Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications
Abstract
1. Introduction
2. Method and Materials
2.1. Materials
2.2. Preparation of Solution
2.3. Preparation of Edible Film
2.4. Characterization
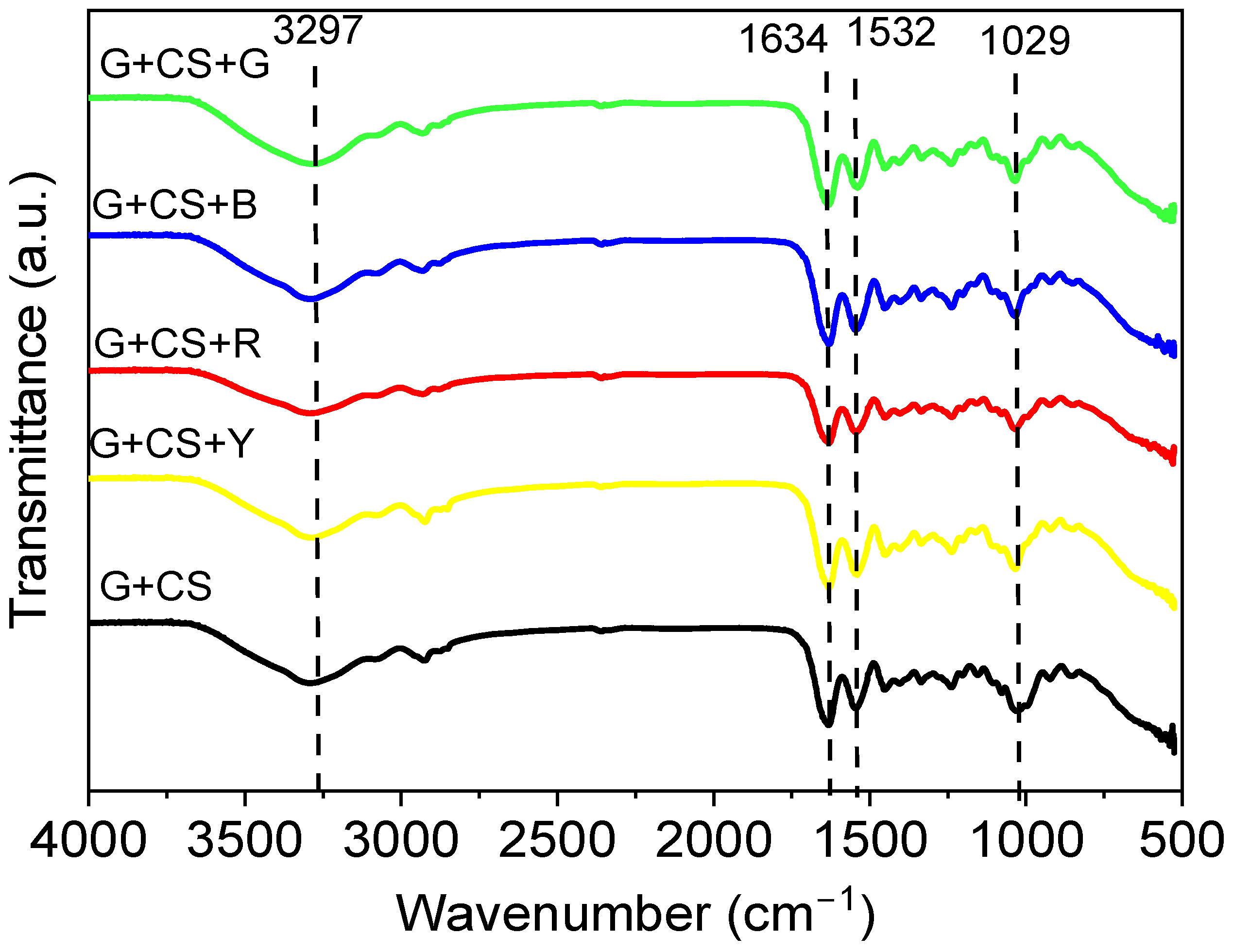
2.4.1. FT-IR Analysis
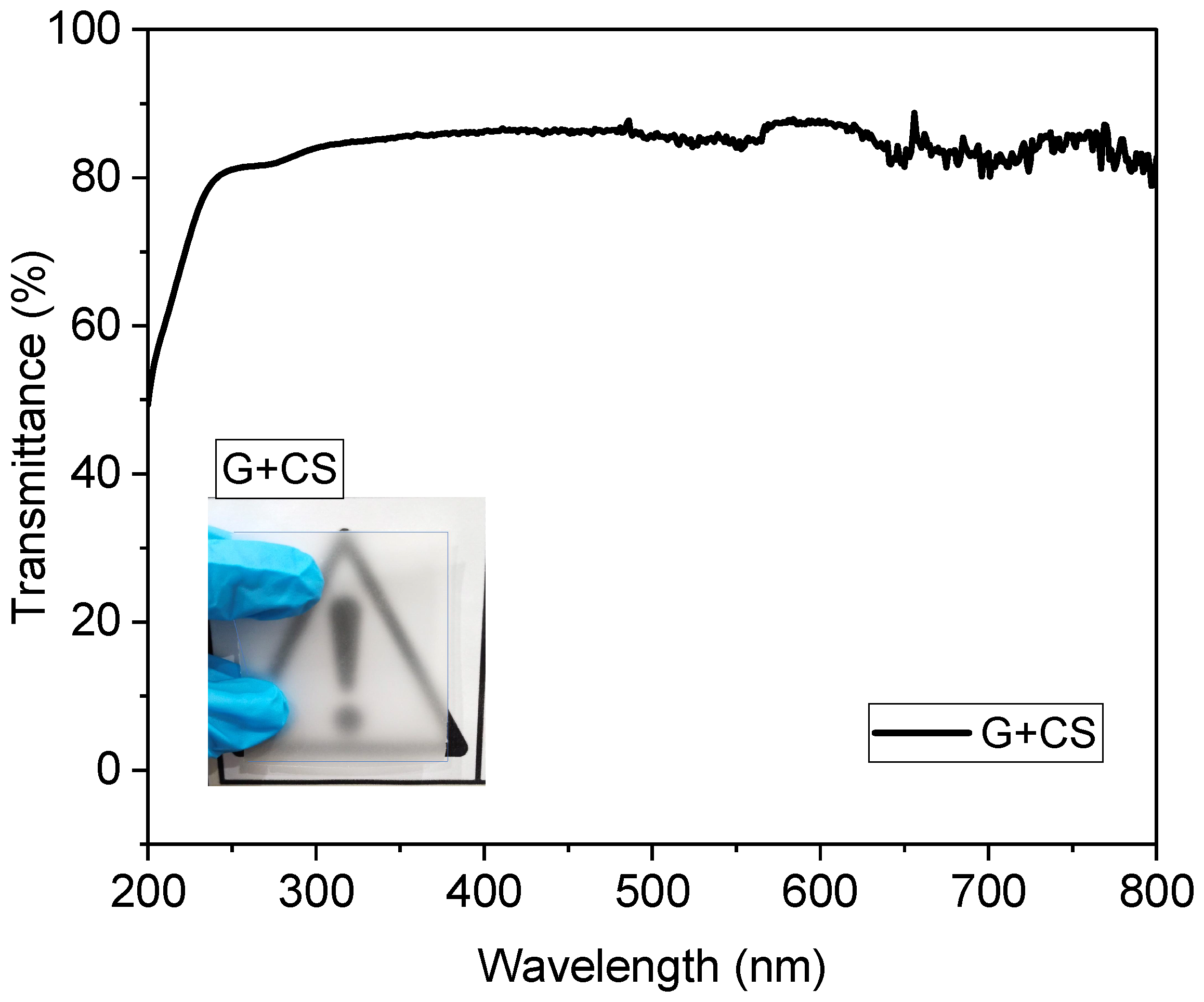
2.4.2. UV-VIS
2.4.3. Contact Angle
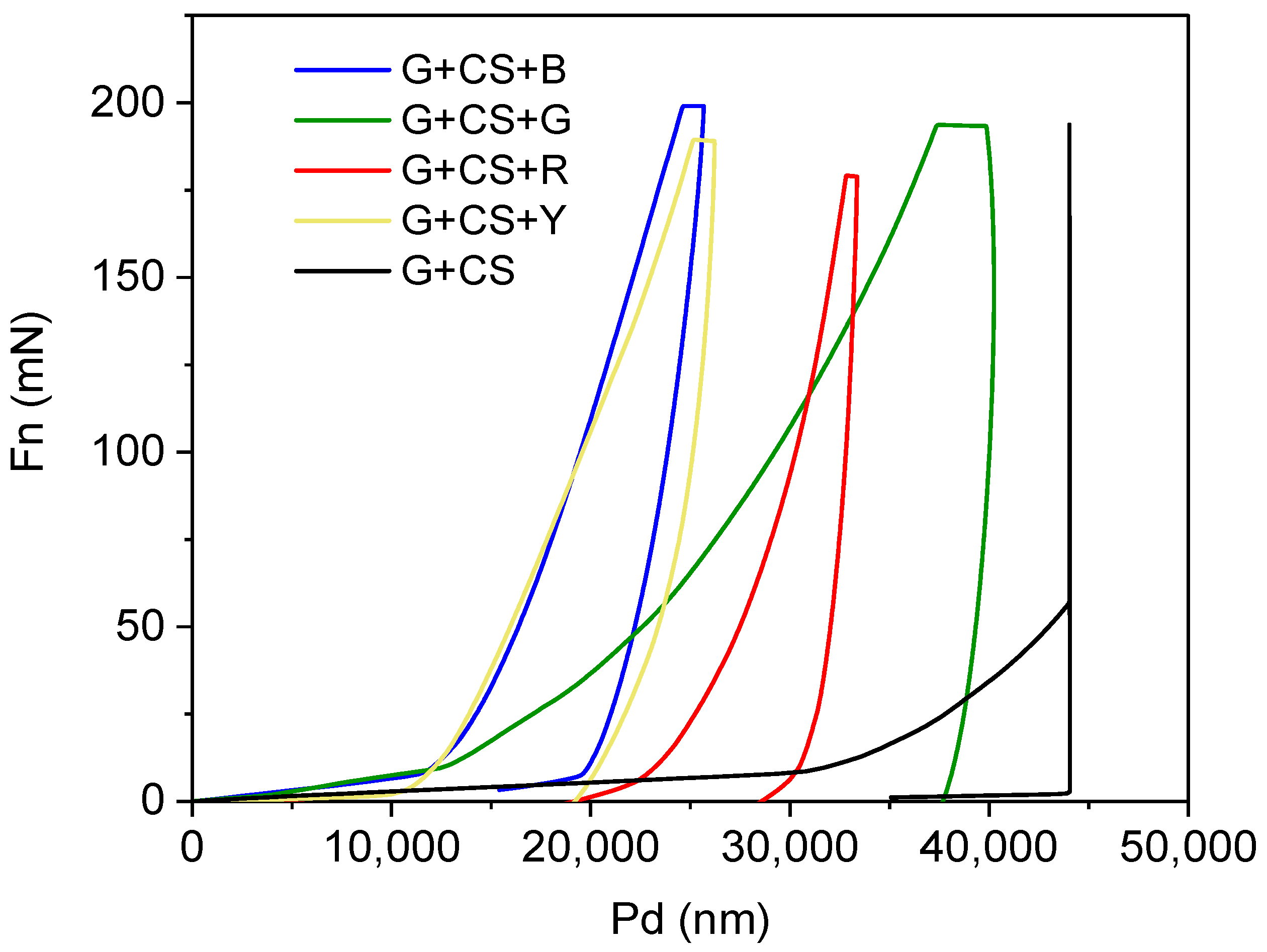
2.4.4. Bending and Hardness
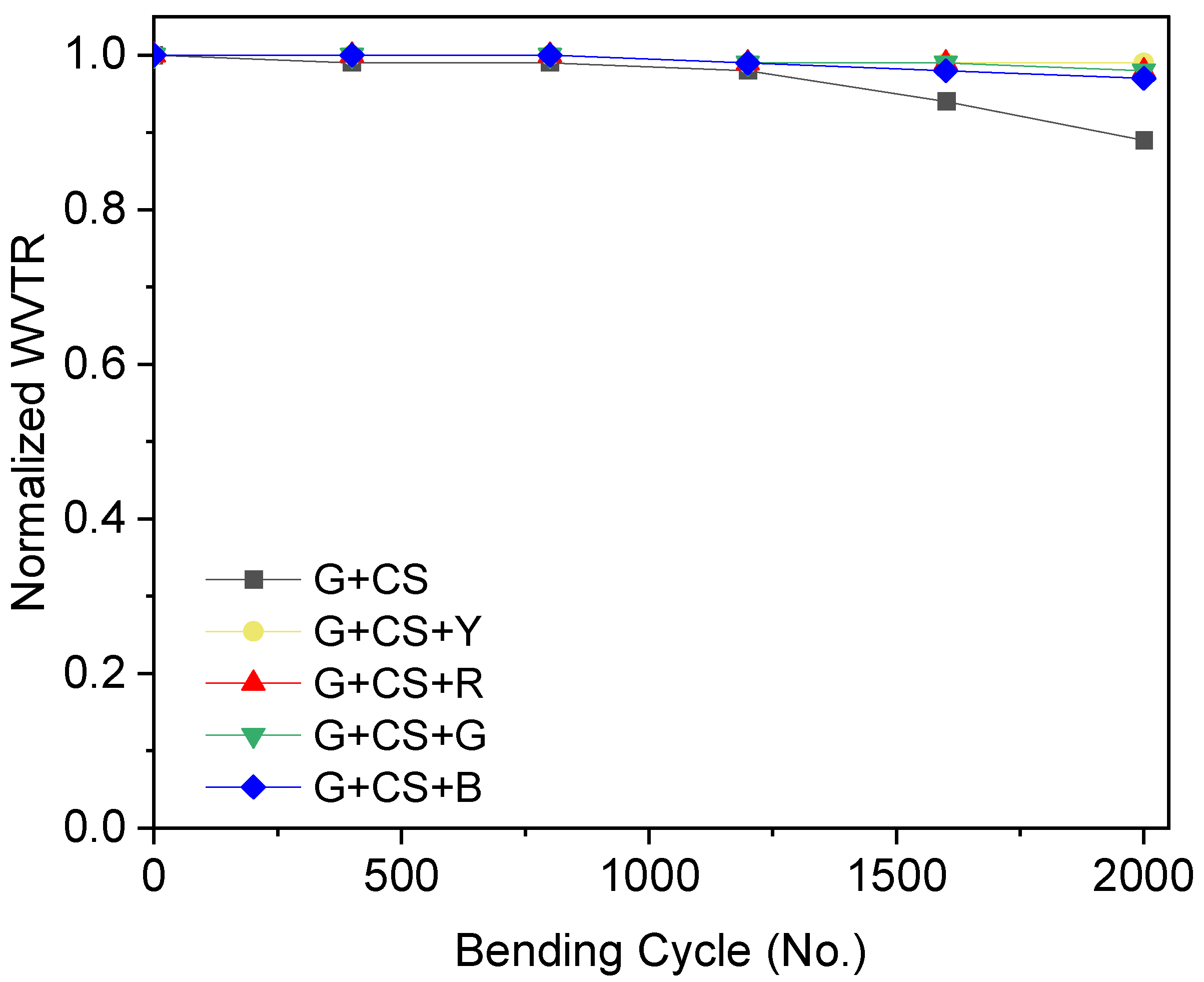
2.4.5. Water Vapor Transmission Rate (WVTR)
2.4.6. Thermogravimetric Analysis (TGA)
2.4.7. Atomic Force Microscopy (AFM)
2.4.8. Shore Hardness
3. Results and Discussion
3.1. Composition Analysis
3.2. Optical Analysis
3.3. Wettability Properties
3.4. Flexibility of Film
3.5. Analysis of Nanoindentation
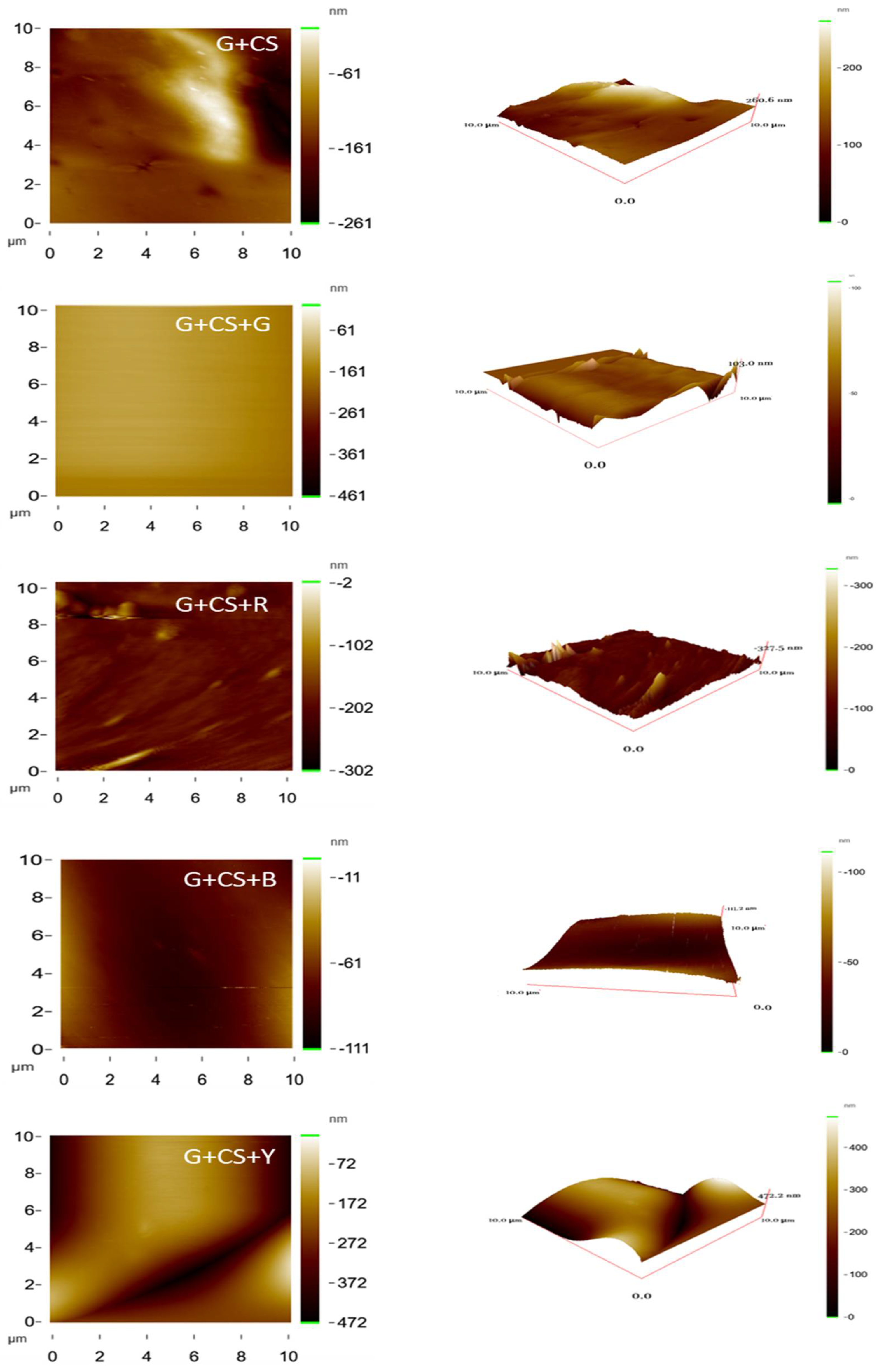
3.6. Surface Analysis
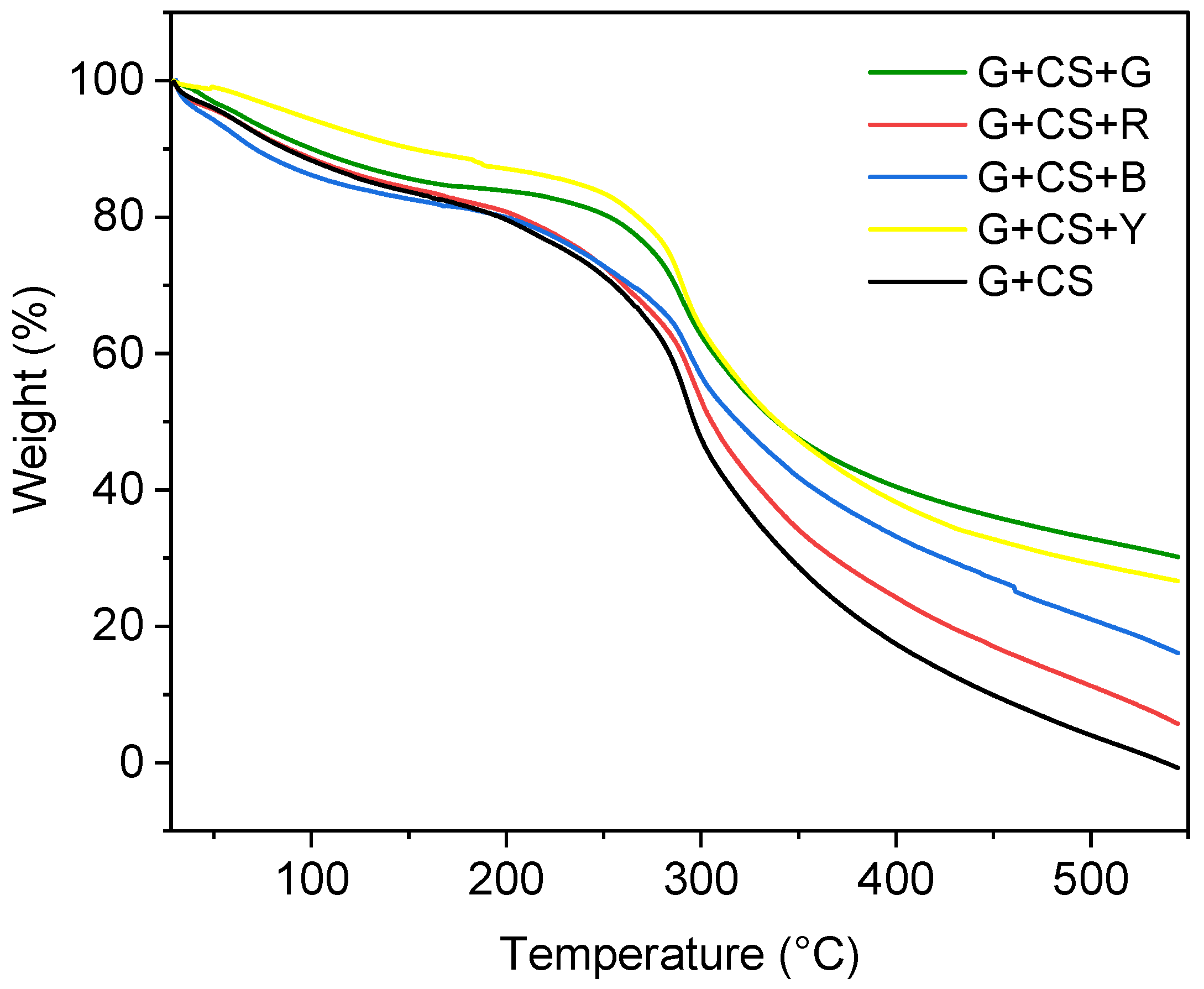
3.7. Thermal Stability of Film
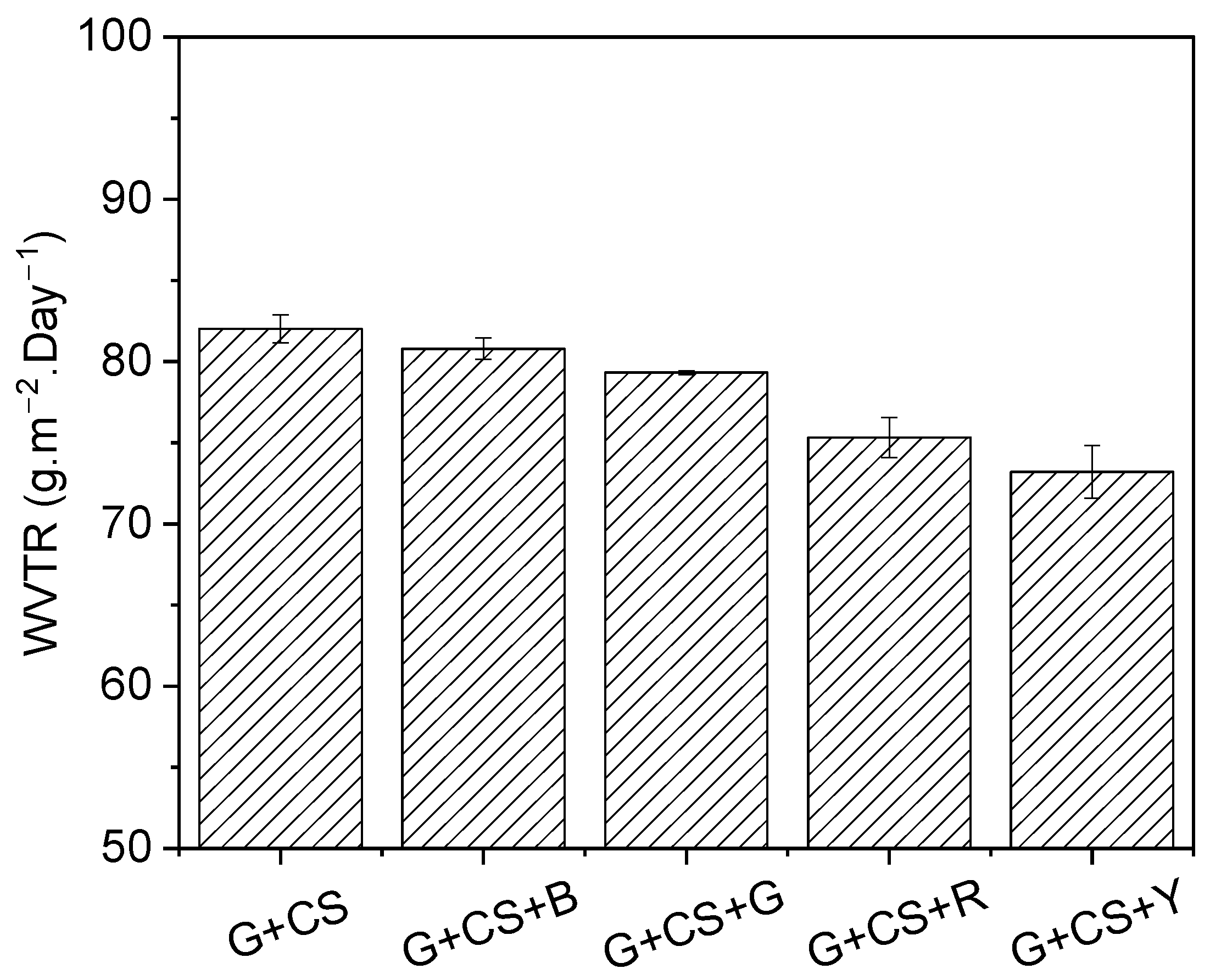
3.8. Barrier Effect on Film
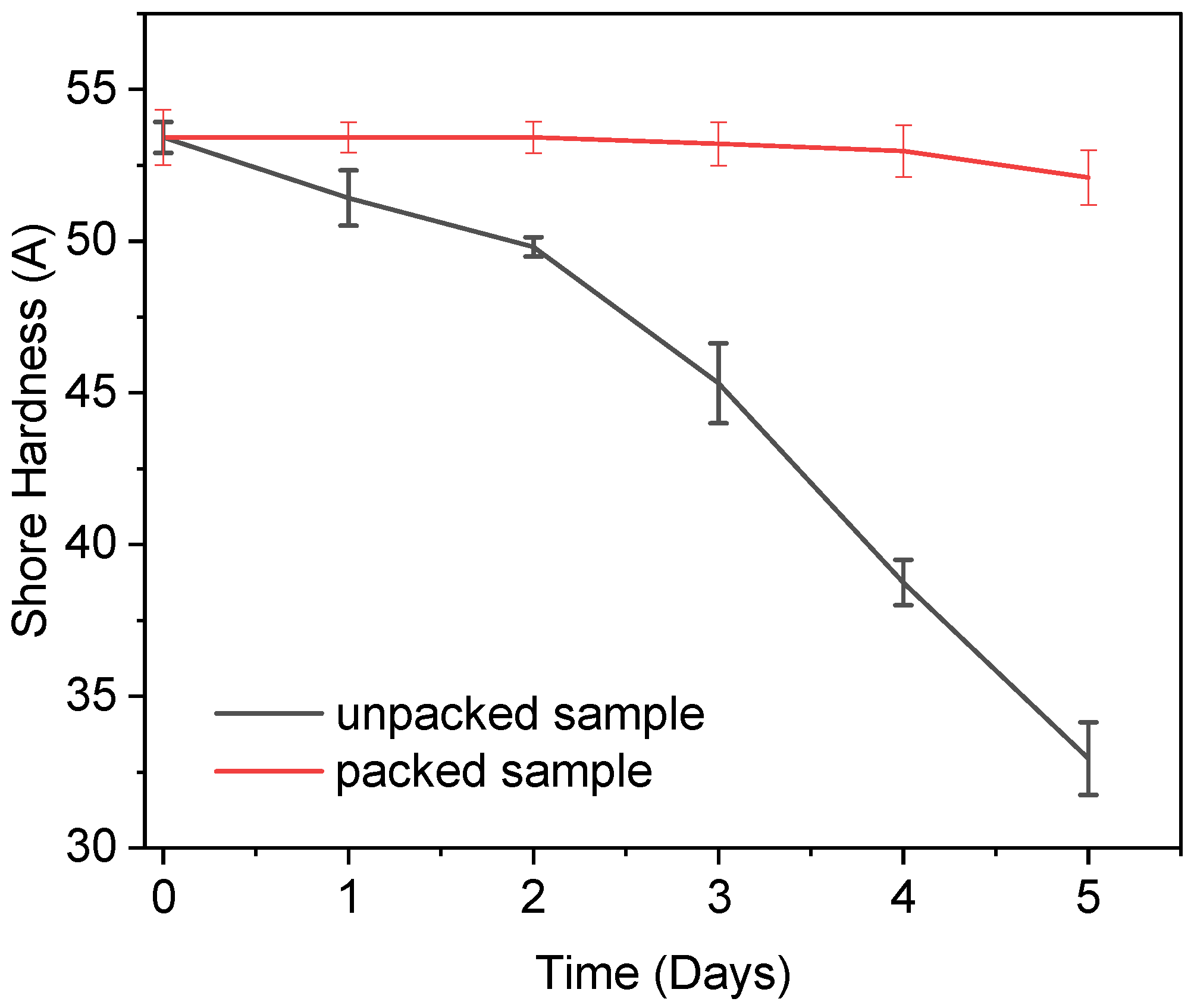
3.9. Real-Time Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siskawardani, D.D.; Warkoyo; Hidayat, R.; Sukardi. Physic-mechanical properties of edible film based on taro starch (Colocasia esculenta L. Schoott) with glycerol addition. IOP Conf. Ser. Earth Environ. Sci. 2020, 458, 012039. [Google Scholar] [CrossRef]
- Said, M.I.; Erwanto, Y.; Abustam, E. Properties of edible film produced using combination of collagen extracts of bligon goatskin with glycerol. Am. J. Anim. Vet. Sci. 2016, 11, 151–159. [Google Scholar] [CrossRef]
