

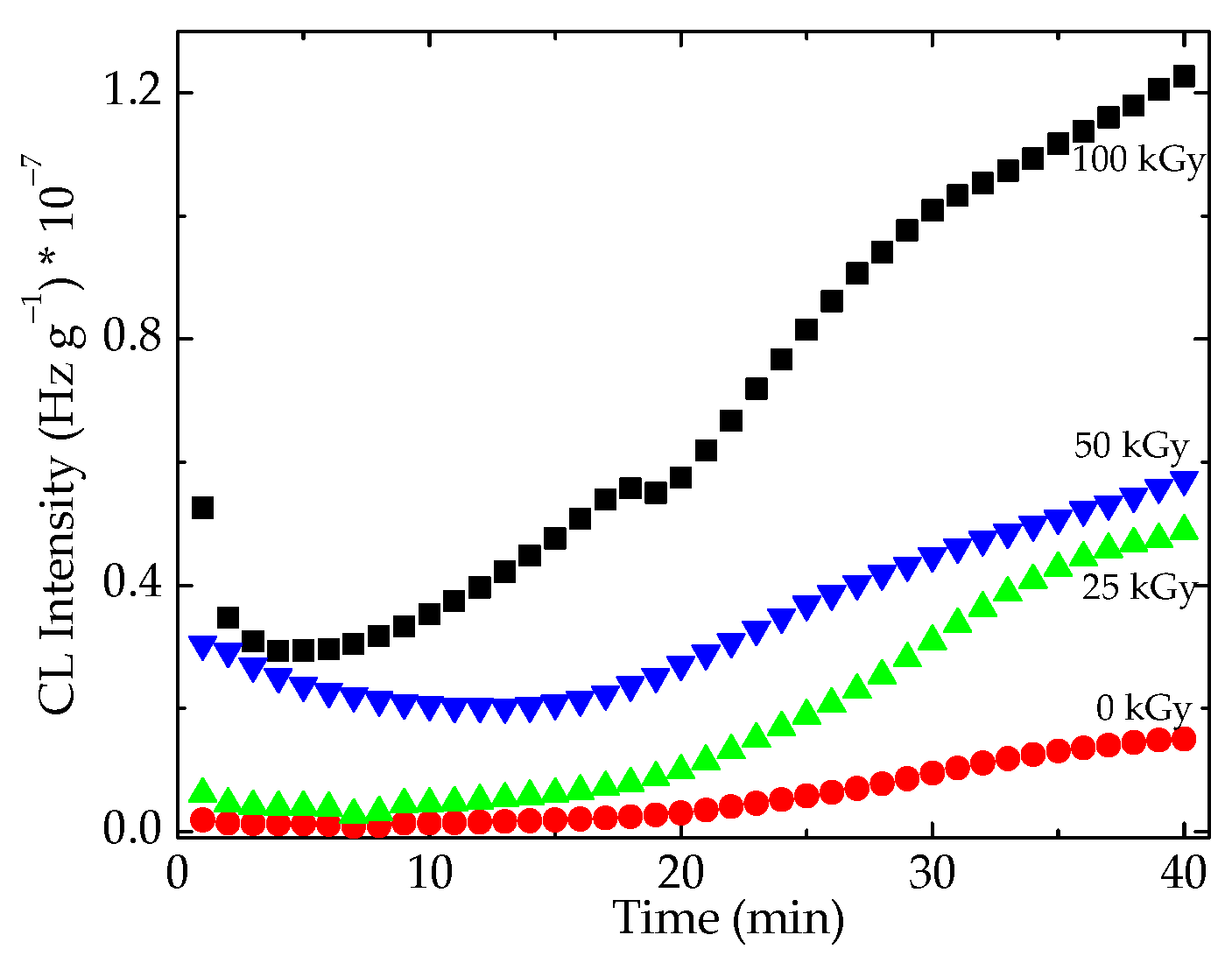
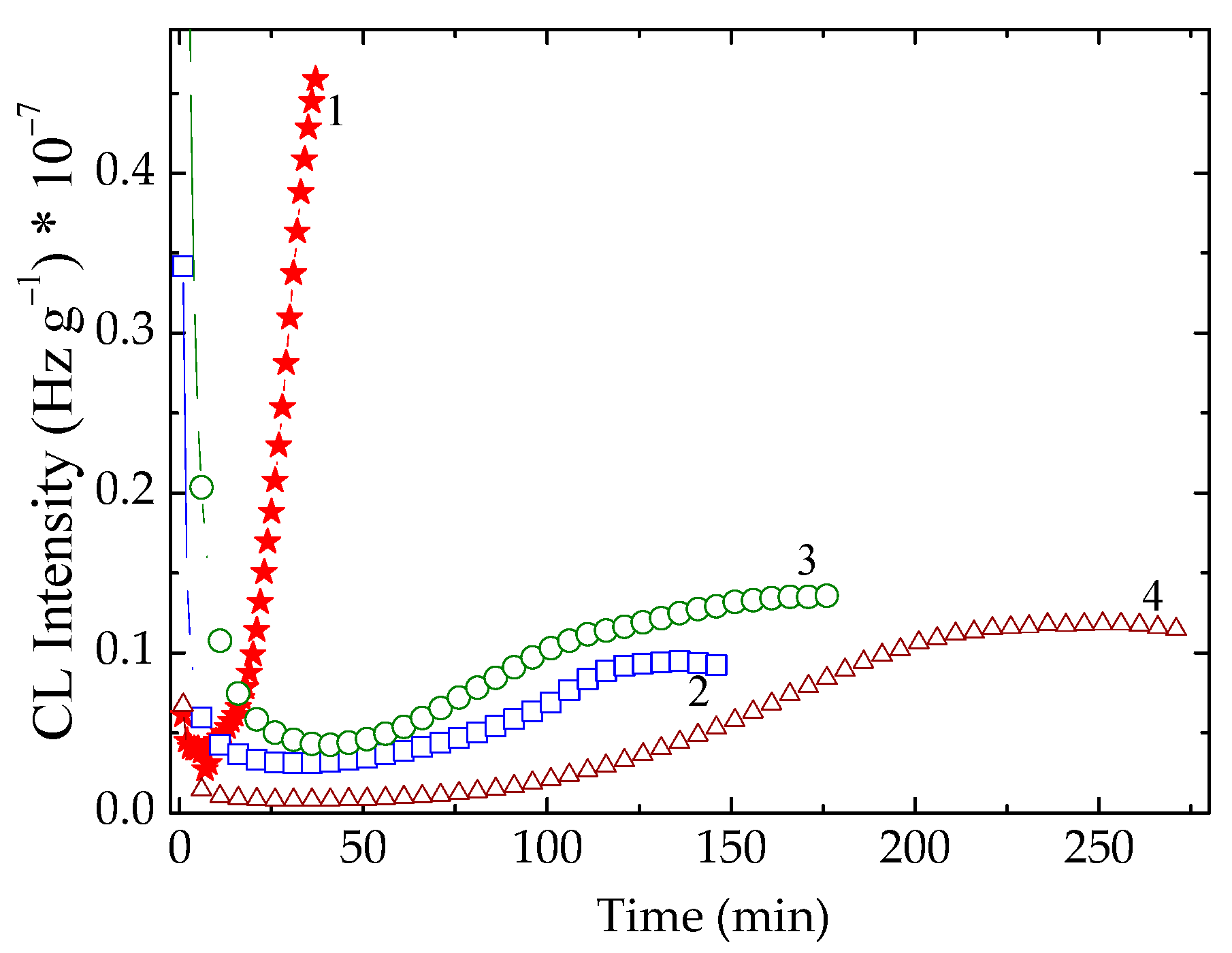
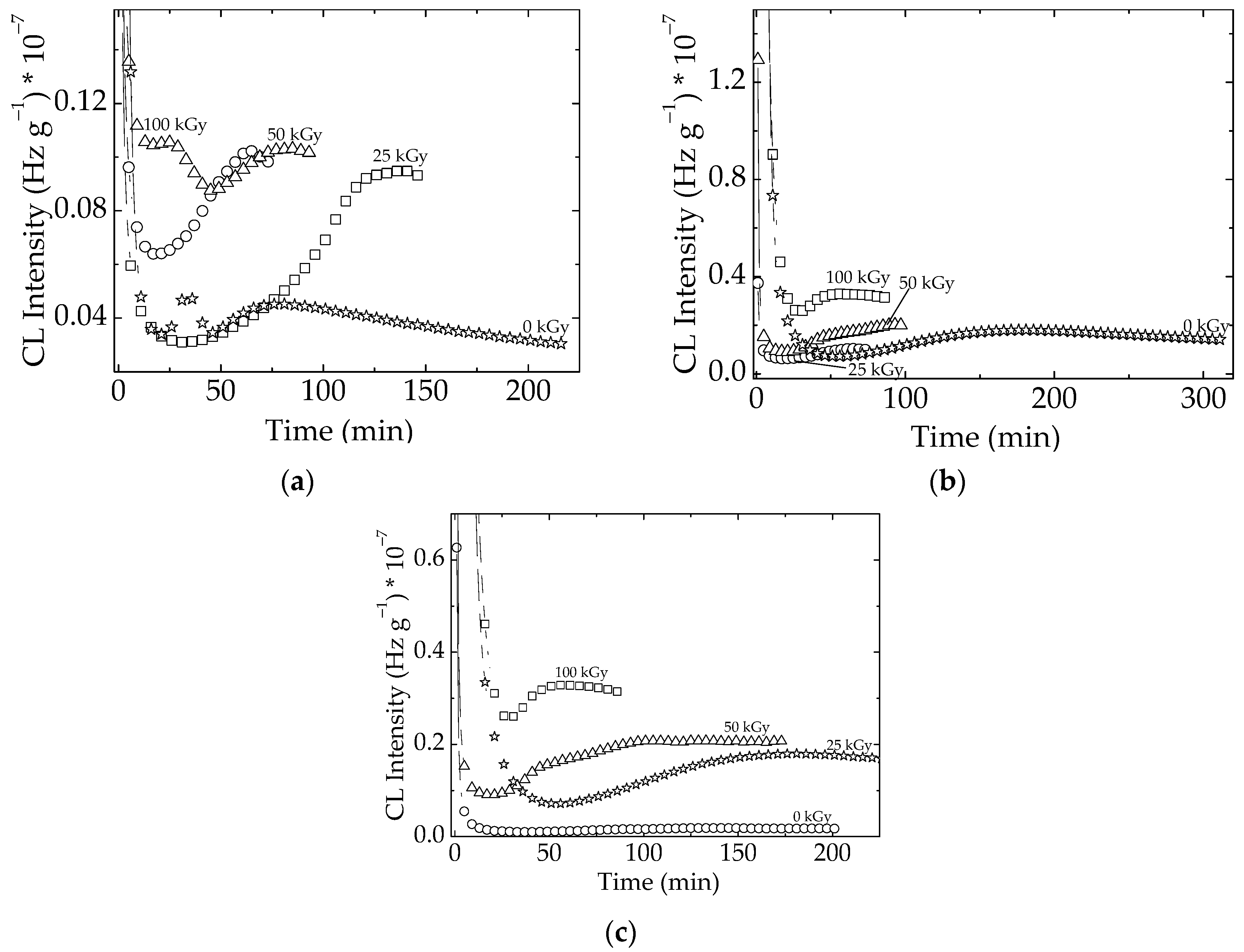



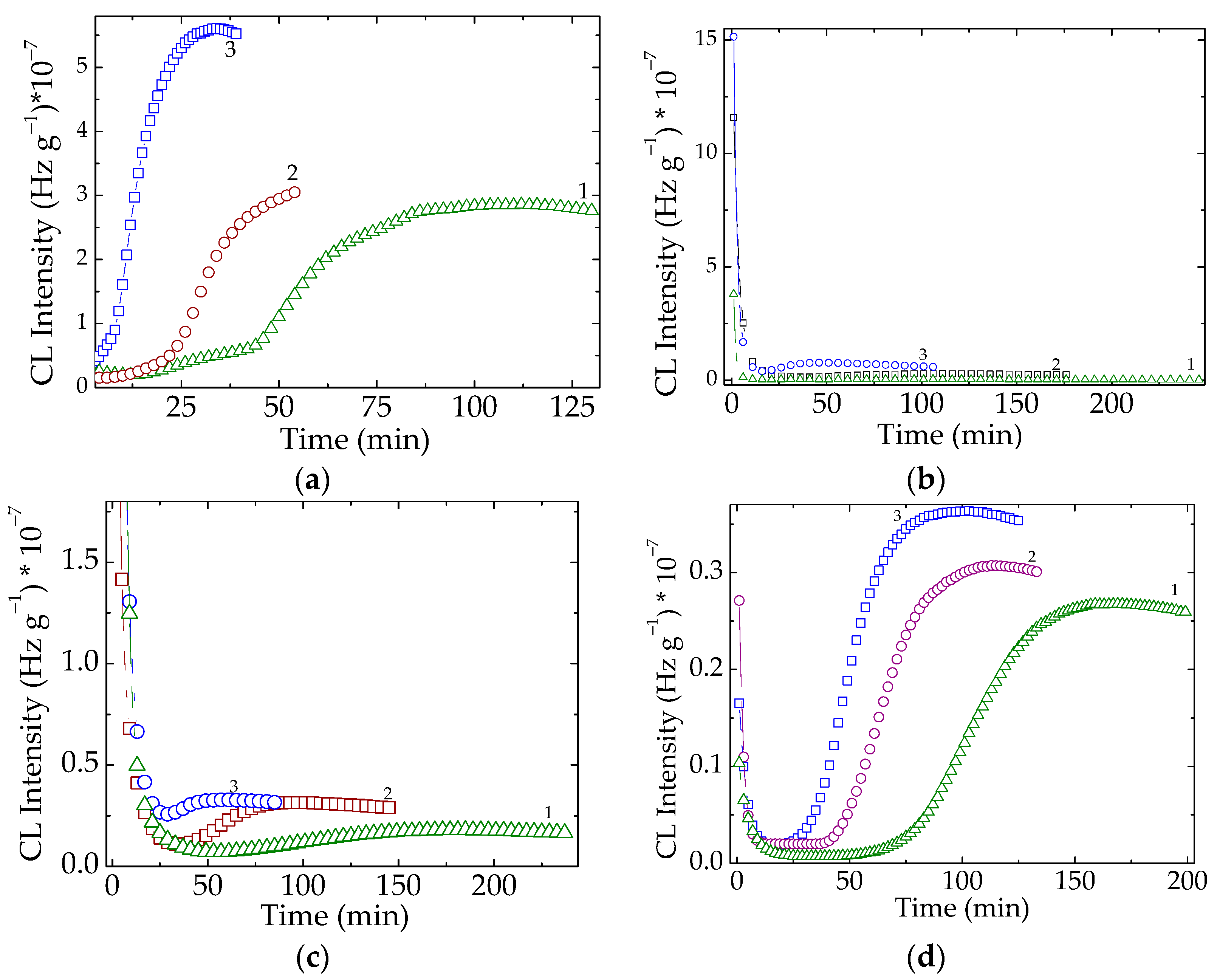
| Sample | Oxidation Induction Time (min) | Correlation Factor | Activation Energy (kJ mol−1) | ||
|---|---|---|---|---|---|
| 130 °C | 140 °C | 150 °C | |||
| free | 32 | 24 | 15 | 0.99682 | 49 |
| Spirulina platensis | 53 | 40 | 25 | 0.98756 | 53 |
| Chlorella vulgaris | 83 | 50 | 28 | 0.99861 | 76 |
| Ascophyllum nodosum | 78 | 35 | 20 | 0.99858 | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharescu, T.; Mateescu, C. Investigation on Some Algal Extracts as Appropriate Stabilizers for Radiation-Processed Polymers. Polymers 2022, 14, 4971. https://doi.org/10.3390/polym14224971
Zaharescu T, Mateescu C. Investigation on Some Algal Extracts as Appropriate Stabilizers for Radiation-Processed Polymers. Polymers. 2022; 14(22):4971. https://doi.org/10.3390/polym14224971
Chicago/Turabian StyleZaharescu, Traian, and Carmen Mateescu. 2022. "Investigation on Some Algal Extracts as Appropriate Stabilizers for Radiation-Processed Polymers" Polymers 14, no. 22: 4971. https://doi.org/10.3390/polym14224971
APA StyleZaharescu, T., & Mateescu, C. (2022). Investigation on Some Algal Extracts as Appropriate Stabilizers for Radiation-Processed Polymers. Polymers, 14(22), 4971. https://doi.org/10.3390/polym14224971