- Khodaei, D.; Álvarez, C.; Mullen, A.M. Biodegradable packaging materials from animal processing co-products and wastes: An overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H. Biodegradation of whey protein-based edible films. J. Polym. Environ. 2000, 8, 135–143. [Google Scholar] [CrossRef]
- Van Long, N.N.; Dantigny, P. Chapter 4—Fungal Contamination in Packaged Foods. In Antimicrobial Food Packaging; Barros-Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 45–63. [Google Scholar]
- Legan, J.D. Mould spoilage of bread: The problem and some solutions. Int. Biodeterior. Biodegrad. 1993, 32, 33–53. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Chapter 8—Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 271–306. [Google Scholar]
- Poonia, A. Antimicrobial edible films and coatings for fruits and vegetables. In Food Science and Nutrition: Breakthroughs in Research and Practice; IGI Global: Hershey, PA, USA, 2018; pp. 177–195. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Egelhaaf, H.; Brabec, C.J. Solution Coated Barriers for Flexible Electronics. In Organic Flexible Electronics, Fundamentals, Devices, and Applications; Cosseddu, P., Caironi, M., Eds.; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Ashfaq, J.; Channa, I.A.; Shaikh, A.A.; Chandio, A.D.; Shah, A.A.; Bughio, B.; Birmahani, A.; Alshehri, S.; Ghoneim, M.M. Gelatin-and Papaya-Based Biodegradable and Edible Packaging Films to Counter Plastic Waste Generation. Materials 2022, 15, 1046. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, L.; Limbo, S. Springer briefs in molecular science chemistry of foods. In Oil Derived Polymers; Parisi, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24732-8. [Google Scholar] [CrossRef]
- Azeem, M.; Jan, R.; Farrukh, S.; Hussain, A. Improving gas barrier properties with boron nitride nanosheets in polymer-composites. Results Phys. 2019, 12, 1535–1541. [Google Scholar] [CrossRef]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, M.A.; Ashfaq, T.; Khan, S.; et al. Polyvinyl alcohol and nano-clay based solution processed packaging coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Kwon, S.; Orsuwan, A.; Bumbudsanpharoke, N.; Yoon, C.; Choi, J.; Ko, S. A Short Review of Light Barrier Materials for Food and Beverage Packaging. Korean J. Packag. Sci. Technol. 2018, 24, 141–148. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Chandio, A.D.; Yousuf, S.; Makhdoom, M.A.; Nasser, M. Sustainable and Eco-Friendly Packaging Films Based on Poly (Vinyl Alcohol) and Glass Flakes. Membranes 2022, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; EL-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Nasser, M. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.-Y.; Bhaduri, B.; Kurkuri, M. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism—A review. Sustain. Mater. Technol. 2022, 31, e00378. [Google Scholar] [CrossRef]
- Sedlarikova, J.; Janalikova, M.; Peer, P.; Pavlatkova, L.; Minarik, A.; Pleva, P. Zein-based films containing monolaurin/eugenol or essential oils with potential for bioactive packaging application. Int. J. Mol. Sci. 2022, 23, 384. [Google Scholar] [CrossRef]
- Thakur, N.; Chaudhary, V. Fruit Purees, Extracts and Juices: Sustainable Source of Edible Packaging. In Edible Food Packaging, Applications, Innovations and Sustainability; Springer: Singapore, 2022; pp. 175–190. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An overview of plasticwaste generation and management in food packaging industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 32–58. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lintang, M.; Tandi, O.; Layuk, P.; Karouw, S.; Dirpan, A. Characterization edible films of sago with glycerol as a plasticizer. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 22070. [Google Scholar] [CrossRef]
- Saklani, P.; Siddhnath; Kishor Das, S.; Singh, S.M. A Review of Edible Packaging for Foods. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2885–2895. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. 23—Plasticizers in edible films and coatings. In Innovations in Food Packaging—Food Science and Technology; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 403–433. [Google Scholar]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Singh, A.; Gu, Y.; Castellarin, S.D.; Kitts, D.D.; Pratap-Singh, A. Development and Characterization of the Edible Packaging Films Incorporated with Blueberry Pomace. Foods 2020, 9, 1599. [Google Scholar] [CrossRef]
- Channa, I.A. Development of Solution Processed Thin Film Barriers for Encapsulating Thin Film Electronics Entwicklung von Lösungsprozessierten Dünnschichtbarrieren für die Verpackung von Dünnschichtelektronik. Ph.D. Thesis, Friedrich Alexander University of Erlangen Nuremberg, Nuremberg, Germany, 2019. [Google Scholar]
- Zuo, G.; Song, X.; Chen, F.; Shen, Z. Physical and structural characterization of edible bilayer films made with zein and corn-wheat starch. J. Saudi Soc. Agric. Sci. 2019, 18, 324–331. [Google Scholar] [CrossRef]
- Andrade, R.M.S.; Ferreira, M.S.L.; Gonçalves, É.C.B.A. Development and Characterization of Edible Films Based on Fruit and Vegetable Residues. J. Food Sci. 2016, 81, E412–E418. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.; Ye, R.; Xiao, J.; Liu, Y.; Ding, J.; Zhang, S.; Liu, A. Mechanical and barrier properties of maize starch–gelatin composite films: Effects of amylose content. J. Sci. Food Agric. 2017, 97, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-H.; Singh, R.K. Detoxification of aflatoxins in foods by ultraviolet irradiation, hydrogen peroxide, and their combination—A review. LWT 2021, 142, 110986. [Google Scholar] [CrossRef]
- Nguyen, T.; Palmer, J.; Loo, T.; Shilton, A.; Petcu, M.; Newson, H.L.; Flint, S. Investigation of UV light treatment (254 nm) on the reduction of aflatoxin M1 in skim milk and degradation products after treatment. Food Chem. 2022, 390, 133165. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—Structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.D.; Rios-Velasco, C.; Olivas Orozco, G.I.; Espino-Díaz, M.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J.; Aguilar-González, M.A.; Lardizábal-Gutiérrez, D.; et al. Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid. Coatings 2018, 8, 384. [Google Scholar] [CrossRef]
- Shah, A.A.; Bhatti, M.A.; Tahira, A.; Chandio, A.D.; Channa, I.A.; Sahito, A.G.; Chalangar, E.; Willander, M.; Nur, O.; Ibupoto, Z.H. Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram. Int. 2020, 46 Pt A, 9997–10005. [Google Scholar] [CrossRef]
- Khoirunnisa, A.R.; Joni, I.M.; Panatarani, C.; Rochima, E.; Praseptiangga, D. UV-screening, transparency and water barrier properties of semi refined iota carrageenan packaging film incorporated with ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 3–8. [Google Scholar] [CrossRef]
- Batool, S.A.; Liaquat, U.; Channa, I.A.; Gilani, S.J.; Makhdoom, M.A.; Yasir, M.; Ashfaq, J.; bin Jumah, M.N.; ur Rehman, M.A. Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications. Bioengineering 2022, 9, 367. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hong, Y.S.; Ho, W.F. Mechanical properties and deformation behavior of as-cast Ti-Sn alloys. J. Alloys Compd. 2009, 479, 390–394. [Google Scholar] [CrossRef]
- Channa, I.A.; Chandio, A.D.; Rizwan, M.; Shah, A.A.; Bhatti, J.; Shah, A.K.; Hussain, F.; Shar, M.A.; Alhazaa, A. Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells. Materials 2021, 14, 2496. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.; Anandan, C.; Rajendran, N. Effect of plasma nitriding on structure and biocompatibility of self-organised TiO2 nanotubes on Ti-6Al-7Nb. RSC Adv. 2015, 5, 41763–41771. [Google Scholar] [CrossRef]
- Radhakrishnan, P.M. A Review on Nano-Indentation of Thin Polymeric Films. IJERT—Int. J. Eng. Res. Technol. 2019, 8, 115–117. [Google Scholar]
- Waheed, H.; Hussain, A.; Farrukh, S. Fabrication, characterization and permeation study of ultrafiltration dialysis membranes. Desalin. Water Treat. 2016, 57, 24799–24806. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Islamipour, Z.; Zare, E.N.; Salimi, F.; Ghomi, M.; Makvandi, P. Biodegradable antibacterial and antioxidant nanocomposite films based on dextrin for bioactive food packaging. J. Nanostruct. Chem. 2022, 12, 991–1006. [Google Scholar] [CrossRef]
- Devi, N.; Hazarika, D.; Deka, C.; Kakati, D.K. Study of complex coacervation of gelatin a and sodium alginate for microencapsulation of olive oil. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 936–945. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Ahmad, A.A.; Al Dairy, A.R.; Al-anbar, A.S.; Al-Bataineh, Q.M. Spectroscopic characterization of optical and thermal properties of (PMMA-PVA) hybrid thin films doped with SiO2 nanoparticles. Results Phys. 2020, 19, 103463. [Google Scholar] [CrossRef]
- Pokatong, W.D.R.; Decyree, J. Characterization and Development of Edible Film/Coating from Lesser Yam Starch-Plasticizer Added with Potassium Sorbate or Cinnamon Oil in Affecting Characteristics and Shelf Life of Stored, Coated Strawberry. Reaktor 2019, 18, 224–234. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Rhim, J.W.; Shellhammer, T.H. 21—Lipid-based edible films and coatings. In Innovations in Food Packaging—Food Science and Technology; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 362–383. [Google Scholar]
- Koch, T.; Bierögel, C.; Seidler, S. Conventional Hardness Values—Introduction. In Polymer Solids and Polymer Melts–Mechanical and Thermomechanical Properties of Polymers; Springer Nature: Cham, Switzerland, 2014; pp. 423–427. [Google Scholar]




| Sample | Composition | |
|---|---|---|
| G+CS | Gelatin (6 wt.%) + Cornstarch (2 wt.%) + Glycerin (3 wt.%) |  |
| G+CS+Y | Gelatin (6 wt.%) + Cornstarch (2 wt.%) + Glycerin (3 wt.%) + yellow color liquid (1 wt.%) |  |
| G+CS+R | Gelatin (6 wt.%) + Cornstarch (2 wt.%) + Glycerin (3 wt.%) + red color liquid (1 wt.%) |  |
| G+CS+G | Gelatin (6 wt.%) + Cornstarch (2 wt.%) + Glycerin (3 wt.%) + green color liquid (1 wt.%) |  |
| G+CS+B | Gelatin (6 wt.%) + Cornstarch (2 wt.%) + Glycerin (3 wt.%) + blue color liquid (1 wt.%) |  |
| Sample | Ra (nm) | Rq (nm) | Rz (nm) |
|---|---|---|---|
| G+CS | 10.77 | 13.18 | 94.83 |
| G+CS+R | 10.83 | 17.55 | 291.41 |
| G+CS+G | 31.23 | 43.98 | 258.72 |
| G+CS+B | 37.95 | 54.60 | 443.92 |
| G+CS+Y | 77.17 | 93.38 | 471.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channa, I.A.; Ashfaq, J.; Siddiqui, M.A.; Chandio, A.D.; Shar, M.A.; Alhazaa, A. Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications. Polymers 2022, 14, 5020. https://doi.org/10.3390/polym14225020
Channa IA, Ashfaq J, Siddiqui MA, Chandio AD, Shar MA, Alhazaa A. Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications. Polymers. 2022; 14(22):5020. https://doi.org/10.3390/polym14225020
Chicago/Turabian StyleChanna, Iftikhar Ahmed, Jaweria Ashfaq, Muhammad Ali Siddiqui, Ali Dad Chandio, Muhammad Ali Shar, and Abdulaziz Alhazaa. 2022. "Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications" Polymers 14, no. 22: 5020. https://doi.org/10.3390/polym14225020
APA StyleChanna, I. A., Ashfaq, J., Siddiqui, M. A., Chandio, A. D., Shar, M. A., & Alhazaa, A. (2022). Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications. Polymers, 14(22), 5020. https://doi.org/10.3390/polym14225020











